Myosin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview

Myosins are a large family of motor proteins found in eukaryotic tissues. They are responsible for actin-based motility.
Structure and Function
Domains
Most myosin molecules are composed of both a head and a tail domain.
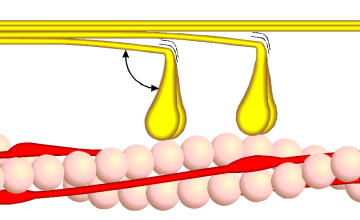
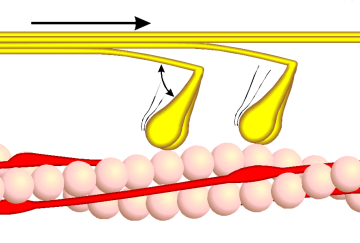
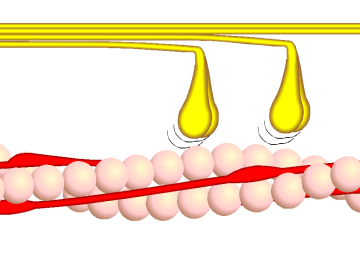
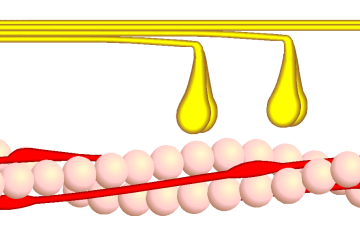
- The head domain binds the filamentous actin, and uses ATP hydrolysis to generate force and to "walk" along the filament towards the (+) end (with the exception of one family member, myosin VI, which moves towards the (-) end).
- The tail domain generally mediates interaction with cargo molecules and/or other myosin subunits.
Myosin I
Myosin I's function is unknown, but it is believed to be responsible for vesicle transport or the contraction vacuole of cells.[1]
Myosin II
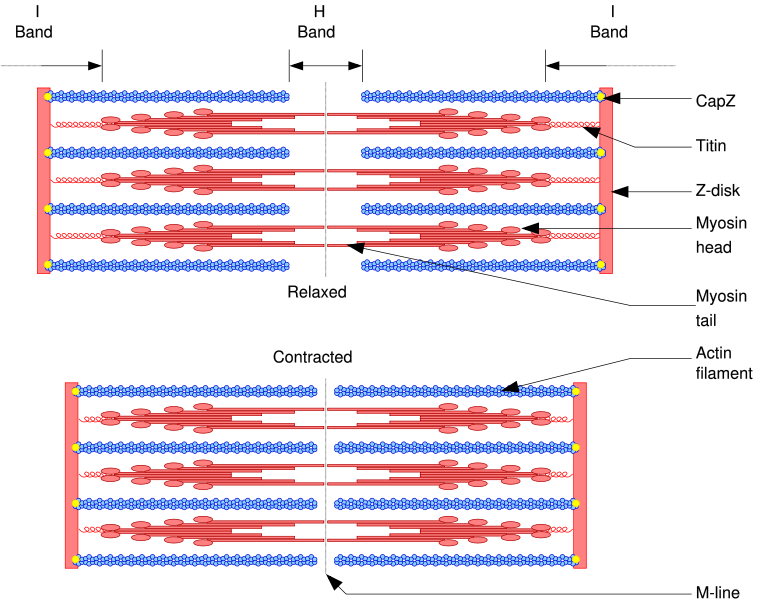
Myosin II, responsible for skeletal muscle contraction, is perhaps the best-studied example of these properties.
- Myosin II contains two heavy chains, each about 2000 amino acids in length, which constitute the head and tail domains. Each of these heavy chains contains the N-terminal head domain, while the C-terminal tails take on a coiled-coil morphology, holding the two heavy chains together (imagine two snakes wrapped around each other, such as in a caduceus). Thus, myosin II has two heads.
- It also contains 4 light chains (2 per head), which bind the heavy chains in the "neck" region between the head and tail.
In muscle cells, it is myosin II that is responsible for producing the contractile force. Here, the long coiled-coil tails of the individual myosin molecules join together, forming the thick filaments of the sarcomere. The force-producing head domains stick out from the side of the thick filament, ready to walk along the adjacent actin-based thin filaments in response to the proper chemical signals.
