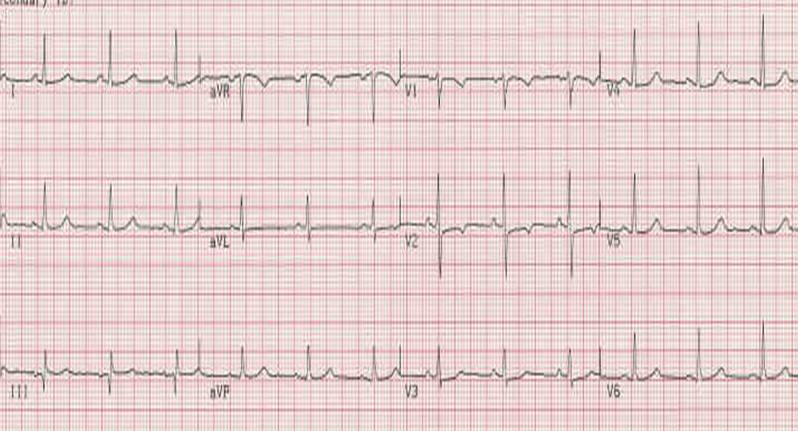
Electrocardiography
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Electrocardiography
Every electrocardiogram (ECG) has nine features that should be examined systematically: [1] [2] [3] [4] [5] [6]

1. Rate and regularity.
2. P wave morphology.
3. PR interval.
4. QRS interval
5. ST segment morphology.
6. T wave morphology.
7. U wave morphology.
8. QT interval.
9. Rhythm.
Rate, regularity, and rhythm are commonly grouped together. However, to accurately assess rhythm, it is necessary to consider not only rate and regularity, but also the various waveforms and intervals on the ECG.
The Normal Adult EKG
- Majority QRS complexes are positive (have tall R waves)
- Except AVR and V1-2:R-wave progression across the precordium.
- T-wave in V1 should be small, flat or flipped.[7]
The P wave Morphology
The P wave represents atrial depolarization (stimulation). At either slow or normal heart rates, the small, rounded P wave is clearly visible just before the taller, more peaked QRS complex. At more rapid rates, however, the P wave may merge with the preceding T wave and become difficult to identify. represents depolarization of the atrial myocardium. Sinus node depolarization is too small in amplitude to be recorded from the body surface so it is not seen.
P waves have four steps to be identified;
A. Examination of the P wave contour. The P wave contour is normally smooth, and is either entirely positive or entirely negative wave (monophasic wave) in all leads except V1.
B. Measurement of the P wave duration. The P wave duration is normally less than 0.12 s.
C. Measurement of the maximal P wave amplitude. The maximal P-wave amplitude is normally no more than 0.2 mV in the frontal plane leads and no more than 0.1 mV in the transverse plane leads.
D. Estimation of the P wave axis. The P wave normally appears entirely upright on leftward and inferiorly oriented leads such as I, II, aVF, and V4 to V6. It is negative in aVR because of the rightward orientation of that lead, and it is variable in the other standard leads.
The direction of the P wave, or its axis in the frontal plane, should be determined on the morphology of the QRS complex. The normal limits of the P wave axis are 0 degrees and +75 degrees
The PR Interval
Definition
The PR interval measures the time required for an electrical impulse to travel from the atrial myocardium adjacent to the sinoatrial (SA) node to the ventricular myocardium adjacent to the fibers of the Purkinje network.
This duration is normally from 0.10 to 0.21 s. A major portion of the PR interval reflects the slow conduction of an impulse through the AV node, which is controlled by the balance between the sympathetic and parasympathetic divisions of the autonomic nervous system. Therefore, the PR interval varies with the heart rate, being shorter at faster rates when the sympathetic component predominates, and vice versa. The PR interval tends to increase with age [8] [9];
- In childhood: 0.10 - 0.20 sec
- In adolescence: 0.12 - 0.16 sec
- In adulthood: 0.14 - 0.21 sec
Background
- Shortens up to a rate of 140 to 150 beats per minute (bpm) through a withdrawal of parasympathetic tone
- PR may increase with increasing rate in the presence of digoxin or if the conducting system is diseased.
- If the atria are artificially paced the PR increases as the paced rate increases.
- Children have shorter PR intervals (0.11 at 1 year).
- Prolongation can be a normal variant: 6700 healthy airmen studied and 0.52% found to have a prolonged PR. 80% of the PR prolongations ranged from 0.21 to 0.24. In a second study 59 of 19,000 (0.31%) airmen had a PR of 0.24 or more.
- In healthy middle aged men, a prolongation of the PR in the presence of a normal QRS does not affect prognosis and is not related to ischemic heart disease.
- PR prolongation often signifies a delay in the AV node but may reflect intra atrial or His Purkinje disease.
Differential Diagnosis of the Shortened PR Interval:
- AV junctional and low atrial rhythms.
- Wolff Parkinson White syndrome.
- Lown Ganong Levine syndrome.
- Glycogen storage disease.
- Hypertension.
- Normal variant.
- Fabry's disease.
- Pheochromocytoma.
Differential Diagnosis of a Prolonged PR Interval:
- AV block due to coronary artery disease, rheumatic disease.
- Hyperthyroidism.
- Normal variant.
Differential Diagnosis of the Depressed PR Interval:
The QRS Interval
The QRS interval represents ventricular depolarization (stimulation).
Duration
- The precordial leads are approximately 0.01 or 0.02 seconds longer than the standard leads.
Amplitude
- Definitions of low voltage:
- If the total amplitude above and below the isoelectric line is < 5 mm in all 3 standard leads.
- An average voltage in the limb leads of < 5 mm with an average of < 10 mm in the chest leads.
- Should be at least 5 mm in V1 and V6 , 7 mm in V2 and V5 and 9 mm in V3 and V4.
- If the total amplitude above and below the isoelectric line is < 5 mm in all 3 standard leads.
- Differential diagnosis of low voltage:
- Maximal acceptable voltage: can be up to 20 to 30 mm in lead 2 and can be up to 25 to 30 mmm in the precordial leads.
References
1. Unverferth, Chest 1979: 75, 157.
The QTc Interval
Bazett's formula
The below image shows the formula for correcting the observed Q-T interval in the electrocardiogram for cardiac rate.
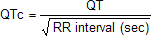
Prolongation of the QTc Interval:
- Represents the time required for completion of ventricular depolarization and repolarization.
- Abnormal when the QTc is > .44 seconds.
- Causes
- Idiopathic long QT syndrome
- The Jervell and Lange-Nielsen syndrome
- Is associated with congenital deafness, syncope, and sudden death.
- Autosomal recessive inheritance
- Heterozygotes may be normal or have a slightly prolonged QT interval
- Incidence among deaf mute children is 0.25%
- The Romano-Ward syndrome
- Clinically similar to the Jervell and Lange-Nielsen syndrome except the hearing is normal
- Autosomal dominant
- Heterozygotes and homozygotes persons may have similar symptoms
- Sporadic long QT syndrome
- Females:males = 2:1
- 57% had a history of syncope
- There was a strong association between syncopal episodes and emotions, vigorous activities and loud noises.
- Pathogenesis
- Imbalance between various components of the cardiac sympathetic innervation.
- Treatment to shorten the QT syndrome:
- Left stellate ganglion block
- Right stellate ganglion stimulation
- The administration of propranolol
- The Jervell and Lange-Nielsen syndrome
Short QT Intervals:
- Digoxin therapy
- Hypercalcemia
- Secondary (acquired) types of QTc prolongation
- Coronary artery disease: Ischemia, infarction
- MVP, cardiomyopathy
- CNS disease, especially intracerebral hemorrhage
- Autonomic nervous system dysfunction secondary to radical neck dissection, carotid endarterectomy, transabdominal truncal vagotomy.
- Metabolic disturbances. Electrolyte imbalance (such as hypocalcemia), liquid protein diet, intracoronary injection of the contrast agents.
- Cardiac medications: Quinidine, procainamide, disopyramide, encainide, flecainide, propafenone, amiodarone.
- Psychotropic drugs. Phenothiazines, tricyclic antidepressants.
- Miscellaneous. Severe bradycardia, high degree AV block, post syncope period of patients with Adams-Stokes syndrome, hypothyroidism, hypothermia, pheochromocytoma, organophosphate poisoning.
The T Wave
Direction
- Normally upright in leads 1 and 2 amd in the chest leads over the left ventricle.
- Normally inverted in aVR.
- Normally upright in aVL and aVF if the QRS is > 5 mm tall but may be inverted if the R waves are smaller.
- In infants and young children precordial T waves may be inverted.
- In adult males it is considered abnormal if the T waves are inverted as far to the left as lead V3.
- In adult females the T in V3 may be shallowly inverted.
- The T wave in V1 may be inverted at any age (is more often inverted than upright) and the T in V2 can normally be inverted.
- When the T in V1 is upright, it is almost never as tall as the T in V6.
Shape
- Notched in children and in adults with Pericarditis
- Can be sharply pointed in ischemia or infarction.
Height
- Normally not > 5 mm in any standard lead and not > 10 mm in any precordial lead
- Differential diagnosis of the tall T wave:
- Myocardial Ischemia
- Potassium intoxication
- Myocardial infarction
- Ventricular overloading
- Psychosis
- Cerebrovascular accident (usually inverted, widely splayed, frequently in subarrachnoid hemorrhages)
- Differential diagnosis of short T waves:
- Obesity Reverses with weight loss
The U Wave
Background
- Ordinarily the U wave has the same polarity as the T wave and is 5 to 25% of the T wave amplitude.
- Tallest in leads V2 and V3, usually not greater than 1.0 mm.
- Considered abnormally large if the U wave is greater than 1.5 mm in any lead.
Causes of Abnormal Prominence
- Bradycardia
- Electrolyte imbalance
Causes of U Wave Inversion
- Left ventricular hypertrophy (LVH): (in I, V5, V6)
- Right ventricular hypertrophy (RVH): (in II, III)
- Ischemic heart disease
- May occur during anginal episode
- U wave inversion during ETT is considered indicative of ischemia by some
Specific Electrocardiographic Abnormalities
EKG Changes Associated with ASDs
- Incomplete and less frequently complete RBBB.
- RVH with strain suggests onset of pulmonary hypertension or associated pulmonic stenosis.
- 2/3 rds of patients with a secundum ASD have right axis deviation.
- Patients with secundum ASDs experience atrial fibrillation or atrial flutter, and this occurs with a higher incidence with increasing age and with pulmonary hypertension.
- Sinus venosus ASDs are often associated with low atrial and junctional rhythms.
- Ostium primum as well as long standing ostium secundum defects may be associated with first degree AV block which reflects prolonged conduction through the atria.
- Ostium primum defects are associated with a marked left axis deviation.
Atrial Arrhythmias
PACs
- Obviously are premature
- Morphology of the P' wave is different than the NSR P wave. If its origin is close to that of the sinus node, then the P' morphology is hard to distinguish from the sinus P wave.
- A PAC differs from a PJC in that the PR interval is > .12 second in a PAC.
- The PR interval may be shorter than that in NSR if it is located closer to the AV node.
- The PR interval tends to lengthen when the coupling time to the PAC is short.
- A PAC may not be conducted to the ventricles and is called a blocked PAC.
- Differential diagnosis includes second degree AV block. In second degree AV block, the PP intervals remain constant.
- Usually the QRS is of normal duration, but occasionally there is aberrant conduction, most frequently of RBBB morphology.
- Aberrancy is more likely to occur when the coupling time is shorter.
- Usually there is not a compensatory pause. The PAC resets the sinus node.
- Most these patients do not have organic heart disease.
- 64% of healthy subjects will have PACs on 24 hour Holter monitoring.
- Frequency higher in the elderly
- In some patients they are related to emotional stress, mental and physical fatigue, excessive smoking, or intake of alcohol and coffee.
- The incidence of PACs is increased in those with organic heart disease.
Ectopic Atrial Rhythm
- Different P wave morphology than NSR
- Rate < 100 BPM
- Called an accelerated atrial rhythm when the rate is faster than the patient's own NSR but < 100.
PAT
- Intraatrial reentrant tachycardia and automatic or ectopic atrial tachycardias are the two forms.
- Used to be thought a common cause of PSVT, but it is now realized the intranodal reentry is more common.
- Accounts for about 10% of PSVTs.
- Abnormal P waves that are different form those in NSR. Often small and hard to identify.
- Atrial rate is 100 to 180 BPM. Rate is usually < 150 in the atrial reentrant form.
- Rhythm is regular
- Paroxysm has three or more beats in succession.
- There is a QRS after each P wave that resembles that of NSR, but aberrancy can occur.
- PR interval is within normal limits or prolonged.
- Secondary STTW changes may occur.
- The rhythm may speed up after the first few beats.
- Not affected by vagal maneuvers.
- If some of the P waves are not followed by a QRS, then this is called PAT with block.
- The patients usually have organic heart disease.
PAT with Block
- P wave morphology different than that of NSR.
- Atrial rate between 150 and 250 BPM
- It is less than 200 in most cases.
- In atrial flutter the rate is > 250 BPM in most cases.
- Isoelectric baseline between P waves in all leads.
- In atrial flutter there is a sawtooth appearance to the P waves.
- AV block
- Usually 2:1, but can be 3:1, and can even be variable and resemble atrial fibrillation.
- Digoxin toxicity caused 73% of cases in the Lown series. Other reports put the number at 40 to 82%.
Multifocal Atrial Tachycardia MAT
- P waves of varying morphology from at least three different foci.
- The absence of one dominant atrial pacemaker.
- Variable PP, RR, and PR intervals.
- Atrial rate is above 100 BPM.
- Can be mistaken for atrial fibrillation if the P waves are of low amplitude.
- High incidence in the elderly and in those with Chronic Obstructive Pulmonary Disease (COPD).
Atrial Flutter
- Rapid regular undulations (F waves) that cause a sawtooth appearance.
- Best seen in 2,3,F and V1.
- Usually inverted in the inferior leads.
- No isoelectric baselines between the F waves.
- Atrial rate of 250 to 350 BPM.
- Can be faster in infants and children.
- Massive dilation of the atria can lead to a rate < 200 BPM.
- Quinidine can reduce the atrial rate.
- Variable ventricular rate depending on the AV conduction.
- Most common response is 2:1
- 3:1 is uncommon
- 4:1 suggests the existence of an AV conduction defect
- May be associated with complete AV block in which case the RR intervals are regular and the F waves have no constant relationship to the QRS. The ventricular response is usually slow.
- 1:1 conduction may be precipitated by excitement, exercise, induction of anesthesia or any increase in sympathetic tone. It may occur in WPW where the impulses are conducted antegrade through the bypass tract. All these are an emergency.
- During treatment with quinidine the atrial rate may slow sufficiently to permit 1:1 conduction.
- Vagal maneuvers increase the degree of AV block.
- QRS either normal or aberrant depending on preexisting IVCD or aberrant ventricular conduction.
Atrial Fibrillation
- Absent P waves.
- Irregularly irregular ventricular response rate.
- Atrial rate ranges from 400 to 700 BPM.
- Sometimes V1 may look as though there is atrial flutter. This may be because the electrode overlies a portion of the RA with rhythmic activity.
- Some authors believe that fine f waves (<.5 mm) are associated with coronary artery disease and that coarse F waves are associated with LA enlargement and rheumatic heart disease.
- The ventricular rate is usually between 100 and 180 BPM.
- If the atrial rate is greater than 200 BPM, then consider WPW.
- In the presence of AV junctional disease, the ventricular rate may be below 70 beats/minute.
- Complete AV block is indicated by a slow ventricular rhythm with a regular RR interval.
- Differential diagnosis includes tremor due to artifact. The oscillations in this case are largest in the limb leads.
- Most common cause of atrial fibrillation is CAD followed by rheumatic and hypertensive heart disease. Seen also in 30% of patients post CABG.
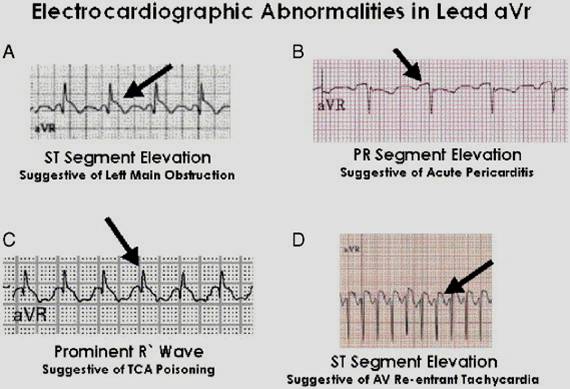
aVr abnormalities and differential diagnosis
- ST elevation in aVr suggestive of left main coronary artery occlusion.
- PR segment elevation suggests acute pericarditis.
- Prominent R wave is a feature of tricyclic antidepressant poisoning.
- Rapid, regular , narrow QRS complex tachycardia with ST elevation is a feature of WPW
Atrioventricular Block; Concealed Conduction
First Degree AV Block
- PR interval is greater than 0.20 seconds (20 msec)
- Each P wave is followed by a QRS
- Range is between 0.21 and 0.40 seconds
- P wave may be mistaken for a T wave or a U wave
- The PR interval is more variable in those without heart disease
- In patients with a narrow QRS, His-Bundle recordings show that the conduction delay is in the AV node, with prolongation of the AH time, rarely is a prolonged HV time responsible
- In patients with PR prolongation and QRS prolongation, then the conduction delay may occur in various regions of the conduction system
Second Degree AV Block
- Type I Second Degree AV Block:
- Also called the Wenckebach phenomenon or Mobitz type I
- Intermittent failure of the supraventricular impulse to be conducted to the ventricles, not every P wave is followed by a QRS
- There is progressive prolongation of the PR interval until a P wave is blocked
- Progressive shortening of the RR interval until a P wave is blocked
- The RR interval containing the blocked P wave is shorter than the sum of 2 PP intervals
- The increase in the PR interval is longest in the second conducted beat after the pause
- These rules may not be followed because of fluctuation in vagal tone and secondary to sinus arrhythmia.
- In patients with normal QRS width, the block is usually located in the AV node
- there is progressive prolongation of the AH interval until the blocked P wave occurs
- When it is associated with bundle branch block, the block may occur in the AV node, His bundle or the contralateral bundle branch
- in 75% the block is in the AV node
- in 25% it is infranodal
- Type II Second-Degree AV Block: Mobitz Type II Block
- There are intermittent blocked P waves
- In the conducted beats, the PR intervals remain constant
- The PR is fairly constant except that slight shortening may occur in the first beat after the blocked cycle. This is the result of improved conduction following the block
- Most patients with type II second-degree AV block have associated bundle branch block.
- In these instances the block is usually located distal to the His Bundle, in approximately 27 to 35% of patients however, the lesion is located in the His bundle itself, and a narrow complex may be inscribed.
- 2:1 AV Block:
- Impossible to determine whether the second-degree AV block is type I or type II.
- A long rhythm strip is helpful to document any change in the behavior of the conduction ratio
- When the atrial rate is increased by exercise or by atropine, the AV block in type I tends to decrease and that in type II tends to increase
- Advanced AV Block or High Grade AV Block:
- When the AV conduction ratio is 3:1 or higher
- In some cases only occasional ventricular captures are observed, and the dominant rhythm is maintained by a subsidiary pacemaker.
- You must compare the PR interval of the rare captured beats, a constant PR interval suggests type II block
- Differential Diagnosis of Second-Degree AV Block:
- Second Degree AV Block may be simulated by blocked PACs. Must be very careful to assure that the P to P intervals are constant
- 2:1 conduction may simulate sinus bradycardia as the blocked P waves may fall on the preceding T waves
Complete or Third-Degree AV Block
- There is complete failure of the supraventricular impulse to reach the ventricles.
- The atrial and the ventricular activities are independent of each other
- The block may be at the level of the AV node, the His bundle or the bundle branches
- If the block is in the main bundle branches, it is called bilateral bundle branch block
- If it involves the right bundle branch and two divisions of the left bundle, then it is called trifascicular block
- The atrial rate is faster than the ventricular rate
- The ventricular rhythm is maintained by either a junctional or an idioventricular pacemaker.