Evolution and Family Tree
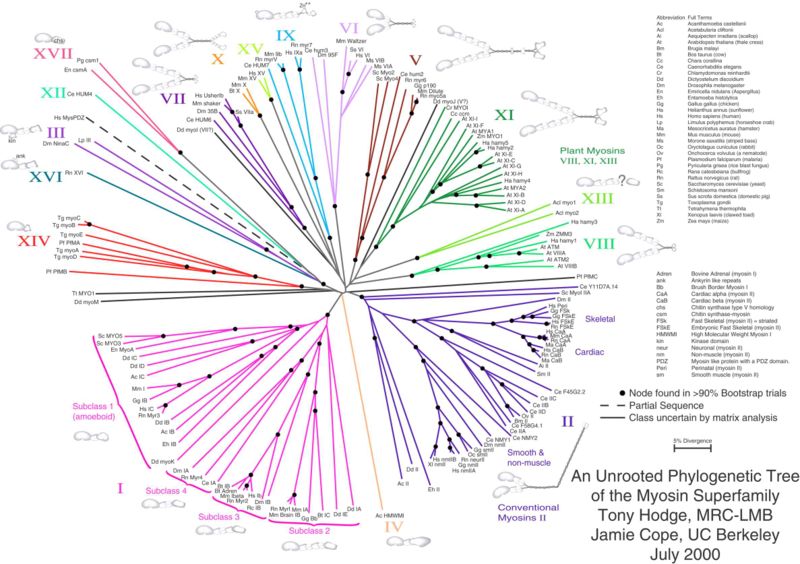
Myosin II, the most conspicuous of the myosin superfamily due to its abundance in muscle fibers, was the first to be discovered. However, beginning in the 1970s researchers began to discover new myosin variants, with one head (as opposed to myosin II's two) and largely divergent tail domains. These new superfamily members have been grouped according to their structural similarities, with each subfamily being assigned a Roman numeral. The now diverse array of myosins has evolved from an ancestral precursor (see picture).
Analysis of the amino acid sequences of different myosins shows great variability among the tail domains but almost perfect retention of the same head sequence. Presumably this is so the myosins may interact, via their tails, with a large number of different cargoes, while the goal in each case - to move along actin filaments - remains the same and therefore requires the same machinery in the motor. For example, the human genome contains over 40 different myosin genes.
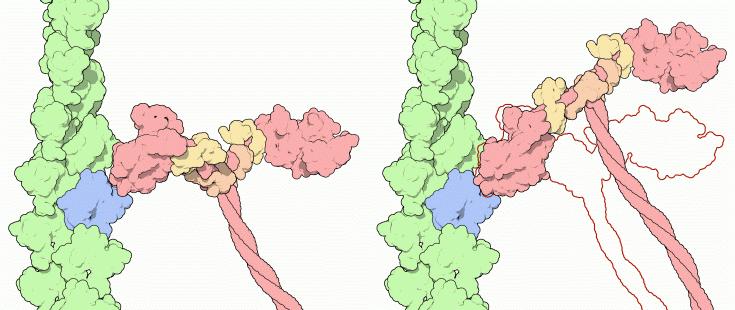
These differences in shape also determine the speed at which myosins can move along actin filaments. The hydrolysis of ATP and the subsequent release of the phosphate group causes the "power stroke," in which the "lever arm" or "neck" region of the heavy chain is dragged forward. Since the power stroke always moves the lever arm by the same angle, the length of the lever arm determines how fast the cargo will move. A longer lever arm will cause the cargo to traverse a greater distance even though the lever arm undergoes the same angular displacement - just as a person with longer legs can move farther with each individual step. Myosin V, for example, has a much longer neck region than myosin II, and therefore moves 30-40 nanometers with each stroke as opposed to only 5-10.
Genes in humans
Note that not all of these genes are active.
- Family I: MYO1A, MYO1B, MYO1C, MYO1D, MYO1E, MYO1F, MYO1G, MYO1H
- Family III: MYO3A, MYO3B
- Family V: MYO5A, MYO5B, MYO5C
- Family VI: MYO6
- Family VII: MYO7A, MYO7B
- Family IX: MYO9A, MYO9B, MYO10
- Family XV: MYO15A
- Family XVIII: MYO18A, MYO18B
- Heavy chain: MYH1, MYH2, MYH3, MYH4, MYH6, MYH7, MYH7B, MYH8, MYH9, MYH10, MYH11, MYH13, MYH14, MYH15, MYH16. See also MYH7.
- Light chain: MYL1, MYL2, MYL3, MYL4, MYL5, MYL6, MYL6B, MYL7, MYL9, MYLIP, MYLK, MYLK2, MYLL1
References
- ↑ Sutherland Macive (6/4/03). "Myosin I". Retrieved 23/05/2007. Check date values in:
|accessdate=, |date=(help)
- T. Hodge and M.J.T.V. Cope (2000). "A Myosin Family Tree". Journal of Cell Science. 113: 3353–3354. Template:Entrez Pubmed
- Molecular Biology of the Cell. Alberts, Johnson, Lewis, Raff, Roberts, and Walter. 4th Edition. 949-952.
External links
Additional images
-
Phase 1
-
Phase 2
-
Phase 3
-
Phase 4
-
Myosin-painting
-
Myosin powerstroke
See also
External links
- Myosin Video A video of a moving myosin motor protein.
- Myosins at the US National Library of Medicine Medical Subject Headings (MeSH)
- The Myosin Homepage
- EC 3.6.4.1
de:Myosin is:Mýósín it:Miosina lt:Miozinas mk:Миозин sr:Миозин sv:Myosin