- The PP and RR intervals are regular, but the P waves bear no relation to the QRS complexes (i.e. the PR interval varies)
- In 30 to 40% of patients with complete AV block there is ventriculophasic sinus arrhythmia can be demonstrated. In this case, there is a decrease in the PP interval in those PP intervals containing a QRS.
- When the underlying rhythm is atrial fibrillation, the presence of complete AV block is manifested by the regularity of the ventricular rhythm.
- In AV block, the atrial rate is faster than the ventricular rate, in AV dissociation the ventricular rate is faster than the atrial rate (likely due to automaticity of a subsidiary pacemaker).
- If the subsidiary pacemaker is above the His bundle, then the escape rhythm is narrow complex and is likely to be AV junctional in origin.
- If the subsidiary pacemaker is below the His bundle, then the escape rhythm is wide. Wide complexes can result from a junctional escape rhythm with superimposed bundle branch block.
- Rate in complete AV block
- AV junctional escape rhythms have a rate between 40 to 60 beats per minute, may be increased by exercise or vagolytic agents
- idioventricular rhythms have a rate of 30 to 40 beats per minute but may be as low as 20 and as high as 50, not affected by exercise or vagolytic agents
- His Bundle recordings:
- allows determination of the site of block
- in chronic acquired complete AV block, most cases (@ 50 to 60%) have block located distal to the His bundle, and the QRS complexes are wide.
- in acute heart block secondary to IMIs, infection, or drugs, the site of the block is usually proximal to the His bundle.
- in acute anterior MIs, the site of the block is distal to the His bundle
Clinical Correlation of AV block
- PR prolongation can be found in 0.5% of healthy patients
- Second degree block type I may be seen in healthy patients during sleep
- Transient AV block can occur with vagal maneuvers
- In acute MI
- First degree block occurs in 8 to 13%
- second-degree block in 3.5 to 10%
- complete block in 2.5 to 8%
- IMIs: AV block is more common in patients with IMIs (1/3rd of patients)
- in 90% of patients the inferior wall is supplied by the RCA which gives off a branch to the AV node
- as a rule the AV block is transient and normal function returns within a week of the acute episode
- AMIs: AV block may be seen in up to 21%
- incidence of second-degree and third-degree AV block is 5 to 7%
- result of damage to the interventricular septum supplied by the LAD
- there is damage to the bundle branches either in the form of bilateral bundle branch block or trifascicular block
- RBBB, RBBB + LAHB, RBBB + LPHB or LBBB often appear before the development of AV block
- the PR is normal or minimally prolonged before the onset of second or third degree AV block
- Although the AV block is mostly transient, there is a relatively high incidence of recurrence or high-degree AV block after the acute event
- in addition to ischemia, fibrosis and calcification of the summit of the ventricular septum that involve the branching part of the bundle branches, play an important role in the genesis of the conduction defect.
- it used to be thought that CAD was the most frequent cause of chronic complete AV block, but it actually causes only 15% of cases
- Degenerative Diseases
- sclerodegenerative disease of the bundle branches first described by Lenegre
- the pathologic process is called idiopathic bilateral bundle branch fibrosis and the heart block is called primary heart block
- is the most common cause of chronic AV block (46%)
- Lev described similar degenerative lesions, which he referred to as sclerosis of the left side of the cardiac skeleton. There is progressive fibrosis and calcification of the mitral annulus, the central fibrous body, the pars membranacea, the base of the aorta, and the summit of the muscular ventricular septum. Various portions of the His bundle or the bundle branches may be involved, resulting in AV block.
- Hypertension
- chronic AV block in patients with HTN is thought to be due to CAD or sclerosis of the left side of the cardiac skeleton exacerbated by hypertension
- Myocardial Diseases
- various degrees of heart block are seen in 15% of patients with dilated cardiomyopathy
- 3% of patients with IHSS
- sarcoid is well known as a cause of block
- amyloidosis
- hemochromatosis
- muscular dystrophy
- SLE
- dermatomyositis
- scleroderma
- ankylosing spondylitis
- tumors, primary and secondary
- myocarditis
- PR prolongation is a common (25 to 95% of cases) sign in patients with acute rheumatic fever
- Type I second degree AV block may occur, but complete AV block is uncommon
- usually transient, disappears when the patient recovers
- diphtheria used to be an important cause
- Chagas disease
- Valvular Diseases
- Calcific aortic stenosis may be accompanied by chronic partial or complete AV block
- there is an extension of the calcification to involve the main bundle or its bifurcation, resulting in degeneration and necrosis of the conduction tissue
- may also occur in rheumatic mitral valve disease, but is less common
- occasionally, massive calcification of the mitral annulus as an aging process may cause AV block
- may also be seen in bacterial endocarditis, especially of the aortic valve
- Drugs
- digoxin is one of the most common causes of reversible AV block
- when second degree AV-block is induced, it is always of the Type I variety
- when complete block occurs, the QRS complexes are narrow because the block is of the AV node
- the ventricular response rate is more rapid than that due to organic lesions, and increased automaticity of the AV junctional pacemaker may be responsible.
- quinidine and procainamide may produce slight prolongation of the PR
- β blockers may cause AV block
- Diltiazem and verapamil may cause AV conduction delay and PR interval prolongation
- digoxin is one of the most common causes of reversible AV block
- Congenital
- occurs in the absence of other evidence of organic heart disease
- site is proximal to the bifurcation of the His bundle, mostly in the AV node
- narrow QRS with a rate > 40 beats per minute
- frequently seen in those with corrected transposition of the great vessels, and occasionally in ASDs and Ebstein's
- Trauma
- may be induced during open heart surgery in the area of AV conduction tissue
- seen in patients operated on for the correction of VSD, tetralogy of Fallot, and endocardial cushion defect.
- may be due to edema, transient ischemia, or actual disruption of the conduction tissue. The block may therefore be permanent or transient.
- also reported with both penetrating and non-penetrating trauma of the chest
Concealed conduction
- Partial penetration into the AV node may occur, but there is no ventricular response.
- Its presence is implied by the behavior of the impulse conduction or impulse formation or both.
- Suspect this when there is unexpected prolongation of the conduction time or block.
- Examples include
- prolongation of the PR interval after a PVC
- the retrograde impulse may penetrate the AV junction and reset its refractory period
- block of the P wave after a PVC
- long RR intervals in atrial fibrillation due to resetting of the junctional escape interval
- prolongation of the PR interval after a PVC
Criteria for Biventricular Hypertrophy
- Suggested if there are voltage criteria for LVH in the chest leads combined with right axis deviation in the limb leads.
- Suggested if there are voltage criteria for LVH in the chest leads combined with prominent R waves in the right chest leads.
- Suggested if there is a shallow S wave in V1 associated with a deeper S wave in V2, the so called shallow S wave syndrome.
- One of the best criteria according to Marriott is LAA as the sole representative of LV enlargement, combined with any of the following clues to RVH:
- S/R ratio of 1.0 or more in V5 or V6
- an S wave in V5 or V6 of 7 mm or more
- right axis deviation
EKG Abnormalities in Central Nervous System Disease
Diseases of the Central Nervous System (CNS)
- EKG changes seen in 71.5% of patients with subarachnoid hemorrhage, and 57.1% of those with cerebral hemorrhage.
- Most common abnormalities are
- large, upright, or deeply inverted T waves
- prolongation of the QTc interval
- prominent U waves
- Can persist for 11 days
- Rarely can have ST segment elevation or depression
- Rhythm disturbances
- sinus bradycardia
- sinus tachycardia
- wandering pacemaker
- AV junctional rhythm
- PVCs
- VT
- Reason for changes is thought to be altered autonomic tone
Drug Effects on the EKG
Digitalis
- Inhibits the transport of Na and K ions across the cell membrane
- It acts indirectly by increasing vagal tone
- Decreases the automaticity of the SA node at therapeutic doses
- The slowing of the sinus rate is due mostly to the vagal effect
- At toxic doses, it may increase the sinus rate as well as impair SA conduction.
- At therapeutic doses, the automaticity of subsidiary atrial pacemakers is reduced.
- Automaticity of subsidiary pacemakers may be increased at higher doses.
- At the AV node there is depression of conduction but enhancement of automaticity.
- This depression of conduction is due to both the vagal and the direct effects of the drug.
- The increase in the PR interval is due primarily to an increase in the AH interval and the HV interval is unchanged.
- causes an increase in the automaticity and a decrease in the excitability of the His-Purkinje system by increasing the phase 4 slope.
- The effects of digitalis on ventricular repolarization are responsible for the characteristic ST segment and T wave changes associated with the administration of the drug.
- The recovery process is shortened and the QT interval is shortened
Effect of Digoxin on the EKG
- ST segment depression
- Decreased amplitude of the T wave, which may become diphasic (initial part is negative and the terminal positive) or negative
- Shortening of the QT interval
- Increase of the U wave amplitude (not as much as hypokalemia or quinidine)
- The changes are most pronounced in the leads with a tall R wave
- The degree of repolarization changes has no correlation with serum digoxin levels
- Changes are more pronounced in tachycardia
- One of the causes of a false positive ETT
- A false positive test is seen in 50% of patients
- In LVH, the QT interval should be prolonged. If it is normal or shortened, then suspect digoxin toxicity.
Digitalis Intoxication
- Reported in 23% of patients on the drug in 1971, lower now
- Can produce all types of arrhythmias from problems with impulse conduction to problems with impulse formation
- Interestingly enough, it does not cause bundle branch block (the level of the block is proximal to the His bundle).
- Frequency of arrhythmias:
- PVCs 48%
- Junctional Tach 13%
- 1 Degree AV Block 12%
- 2 Degree AV Block 11%
- 3 Degree AV Block 11%
- PAT 13%
- PAT with Block 10%
- VT 10%
- Sinus Bradycardia 2.3%
- Sinus arrest 1.6%
- A regular rhythm in the presence of atrial fibrillation suggests complete AV block
- In cases of enhanced impulse formation from the AV node, the rate of junctional discharge is between 70 and 130 beats per minute, and the rhythm is regular. This rhythm can be seen in patients with an MI or myocarditis
- Lown had reported in 1960 that 73% of cases of PAT with block were due to digoxin. The number is probably lower currently. The atrial rate is between 150 to 200 BPM.
Quinidine
- Direct effect is to reduce the automaticity, excitability and conductivity, but this is counterbalanced by the indirect vagolytic effect of the drug which tends to increase the rate.
- With higher toxic doses, the automaticity of the SA node may be depressed resulting in sinus bradycardia or sinus arrest. SA and AV conduction may be impaired and intraventricular conduction time markedly prolonged. The automaticity of ventricular tissue may be paradoxically increased with the appearance of ventricular arrhythmias.
EKG Findings
- Decrease in the amplitude of the T wave or T wave inversion
- ST depression
- Prominent U waves (an early finding)
- Prolongation of the QTC
- Notching and widening of the P waves
EKG Findings with Toxic Effects
- Widening of the QRS
- Various degrees of AV block
- Ventricular arrhythmias, syncope and sudden death
- Marked sinus bradycardia, sinus arrest or SA block
Procainamide
- Unlike Quinidine it is not anticholinergic
- EKG changes are less pronounced than those accompanying quinidine
- The QRS interval is increased in proportion to the plasma level
- The Qtc interval is prolonged but to a lesser degree than quinidine
- U waves become more prominent
- In high or toxic doses, it may cause high-degree block and marked widening of the QRS duration, PVCs, VT, Torsades, VF or asystole. These effects are seen more frequently with the IV preparation than the oral preparation
Disopyramide
- EKG changes are infrequent at the usual therapeutic doses
- Occasionally there is QTc prolongation and QRS widening
Lidocaine
- Like all type 1B agents, this shortens the action potential duration
- May slow the rate or cause sinus arrest in high doses, the automaticity of ectopic atrial pacemakers may be reduced.
- In usual doses, it is not associated with any noticeable changes in the P wave, the PR interval, the QRS complex, The QT interval, or the T wave.
Tocainide and Mexiletine
- Tocainide may slightly shorten the QT
- Mexiletine in therapeutic doses has no effect on intervals
Phenytoin
- QTc may be decreased
- In patients with heart disease, IV phenytoin may produce bradycardia, AV block, asystole, or ventricular fibrillation.
Encainide, Flecainide and Propafenone
- Prolong the PR and the QRS
- Propafenone may also slow the sinus rate due to its β-blocking effects
β-blockers
- Decrease the sinus rate but have no effect on the intervals.
- May cause AV block in those with preexisting disease of the AV conduction system or in those receiving digitalis
Amiodarone
- Sinus bradycardia
- Occasional cases of SA block or sinus arrest
- Prolonged QTc
Bretylium
- A brief sympathomimetic phase followed by adrenergic blockade.
- Heart rate initially increases and then falls.
- No change in the intervals
Ca Channel Blockers
- In patients with sinus node disease, verapamil and diltiazem may cause severe bradycardia and even sinus arrest.
- Effect on rate depends on the balance between the effect on the conduction system and a reflex increase in the heart rate due to vasodilation.
- Diltiazem and verapamil prolong the PR interval, nifedipine has no effect.
- No effect on the QRS or the QTc
Adenosine
- Decreases the automaticity of the sinus node and AV nodal conduction
- Caused by an increase in the AH interval and not by an increase in the HV interval
- Blocks occur within 20 seconds of i.v. administration
- The incidence of transient second degree AV block is high
Definition of Proarrhythmia
- Fourfold increase in the hourly frequency of PVCs compared with the control period.
- A tenfold increase in the hourly frequency of couplets or VT compared with the control period
- The first appearance of VT that was not present during the control period
- Occurs in 3.4% to 16.4% of patients. Incidence is highest with class 1c drugs and lowest with class 1B.
Phenothiazines
- Widening, flattening, notching or inversion of the T wave
- Prolongation of the QTc interval
- Prominence of the U wave
- Incidence with Mellaril > Thorazine > Stelazine.
- Dose related QT prolongation
Tricyclic Antidepressants
- EKG changes in 20% of patients on therapeutic doses
- Prolongation of the QTc, displacement of the ST, T wave abnormalities, QRS widening, supraventricular and ventricular tachycardias can all occur.
- In overdose, widening of the QRS is frequent, there is also QTc prolongation
Lithium
- Flattens or inverts the T waves in 20 to 30% of patients
- QTc is not prolonged
- Sinus bradycardia, sinus pauses, and SA block may be caused by lithium
EKG in Electrolyte Disturbances
Hyperkalemia
- Tall, narrow, and peaked T waves
- earliest sign
- K > 5.5
- ddx includes the T waves of bradycardia, CVA. Prominent U waves and QTc prolongation are more consistent with CVA than hyperkalemia.
- Intraventricular conduction defect
- seen with K > 6.5
- there is some correlation of QRS duration with K
- as this progresses, the QRS complexes may resemble sine waves
- generally the widening is diffuse and usually there is no resemblance of the morphology to that of either LBBB or RBBB
- Decrease of the amplitude of the P wave or an absent P wave
- decreased amplitude when K is > 7.0
- absent when K is > 8.8
- impulses are still being generated in the SA node and are conducted to the ventricles through specialized atrial fibers without depolarizing the atrial muscle
- ST-segment changes simulating current of injury
- have been called the dialyzable current of injury
- Cardiac arrhythmias: bradyarrhythmias, tachyarrhythmias, atrioventricular conduction defects
- occurs with severe hyperkalemia, not mild to moderate hyperkalemia
Hypokalemia
- ST segment depression, decreased T wave amplitude, prominent U waves
- seen in 78% of patients with a K < 2.7 meq
- seen in 35% of patients with a K > 2.7 and < 3.0
- seen in 10% of patients with a K > 3.0 and < 3.5
- U waves are also prominent in bradycardia and LVH
- Prolongation of the QRS duration
- uncommon except in severe hyperkalemia
- Increase in the amplitude and duration of the P-wave
- Cardiac arrhythmias and AV block
- Contrary to popular belief there is not prolongation of the QTc, this is artifactually prolonged due to the U wave. In some cases there is fusion of the T and the U wave making interpretation impossible.
Hypercalcemia
- A decrease in the QTc interval
- The decrease is at the expense of the ST segment which becomes shortened or absent.]
- This is true for Ca of up to 16, after this QTc prolongation occurs
- Administration of I.V. calcium to a digitalized patient may be dangerous.
Hypocalcemia
- Prolongation of the QTc interval is the major EKG finding
- There is a lengthening of the interval between the end of the QRS and the beginning of the T wave (i.e. ST-segment lengthening).
EKG Changes of Hypothermia
Changes Associated with Hypothermia
- Slowing of the sinus rate
- Prolongation of the PR and the QTc
- Prolongation of the QRS is often due to the appearance of the J wave
- The most typical finding is the appearance of the Osborne J wave, an extra deflection between the QRS and the T wave.
- Consistently found when the temperature falls below 25 degrees Centigrade.
- More prominent in the left precordial leads
- Increases in size with decreasing temperature
- Caused by a current of injury, delayed ventricular depolarization, or early repolarization
- About 50 to 60% of these patients develop atrial fibrillation. VF may also occur.
AV Junctional Rhythms
EKG findings of Junctional Rhythms
- The P wave axis is -60 to -80 degrees (normal is 0 to 75 degrees)
- The P wave of the junctional beat may
- Precede the QRS in an "upper" nodal rhythm
- Superimpose on the QRS in a "middle" nodal rhythm
- Follow the QRS in a "lower" nodal rhythm
- This depends not only on the location of the pacemaker (upper, middle, or lower) but also on the retrograde conduction of the impulse.
- There could be a pacemaker located in the upper portion of the node, but if retrograde conduction was slow, then the P wave would not precede the QRS
- Thus these terms pertaining to the nodal location may be misleading and are no longer used.
- Typically the PR interval is < .11 second, the RP interval may be up to .20 seconds
- The morphology of the QRS is not altered.
"Passive" Junctional Rhythms
- AV junction is the site of impulse formation when there is depression of the SA node, SA block, sinus bradycardia, sinus arrhythmia.
- In this case the rhythm is an escape rhythm
- Occurs if the sinus rate is slower than that of the junctional pacemaker (35 to 60 BPM)
- May occur after the postextrasystolic pause on an atrial or ventricular premature beat.
- Occasionally the sinus and the AV junctional rhythm ore at similar rates and the P waves and the QRS complexes are in proximity to each other but are unrelated to each other. This phenomenon is called isorhythmic AV dissociation.
- The ventricular rate increases with atropine
- The QRS morphology is similar to that in NSR, including any aberrancy
- May be seen in patients with SA nodal, AV nodal disease, digoxin, healthy people with sinus bradycardia.
"Active" Junctional Rhythms
- Junctional tachycardia at a rate > 60 BPM
- When there is a junctional pacemaker the P waves are inverted in leads 2,3,F.
- includes premature junctional beats
- Are premature
- Morphologic characteristics of an AV junctional beat
- Usually have a constant coupling interval
- In most cases the postextrasystolic pause is not fully compensatory. The retrograde conducted impulse discharges the SA node and resets its rhythmicity.
- Differential diagnosis:
- PACs: PJCs more likely if the P waves are inverted inferiorly, if the PR is < .12, and if the QRS is normal in duration.
- PVCs: if a retrograde P occurs after the beat, and the RP is < .11, then it is unlikely to be a PVC because the interval is too short to complete VA conduction.
- Includes paroxysmal AV junctional tachycardia (AV nodal reentrant and automatic junctional tachycardia)
- May be due to reentry or increased automaticity.
- Onset and termination are abrupt. May last seconds, hours or days.
- Rate 140 to 220 BPM and is regular.
- The P-QRS complex has the morphologic characteristic of a junctional beat.
- P waves are inverted in 2,3,F. In many cases they are buried and cannot be identified.
- QRS can be wide if there is preexistent IVCD.
- In AV junctional tachycardia, vagal stimulation has little effect on this rhythm
- Can be seen in healthy patients, those with CAD, and with dig toxicity
- Includes nonparoxysmal junctional tachycardia (accelerated AV junctional rhythm)
The Frequently Used Term "Paroxysmal Supraventricular Tachycardia"
- Sudden onset of a regular, narrow complex tachycardia
- Two basic mechanisms: reentry and automaticity
- Differential diagnosis includes
- Sinus node reentry
- Uncommon, < 5% of cases of SVT
- Suggested if the P waves are identical those to the P waves of NSR
- Rate is between 100 and 160 BPM (average 130 BPM)
- Slower than other forms of PSVT
- May be slowed and terminated by CSM
- Intraatrial reentry
- Uncommon with same incidence as sinus node reentry tachycardia
- P waves usually upright in inferior leads, have a different morphology than in NSR
- Not influenced by CSM
- AV nodal reentry
- Causes 60% of PSVTs
- P waves are inverted in the inferior leads
- In 2/3rds of these cases they are superimposed on the QRS
- In other cases they appear immediately after the QRS
- Rate is fast, 140 to 200 BPM
- As a rule vagal maneuvers terminate the tachycardia
- Reentry using an accessory pathway (WPW):
- The accessory pathway is either the anterograde or the retrograde pathway of the reentry circuit
- If conduction is down the regular AV node, then the QRS is not widened, this is more common.
- If conduction is down the accessory pathway, then the QRS is widened.
- Reentry using a concealed AV bypass tract:
- The bypass tract conducts only retrograde, resting EKG is unrevealing
- Narrow QRS complex during tachycardia.
- In both this and in WPW there are always inverted P waves that follow the QRS.
- The fact that P waves can be identified in these tachyarrhythmias is how WPW and bypass tracts can be distinguished for AV nodal reentry tachycardias.
- The rate of tachycardias associated with bypass tracts is faster than that due to AV nodal reentry and is 150 to 240 BPM, suspect this when the rate is > 200 BPM.
- Although patients with WPW frequently experience tachyarrhythmias, it is more common for a person with a narrow complex tachycardia to have a concealed bypass tract as a cause. Concealed bypass tracts cause 15 to 30% of PSVTs
- Enhanced automaticity of an atrial focus
- P waves always precede the QRS.
- May be inverted in the inferior leads if there is a low atrial focus.
- Relatively slow, 100 to 180 BPM.
- The PR is > .12 seconds.
- After a few beats the tachycardia accelerates.
- The tachycardia may be associated with AV block (i.e. PAT with block).
- Accounts for < 5% of PSVTs.
- Vagal maneuvers do not terminate these.
- Enhanced automaticity of an AV junctional focus
- Rare, similar characteristics to that of an atrial focus
- Sinus node reentry
Nonparoxysmal Junctional Tachycardia (Accelerated AV Junctional Rhythm)
- Abnormal impulse formation at the AV junction.
- Rate is only moderately increased to about 70 to 130 BPM.
- Lacks the sudden onset and termination characteristic of the paroxysmal type.
- Often the result of dig intoxication, acute MI, CT surgery, myocarditis.
Chou's Definition of AV Dissociation
- Atrial and ventricular rates are independent of each other.
- The ventricular rate is faster than the atrial rate.
- There is no retrograde conduction of the ventricular impulse to the atria.
- In complete AV dissociation, both the atrial and the ventricular rates remain constant (PP and RR intervals are constant although the PR varies).
- In incomplete AV dissociation, some of the impulses arriving at the AV junction are conducted.
- In isorhythmic AV dissociation, the rates of the dissociated impulses are nearly the same.
Reciprocal or Echo Beats
- Occurs when the impulse activates a chamber, returns, and reactivates the chamber again.
- Used to refer to the phenomenon of one or two beats.
- If the process continues, it is called reentrant tachycardia.
- An anterograde and a retrograde pathway are required, and both are usually in the AV node.
- In Echo beats of atrial origin, there is a P-QRS-P sequence.
- In Echo beats of ventricular origin, there is a QRS-P-QRS sequence.
Reading Ischemia In The Presence Of LBBB
LBBB can simulate an MI due to the associated secondary ST changes and pseudoinfarct q waves that it causes, and furthermore it can mask the EKG changes of an MI.
Pseudoinfarct Patterns: Simulation of an Anterior MI
- Can cause poor R wave progression. Often see a decrease in the amplitude of R waves to the midprecordium in the absence of a septal infarct.
- QS complexes are often seen in the right precordial leads in uncomplicated LBBB and they may even extend as far out as V5 or V6.
- Noninfarctional Q waves may be seen in AVL.
- The Reason: LBBB causes a loss of the normal septal r waves in the right precordial leads. The septum is no longer being depolarized from left to right as it normally does because of the delay down the left bundle.
- There can occasionally be Rs complexes in V1. These unanticipated initial positive forces are due to early RV depolarization and may actually mask the q waves (i.e. loss of initial septal forces) that accompany an anteroseptal MI.
Simulation of an Inferior MI
- Noninfarctional QS complexes can be seen in leads II, III, and AVF in LBBB.
- There are a number of autopsy cases were there are QS waves inferiorly without evidence of an MI.
- There are several reported cases of intermittent LBBB in which the QS waves inferiorly were present only in the aberrantly conducted beats.
- Conversely, LBBB may mask the development of Q waves in an IMI.
Secondary ST T Wave Changes
- Primary ST T wave changes are repolarization changes that are seen with ischemia or electrolyte imbalance and reflect actual changes in the myocardial action potentials.
- Secondary ST T wave changes occur when the sequence of ventricular activation is altered without any disturbance in the electrical properties of the myocardial cells such as is seen in LBBB.
- As a result of secondary ST T wave changes, the QRS and the T wave vectors are oriented in opposite directions which is known as discordance of the QRS and T wave vectors.
- Thus, the QRS is often predominantly negative in the right precordial leads while the T wave is oriented positively. In those leads where there is a tall positive R wave there is a negative T wave.
- These secondary ST T wave changes often mimic infarction, and furthermore they may mask the ST T wave changes of an MI.
- Sometimes primary ST T wave changes will be superimposed on the LBBB pattern and the following suggests the diagnosis of ischemia or infarction:
- ST segment elevation in leads with a predominant R wave. In uncomplicated LBBB, the ST segment is isoelectric or depressed.
- T wave inversions in the right to midprecordial leads or in other leads with a predominantly negative QRS. In other words there is an absence of discordance, and there is the presence of concordance.
- Morphology: In leads with a predominant R wave, the ST segment begins to slope downwards and blends into the T wave. The ascending limb of the T wave ascends back to the baseline at a more acute angle.
- The ischemic T waves have a more symmetric appearance and a slightly upwardly bowed ST segment.
- ST T elevations simulating acute infarction: The ST segment can be markedly elevated ( up to 10 mm or more at the J point ) in leads with a QS or rS segment in uncomplicated LBBB. In addition, there can be a loss of R wave progression.
- T wave inversions in intermittent LBBB: May develop deep T wave inversions in the right to midprecordial leads of normally conducted beats in the absence of CAD. These T wave inversions are deepest in leads V1 to V4 with a symmetric or coved appearance.
Etiology of Q Waves
- As described earlier, in LBBB there is a loss of depolarization from left to right, which produced an initial r wave in the right precordial leads.
- Now there is depolarization from right to left. Consequently the initial r wave is lost, and the noninfarctional QS complexes may appear in the precordial leads.
- The reversal of septal activation results in RS complexes in the left precordial leads.
Can You Read a Left Ventricular Free Wall Infarction In the Presence of a LBBB?
- No. This pattern of infarction results in abnormal q waves in the midprecordial to lateral precordial leads.
- In LBBB the initial septal depolarization forces are directed from right to left. These leftward septal forces will produce an initial R wave in the midprecordial to the lateral precordial leads, masking the loss of potential q waves produced by the infarction.
- Therefore left ventricular free wall infarction by itself will not produce diagnostic q waves in the presence of a LBBB.
- Poor R wave progression is seen in uncomplicated LBBB.
Can You Read a Septal Infarction in the Presence of LBBB?
- Yes. Again the septal forces are directed to the left in LBBB.
- If enough of the septum is infarcted to eliminate these initial leftward septal forces, abnormal QR, QRS, or qrs types of complexes may appear in the midprecordial to lateral precordial leads.
- These initial q waves may reflect posterior and superior forces from the spared basal portion of the septum.
- Small q waves of .03 sec or less may be seen in leads I, V5 to V6 in uncomplicated LBBB.
- The presence of q waves laterally is an example of false localization.
References
1. Myocardial Infarction, Electrocardiographic Differential Dx, Ary L. Goldberger, 3rd ed., Mosby Co., St. Louis, 1984, p.85 93.
Criteria For LVH
Sokolow and Lyon Criteria[10]
- Add the depth of the S wave in V1 to the height of the R wave in lead V5 or V6 (whichever is taller) and if the sum is greater than 35 mm then LVH is present.
- This criterion correlates well with the thickness of the LV walls and the diameter of the LV cavity as determined by ECHO.
- Sensitivity 22% and specificity of 100%.
Cornell Voltage Criteria[11]
- Add the height of the R wave in lead aVL to the depth of the S wave in lead V3.
- LVH if the sum is > 28mm in men or > 20 mm in women.
- Sensitivity of 42% and specificity of 96%.
Roberts Criteria[12]
- Add the QRS voltage in all 12 leads and LVH is present if the voltage exceeds 175 to 225 mm.
Estes Criteria[13]
- R or S in limb lead: 20 mm or more
- S in V1, V2, or V3 25 mm or more 3 points
- R in V4, V5, or V6 25 mm or more
- Any ST shift (without digitalis) 3 points
- Typical "strain" ST T (with digitalis) 1 points
- LAD: 15 degrees or more 2 points
- QRS interval: .09 seconds or more 1 point
- Intrinsicoid deflection in V5 or V6 of .04 seconds or more 1 point
- Pterminal force in V1 more than .04 sec 1 point
Total possible 13 points
Total of 5 points = LVH, 4 points = probable LVH
References
- ↑ Chou's Electrocardiography in Clinical Practice.
- ↑ Atterhog, J. Electrocardiol. 1977:10,331.
- ↑ Lister,J., Am.J. Cardiol 1965:16, 516.
- ↑ Johnson, R, Am. J. Cardiol. 1960:6, 153.
- ↑ Manning, G., Am. J. Cardiol.1962:9, 558.
- ↑ Erikssen, J., Clin.Card. 1984:7, 6.
- ↑ http://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&ved=0CCYQFjAA&url=http%3A%2F%2Fwww.medschool.lsuhsc.edu%2Femergency_medicine%2Fdocs%2FTop%2520Ten%2520(or%252011)%2520EKG%2520Killers.ppt&ei=PXJeUJ0xp9rRAbOOgagK&usg=AFQjCNGGRSrJfRVl3cMy5au0lnmpeaBRQA
- ↑ Beckwith JR. Grant's clinical electrocardiography. New York: McGraw–Hill, 1970:50.
- ↑ Marriott's Practical Electrocardiography, Wagner G.S., 10th edition, 2001
- ↑ Sokolow, M, and Lyon, T.P.: The Ventricular Complex As Obtained By Unipolar Limb Leads. Am. Heart J. 1949:37,161.
- ↑ Casale, P., Electrocardiographic detection of left ventricular hypertrophy: Development and prospective evaluation of improved criteria. J. Am. Coll Cardiol. 1985:6,572
- ↑ Roberts, W. and Podalak, M: The king of hearts: Analysis of 23 patients with hearts weighing 1,000 grams or more. Am J. Cardiol. 1985:55,485.
- ↑ Surawicz, B.: Electrocardiographic diagnosis of chamber enlargement. J. Am. Coll. Cardiol. 1986:8,711.
Nonspecific ST-Segment and T-Wave Changes
Causes of NSSTWs
- Ischemic heart disease
- LVH or RVH
- PE
- Acute and chronic pericarditis
- Electrolyte imbalance
- MVP
- Ventricular pacing
- CNS diseases
- Pheo, adrenal insufficiency, hypothyroidism, hypopituitarism
- Fear tension and stress
- Gallbladder disease
- Acute pancreatitis
- Truncal vagotomy
- Hyperventilation (in 70% of patients after 30 to 60 seconds)
- Orthostatic changes (3 to 23%), most prominent in the inferior leads
- Postprandial T-waves changes. One of the most common causes. 50% of patients will have normal EKGs when repeated in the fasting state.
Pacemakers
Pacemaker Code
- The first letter of the code indicates the chamber paced
- V - Ventricular
- A - Atrial
- D - Dual
- The second letter of the code indicates the chamber sensed
- V - Ventricular
- A - Atrial
- D - Dual
- 0 - none
- The third letter of the code indicates the mode of response
- I - Inhibited
- T - Triggered
- D - Double
- 0 - None
- VVI means that the ventricle is paced, the pacemaker senses the ventricle, and the pacemaker can be inhibited
- Now a fourth letter means that there is a telemetry function or programmability:
- P - Programmable
- M - Multiprogrammable
- C - Communicating
- 0 - None
- The fifth letter indicates special antitachycardia functions
- B - Bursts
- N - Normal rate competition
- S - Scanning
- E - External
Pacemaker Spikes
- Duration less than 2 msec, except when there is a long decay curve, and this decay curve can distort the QRS.
- Pacemakers with unipolar leads give much larger pacing spikes than those produced by bipolar leads
- In a bipolar lead, the negative electrode is located at the tip of the catheter and the positive electrode is 1 cm proximal to the tip. The current flows from the negative electrode to the positive electrode.
- In a unipolar lead, the active electrode, the cathode, is located at the tip of the catheter, and the indifferent electrode, the anode, is the exposed metal portion of the pulse generator. The electrical current flows form the tip of the catheter to the indifferent electrode through body tissue and fluid.
- Because of the greater distance between the two electrodes and therefore the bigger the dipole, the magnitude of the spike from unipolar pacemakers is larger.
- The longer distance between the electrodes makes is more vulnerable to extrinsic signal interference, such as electromagnetic and myopotential interference from skeletal muscles.
- The orientation of a unipolar spike will depend upon the location of the pulse generator since it is the positive pole.
Atrial Pacing
- If the site of stimulation is near the SA node in the RA, then the morphology of the P waves resembles that of sinus beats.
- If the site of stimulation is in the coronary sinus, then the morphology of the P waves is inverted in leads 2,3, and F.
Ventricular Pacing
- If the transvenous site of stimulation is in the RV, then a LBBB pattern appears. The sequence of activation is from the right to the left and from the apex to the base (i.e. a superior orientation, LAD). Unlike the usual LBBB, V6 may be predominantly negative.
- If the transvenous site of stimulation is at the inflow or the outflow tract of the RV, then the QRS axis is normal.
- If there is left ventricular epicardial-myocardial pacing there is RBBB.
Pacing-Induced T Wave Changes
- Inverted broad T waves associated with QT prolongation. They are seen most commonly in the inferior leads and in the left precordial leads. May resemble ischemic T waves.
Various types of pacemakers
- AOO (Atrial asynchronous):
- Also called a fixed-rate atrial pacemaker.
- No sensing function.
- A lead is connected to the RA, which it paces at a preset rate, regardless of the patient's own heart rate or rhythm.
- Requires an intact AV conduction system.
- Rarely used.
- AAI, and AAT (Atrial demand pacemakers):
- In AAI pacemakers, if there is spontaneous atrial activity at a rate greater than (or the PP interval is less than) a predetermined level, then no stimulus is delivered.
- In AAT pacemakers, the pacer senses the atrial impulse and stimulates the atrium when the atrial rate falls below a certain level, but it also delivers stimuli when it senses native P waves.
- Also rarely used.
- VOO (Ventricular asynchronous):
- Also called fixed-rate ventricular pacemakers.
- Delivers stimuli at a constant rate regardless of the patient's own heart rate or rhythm.
- Competes with the patient's native QRS, if the latter are present.
- Each stimulus is followed by a QRS complex unless the stimulus occurs during the ventricular refractory period.
- Fusion beats may be seen, part of the ventricle depolarized by the patient's own impulse and part by the artificial pacemaker.
- There is a risk that the spike may fall during the vulnerable period, resulting in ventricular fibrillation.
- Risk is low in most patients but increases in the presence of ischemia, electrolyte imbalance, or drug toxicity.
- A magnet converts demand pacemakers to this mode of pacing.
- VVI or VVT (Ventricular Demand Pacemakers):
- Most popular in use, particularly VVI.
- The pacemaker generates impulses whenever the patient's own ventricular rate falls below a predetermined level. The stimulus is suppressed of the patient's own ventricular rate is above this level.
- Pacing rate is usually 72 beats/minute.
- The interval from a native complex to that of a pacemaker spike is called the escape interval. This interval may not be the same as the pace interval (the spike-to-spike interval) and is usually much longer because depending on the site of origin of the spontaneous impulse, its potential may not be sensed by the pacemaker until the later portion of the depolarization.
- In some pacemakers, the escape interval is deliberately set longer than the pacing interval a feature which is known as hysteresis.
- Fusion beats may occur.
- Occasionally there are pseudofusion beats in which case the pacemaker spike falls on the spontaneous beat. The pacemaker spike does not cause depolarization, and other than the superimposed spike, it has the same morphology as a pure spontaneous beat. occurs when the pacemaker fires during the absolute refractory period of the heart.
- Pseudofusion beats are more likely to be seen in those patients with an IVCD or PVCs.
- May be seen in patients with a RV pacemaker and left-sided PVCs or an LV pacemaker and a right-sided PVC.
- VVI pacemaker in the magnet mode:
- Converts the pacemaker to a fixed-rate mode.
- A significant decrease in the pacing rate indicates a decrease in the pacemaker's battery voltage.
- Rate-responsive pacemakers:
- Increase the pacing rate in response to physiologic demands.
- Methods of detecting the need for an increased rate include a piezoelectric crystal that detects vibration generated by body motion, an RV body temperature monitor, the measurement of the QT interval, mixed venous O2 saturation.
- VVT (Ventricular triggered pacemakers):
- Functions similarly to the inhibited type (VVI) of pacemaker in that it delivers a stimulus at a preset interval if no spontaneous ventricular depolarization is detected.
- It differs from the ventricular inhibited pacemakers by also delivering a stimulus immediately after a spontaneous QRS is sensed.
- When there is a spontaneous ventricular complex, the pacing spike comes shortly after the QRS.
- In the paced beats, the stimulus spike initiates rather than falls on the QRS complexes.
- Were originally developed to avoid the potential problem of pacemaker inhibition by extracardiac signals such as those that originated from skeletal muscles or radio transmitters. Modern circuitry has reduced the likelihood of such interferences.
- Because of
- power drain
- distortion of the native QRS
- This mode is infrequently used.
Pacemaker Malfunction Detection by Routine EKG
- With a demand pacemaker there may be a loss of either sensing or capturing
- Loss of sensing occurs before loss of pacing
- Sources of malfunction:
- pulse generator
- lead
- junction of the electrode and the cardiac tissue
Sensing Malfunction
- Undersensing:
- When it has lost its ability to sense it functions as a fixed-rate asynchronous pacemaker.
- Sometimes exercise will increase the native heart rate and expose a failure to sense.
- May not always be due to pulse generator malfunction. It may be due to scar tissue, inadequate myocardial or endocardial contact, poor orientation of a bipolar electrode, or inappropriate programming of amplifier sensitivity in a newly placed pacemaker.
- Late sensing loss may be due to acute MI, growth of insulating tissue around the electrode, or myocardial perforation with the electrode migrating to a poor signal area. Broken leads or wire insulation are now uncommon causes as the quality of the wires has improved.
- Oversensing:
- Uncommon to oversense physiologic intracardiac voltage.
- Occasionally very tall p waves or t waves may be mistaken for qrs complexes.
- Myopectoral inhibition may occur when the pectoralis muscle inhibits the pacer.
- Radar, TV, radiotransmitters and electrocautery may inhibit the pacemaker.
- Microwave and weapon detector equipment no longer cause a problem in modern equipment.
- DC countershock and XRT may damage pulse generators.
Pacing Malfunction
- Can only be recognized of the patient's rate is slow enough to permit pacer spikes.
- If the patient's rate is too rapid, then a magnet can be applied to convert the pacer to a fixed-rate mode.
- A change in the spike to spike interval of more than 10 msec may indicate early battery depletion. Such a change in the rate is difficult to determine from the routine EKG, and a change of 1 to 2 beats per minute may be due to a change in the paper speed.
- Generally, a slowing of more than 4 beats per minute is an indication of battery depletion.
- Some pacemakers now allow the detection of a reduction in the pulse width or pulse amplitude of the spike.
- Myocardial perforation may be responsible for pacing failure.
- RV endocardial leads may perforate either the interventricular septum or more commonly the free wall of the RV, ECG would then change from a LBBB to a RBBB.
VAT (Atrial Synchronous Pacemakers)
- This is a ventricular stimulating pacemaker with its pacing rate dependent on the atrial rate.
- Rarely used.
- If the atrial potential is inadequate or absent within a preset interval, then the pacemaker becomes an asynchronous ventricular pacemaker.
- If the atrial rate becomes too rapid then the pacer is programmed to produce 2:1, 3:1 or 4:1 block.
VDD (Atrial Synchronous Ventricular Inhibited Pacemakers)
- It senses and paces the ventricle.
- It has been refined by the addition of ventricular sensing and provides for ventricular pacing during sinus bradycardia.
DVI (Atrioventricular Sequential Pacemakers)
- Dual-chamber pacemaker that paces both the atrium and the ventricle and senses only the ventricle.
- If conducted supraventricular beats occur at a rate above a preset level, all activities of the pacemaker are suppressed.
- If no ventricular potential is sensed by the ventricular electrode at a predetermined interval, atrial pacing occurs.
- Subsequent ventricular pacing activity depends on the type of the AV sequential pacemaker, committed or uncommitted.
- uncommitted: ventricular pacing is inhibited by the native QRS interval if the spontaneous AV conduction time is shorter than the programmed pacemaker AV interval.
- committed: ventricular pacing occurs at the programmed AV interval regardless of whether there is spontaneous AV conduction.
- In both types, the pacemaker restores normal AV sequence only when the native atrial rate is slower than the pacemaker's automatic rate.
DDD (Atrioventricular Universal Pacemakers)
- The most common dual chamber device implanted today.
- Senses both the atrium and the ventricle.
- It may be inhibited or triggered to pace the atrium or ventricle or both according to the programmed rate limits and AV interval.
- The rate is often programmed to be 60 beats/min, the upper rate is 150 beats/min and the AV interval is 200 msec.
- If the rate is faster than 60 beats/min (if a P wave appears is less than 800 msec from the preceding QRS) and the AV conduction time is less than 200 msec (the programmed intervals) then no pacing stimulus is seen.
- There are built in features to prevent pacemaker-induced arrhythmias so that no ventricular stimulus is delivered in less than 400 msec from the preceding QRS interval. This is the same as an upper rate limit of 150 beats/min.
- If a PVC is sensed less than 800 msec after the preceding QRS, both atrial and ventricular pacing is inhibited. The atrial and the ventricular response is reset by the PVC.
- Application of a magnet results in DOO pacing (asynchronous pacing in both the atria and the ventricles).
DDD Pacemaker Related Tachycardias
- Wenckebach pacemaker response
- Occurs when the atrial rate is faster than the programmed DDD pacemaker upper rate limit.
- In this situation, the atrial pacemaker is inhibited.
- The ventricle does not necessarily fire at the preset AV interval, it will wait until the upper rate limit (the upper VV interval) is reached.
- Therefore there is prolongation of the P to V interval , the degree to which is proportional to the preceding VP interval.
- Pacemaker Circus Movement Tachycardia
- Also called endless loop tachycardia
- The cause is retrograde P waves.
- Such a retrograde P wave is sensed by the atrial component of the pacemaker, which in turn triggers the delivery of a ventricular stimulus, resulting in a paced QRS complex with retrograde atrial capture.
- The rate is limited by the upper rate of the pacemaker.
- Lengthening the atrial refractory period of the pacemaker may decrease the incidence of, but does not eliminate this potential complication.
Hysteresis, Fallback Response, Cross Talk
- With regard to atrial hysteresis, there are two AV intervals. The AV interval of a sensed atrial event is shorter than that which follows an atrial stimulus.
- The reason for this is to make the AV intervals the same in actuality to maintain the hemodynamic benefit of the atrial contraction.
- The reason that the interval following a sensed atrial beat is that there is a time delay between when the pacemaker senses the atrial activity. When atrial activity is sensed, atrial contraction is likely to have begun.
- Rate hysteresis is present when the pacemaker escape interval is longer than the pacing interval. This allows the patient's own sinus rhythm, which is more physiological, to be maintained at a lower rate than the pacing rate.
- In patients who cannot tolerate a sustained upper rate, the fallback response mechanism returns the ventricular response rate to more tolerable levels. AV synchrony may not be maintained during the fallback response.
- Cross talk refers to the phenomenon whereby the ventricular channel senses the atrial impulse and is thereby inhibited. To overcome this, the ventricular sensing circuit is turned off shortly after the atrial stimulus.
Pacemaker Syndrome
- Refers to dizziness, near syncope, and fatigue even though there is no pacemaker malfunction. This syndrome is the result of unfavorable hemodynamic consequences of AV asynchrony.
- The incidence is higher in patients with VA conduction.
Electrocardiographic Findings In RVH
Differential Diagnosis of R>S in V1
- RVH
- Posterior MI
- WPW
- Hypertrophic CM (septal hypertrophy)
- Kulbertus' block (septal fascicular block)
- Duchennes Muscular dystrophy
- Normal variant
- V4r may be a more useful and reliable than lead V1 in that it often reveals an r>s while v1 remains normal
- An incomplete right bundle branch block in the right precordial chest leads may signal the development of RVH
- In the limb leads right axis deviation develops and at times prominent Q waves simulating an IMI appear in leads 2,3, and F.
- In children an S1S2S3 pattern (i.e an S wave deeper than R in all 3 standard leads) is a reliable index of RVH.
- RV strain can be seen in leads V1 and V2 but also in leads 2,3,F
Summary of Clues to RVH
- Right axis deviation of +90 degrees or more
- RV1 = 7 mm or more
- RV1 + SV5 or SV6 = 10 mm or more
- R/S ratio in V1 = 1.0 or more
- S/R ratio in V6 = 1.0 or more
- Late intrinsicoid deflection in V1 (0.035+)
- Incomplete RBBB pattern
- ST T strain pattern in 2,3,F
- P pulmonale or P congenitale
- S1 S2 S3 pattern in children
Torsades De Pointes and Polymorphic VT
Background
- The peaks of the QRS complexes appear to twist around the isoelectric axis.
- Polymorphic VT is distinguished from Torsades by the absence of QT prolongation in polymorphic VT.
EKG Findings
- Paroxysms of VT with irregular RR intervals.
- A ventricular rate between 200 and 250 beats per minute.
- Two or more cycles of QRS complexes with alternating polarity.
- Changing amplitude of the QRS complexes in each cycle in a sinusoidal fashion.
- Prolongation of the QT interval.
- Is often initiated by a PVC with a long coupling interval, R on T phenomenon.
- There are usually 5 to 20 complexes in each cycle.
Clinical Correlation
- Drugs: quinidine, PCA, norpace, amiodarone, phenothiazines, TCAs, pentamidine.
- with quinidine majority of the cases occur within one week of initiation, and with therapeutic levels
- Electrolyte imbalances: Hypokalemia, hypomagnesemia, hypocalcemia
- CAD
- MVP
- Variant angina
- Myocarditis
- Subarachnoid hemorrhage
- Congenital QT prolongation
- Liquid protein diets
- Hypothyroidism
- because of bradycardia and a prolonged QT syndrome
- Organophosphate poisoning
The EKG of Cardiac Transplantation
General
- The recipient retains the posterior walls of his own atria.
- The recipient's atria are vascularized by bronchial collateral vessels.
- The transplanted heart is denervated and lacks autonomic neural control.
- The EKG changes in these patients are mainly due to the remnant activity of the recipients atria, injury to the donor heart during the transplant procedure, and increased PVR in the recipient.
- Atrial arrhythmias and ventricular conduction defects are common.
- Role of the ECG in acute rejection is controversial.
- MI may occur as a result of accelerated atherogenesis.
EKG Findings
- Two sets of P waves
- the recipients native P waves are of a small amplitude
- donor P waves have normal amplitude and configuration
- the suture line between the donor and the recipient atria blocks any interchange of the electrical impulses from the two sources. Atrial dissociation is present.
- the donor P wave is conducted and stimulates the ventricles. The recipients atrial impulses are not conducted.
- the rate of the denervated donor's heart is faster than that of the recipient's atrial remnant rate.
- in one series, two sets of P waves were identified in 86% of patients.
- P waves of the recipient atrial remnants may not be seen because of their small amplitude or the presence of sinus node dysfunction or atrial fibrillation prior to the transplantation. May also lose the sinus node artery during surgery.
- a. fib/flutter may be present in one set of atria while NSR is present in the other pair making interpretation difficult.
- RBBB (complete or incomplete)
- occurs in 83% of patients in Chou's experience, in 45% in another series.
- LAHB
- occurs in about 25% of patients
- Bradyarrhythmias due to sinus node dysfunction
- cause not explained by rejection or ischemic time during transplant
- subsidiary pacemakers may be unreliable in providing an escape rhythm and sudden death may occur.
- permanent pacemaker may be required.
- PACs, PSVTs, afib and aflutter are seen postoperatively. Some investigators have felt that these episodes are associated with rejection episodes.
- Presence of complex VEA is associated with accelerated atherosclerosis and early death.
- ST-segment and T-wave changes
- some patients may develop diffuse ST-segment elevation followed by evolutionary changes of the ST segment and T wave changes consistent with acute pericarditis in the early post-operative period.
- as a rule these changes are transient
- some patients develop ischemic appearing ST T wave changes and these are not associated with evidence of acute rejection and their angiograms are normal.
ECG Evidence of Rejection
- Originally though that low voltage was indicative of rejection, but this has been called into question.
Accelerated Atherogenesis
- 40% of patients have this after three years.
- Equally frequent in those who did and did not have ischemic heart disease at the time of transplantation.
- Immunologic damage to the coronary endothelium is thought to be the mechanism of injury.
- Is a mixture of ordinary proximal lesions and diffuse obliterative disease.
- Usually silent because of denervation.
Electrocardiography of Traumatic Heart Disease
General Principles
- Injury may be divided in to penetrating and non-penetrating.
- Presentation depends upon the location of the injury and the cardiac structures involved.
- EKG is usually not as helpful as the physical exam and the CXR in the evaluation of penetrating injuries.
- In the evaluation of non-penetrating injuries, the EKG is helpful.
Non-Penetrating Injuries
Causes
- MVA. Most common cause. Heart can be compressed between the sternum and the spine.
- Sudden acceleration and deceleration.
- Fist, a kick, a blunt object or an animal.
- CPR.
- Serious damage may be present in the absence of fractures.
Potential Damage
- Pericardium
- disruption
- hemopericardium and tamponade
- pericarditis
- Myocardium
- contusion
- rupture
- septal perforation
- late aneurysm
- Valves
- chordae tendineae
- papillary muscle rupture
- Coronary arteries
- contusion
- thrombosis
Potential EKG Changes
- ST and T wave changes
- the most common change (17 to 58%)
- develop within 24 to 48 hours of the injury and mimic the changes due to myocardial ischemia.
- in most, the changes are transient, but they may persist.
- myocardial contusion or traumatic pericarditis is the usual underlying abnormality.
- if the abnormality persists, then extensive myocardial scarring may be present.
- CK MB and technetium-99 pyrophosphate scintigraphy have been found to be even less sensitive than the EKG in the diagnosis of myocardial contusion.
- Reduction of QRS voltage
- suggests effusion, possible tamponade
- Pseudoinfarction pattern
- rare
- IVCD
- reported to be as high as 23% in one series, Chou feels this is an overestimate
- RBBB is the most common abnormality
- SVTs and VT
- VF may be responsible for sudden death
Electrical Injury
- Sudden death due to electrocution is usually secondary to VF or cardiac standstill.
- The heart s most sensitive to a low frequency current of 40 to 60 cycles/second
- Current flow causes tissue coagulation by heat damage.
- Damage is proportional to voltage, resistance of the tissue, and the duration of the flow.
- EKG abnormalities are present in 10 to 46% of patients with electrical injury.
- Arrhythmias (a. fib, VT, VF) may appear hours after the injury and may be recurrent for several months.
- ST segment changes and T wave changes some of which resemble those of myocardial ischemia or injury may occur.
- The QT interval may prolong.
- Pseudoinfarct patterns have been observed.
Ventricular Arrhythmias
EKG Findings
- They are premature in relation to the expected beat of the basic rhythm
- Ectopic beats from the same focus tend to have a constant coupling interval (the interval between the ectopic beat and the preceding beat of the basic sinus rhythm).
- They do not vary from each other by more than .08 seconds if the focus is the same.
- PVCs with the same morphology but with a varying coupling interval should make one suspect a parasystolic mechanism.
- A longer RR interval is followed by a relatively longer coupling interval
- The QRS complex is abnormal in duration and configuration. There are secondary STT wave changes. The morphology of the QRS may vary in the same patient.
- If the PVC originates from the RV then the QRS has a LBBB morphology
- The duration of the QRS is > .12 seconds, but a narrower QRS may occur if the focus is higher in the septum.
- The T wave is inverted and the ST segment is depressed.
- There is usually a full compensatory pause following the PVC.
- The sum of the RR intervals that precede and follow the ectopic beat (or the RR interval that contains the PVC) equals two RR intervals of the sinus beats.
- Because of sinus arrhythmia, the RR interval that contains the PVC may not be exactly twice the duration of the RR interval of the adjacent sinus beat, even though a full compensatory pause does exist).
- Retrograde capture may or may not occur.
- They may occur in various frequency and distribution patterns such as bigeminy, trigeminy, quadrigeminy, and couplets. These are called complex PVCs.
- The Rule of Bigeminy:
- PVCs frequently occur after a long RR interval.
- The compensatory pause of the precipitated PVC constitutes another long RR interval, which in turn favors the appearance of another PVC.
- Therefore bigeminy tends to perpetuate itself.
- The Rule of Bigeminy:
- Occasionally PVCs may be interpolated.
- Between 2 beats without disturbing NSR.
- Occurs mostly when the NSR is slow and the PVC is early.
- The PR following the PVC is nearly always prolonged because of concealed retrograde conduction of the ectopic ventricular impulse, which renders the AV junction partially refractory.
Grading of Frequency
- Called frequent if there are 5 or more PVCs per minute on the routine ECG
- Lown and Graboys proposed the following grading system which is used for prognostic purposes:
- Grade 0 = No PVCs
- Grade 1 = Occasional (< 30 per hour)
- Grade 2 = Frequent (> 30 per hour)
- Grade 3 = Multiform
- Grade 4 = Repetitive
- A = Couplets
- B = Salvos of > 3
- Grade 5 = R-on-T
Differential Diagnosis
- Aberrant ventricular conduction:
- A transient form of abnormal IVCD
- Occurs when there is unequal refractoriness of the two bundles
- The right bundle has a longer action potential duration, and is more vulnerable to conduction delay or failure.
- The refractory period is affected by the preceding cycle length.
- The refractory period is longer when there is a long preceding RR interval
- aberrant ventricular conduction is favored when a premature supraventricular impulse comes after a long preceding RR interval (Ashman's phenomenon).
- If the underlying rhythm is sinus in origin, and if the abnormal QRS is preceded by a premature P wave, then the ectopic beat is likely to be supraventricular in origin.
- The absence of a fully compensatory pause further supports this diagnosis.
- If a retrograde P wave is identifiable after the QRS complex and the RP interval is less than .11 second, the premature beat is likely to have originated from the AV junction, since the RP interval is too short for VA conduction (unless an accessory pathway is present).
- A long RP interval of .20 seconds or longer is suggestive but not diagnostic of a PVC, since the retrograde conduction time of a junctional beat is less likely to exceed this duration.
- The beat is more likely to be due to aberrancy if the initial forces are similar to those of the sinus beat and if it has an RSR' configuration in lead V1.
- If the QRS complexes in all the precordial leads are positive or all negative,then a PVC is more likely.
- Diagnosis of PVCs in the presence of atrial fibrillation:
- Absence of P waves and the irregularity of the rhythm are the handicaps.
- A constant coupling time is suggestive of PVCs.
- Ashman's phenomenon. Keep in mind that a long cycle length also favors the precipitation of a PVC, therefore this sign is helpful but not diagnostic of aberrancy.
- PVC is favored if the abnormal complex terminates a short-long cycle.
Clinical Correlation
- Healthy patients
- The most common arrhythmia in patients with and without CAD.
- Less common in infants and children, more common in the elderly.
- Usually originate from the RV.
- In normal patients, they may be either precipitated or suppressed by exercise.
- No relationship to coffee or smoking has been established.
- Frequency decreases with sleep.
- Coronary artery disease
- Routine ECGs demonstrate PVCs in 10% of patients with CAD.
- Incidence inreases to 60 to 88% when the monitoring is increased to 12 to 24 hours.
- The frequency of complex VEA increases with increasing numbers of vessels involved. (40% with one, 53% with two, and 78% with three vessels involved has VEA).
- Patients with CAD are more prone to develop VEA with exercise (incidence 4 times higher than age matched controls).
- Reported incidence in acute MI varies, but is near 100%.
- After the initial 6 hours, the frequency decreases.
- Persistence of VEA is associated with larger infarct size.
- In one study, patients with EFs of greater than 50% had no persistent VEA, and patients with EFs of less than 30% had frequent PVCs.
- Other Organic Heart Diseases:
- occur on routine EKG in 1/3rd of patients.
- 12% of patients with congested cardiomyopathy have PVC on routine tracings.
- 1.6% of patients with IHSS have PVCs on routine EKG.
- Drugs:
- PVCs are the most common arrhythmia in patients with digoxin toxicity.
- Other drugs that cause PVCs are quinidine, PCA, norpace, phenothiazines and tricyclic antidepressants.
- Electrolyte Imbalance:
- Hypokalemia, hypomagnesemia, and hypercalcemia are frequently associated with the appearance of ventricular arrhythmias.
Prognostic Significance
- In the absence of CAD or HTN, there is no excess mortality in patients with PVCs.
- On the other hand, PVCs in the presence of other cardiac abnormalities or hypertension is associated with a mortality twice that expected.
- The development of VT is most likely in those with greater than 12 PVCs/min, couplets, and multifocal PVCs.
- Complex VEA during acute MI does not have any prognostic significance.
- Their presence 2 to 3 weeks after acute MI is associated with a 3 fold increase in the risk of sudden death.
Ventricular Parasystole
- An ectopic ventricular pacemaker activates the ventricle concurrently with, but independent of, the impulse of the basic rhythm.
- It is protected from the impulse of the basic rhythm by entrance block.
- Its own impulse is able to depolarize the ventricle.
- Probably due to increased automaticity.
- EKG findings:
- varying coupling intervals
- With PVCs there is usually fixed coupling
- RR intervals of the ectopic beats being mathematically related to each other
- The interectopic forum is variable, are multiples of the shorter interval
- Although the ectopic focus fires regularly, only those that find the ventricles responsive are manifested electrocardiographically.
- The ratio between the intervals is seldom exact whole numbers (3:1) and more often 3:2 etc.
- Parasystolic rates vary from 20 to 400 BPM
- In most patients the rate varies between 30 to 56 BPM
- Parasystolic rates greater than 70 BPM is called parasystolic VT.
- the presence of fusion beats
- May discharge at the same time that an impulse of the basic rhythm arrives at the ventricles.
- The ventricles are then depolarized by the two wavefronts.
- Can occur with regular PVCs, but with parasystole more frequently.
- varying coupling intervals
- It is relatively uncommon
- Seen in 1 to 1.5/1000 EKGS
- Long rhythm strip required to demonstrate variability in coupling interval
Ventricular Tachycardia
Computer model of re-entry in VT
Paroxysmal Ventricular Tachycardia
- Rapid succession of three or more ectopic beats.
- Sustained if it lasts longer than 30 seconds.
- Called incessant if the tachycardia is recurrent and the episodes are interrupted by only a few sinus beats.
EKG Findings
- Abnormal and wide QRS complexes with secondary ST-segment and T wave changes.
- Usual QRS duration is > .12 seconds, may be shorter if the ectopic focus is located in the ventricular septum.
- The secondary STTW changes are in a direction that is opposite the major deflection of the QRS.
- A ventricular rate between 140 and 200 BPM.
- When the rate is > 200 and has a sine wave appearance, it is called ventricular flutter.
- When the rate is < 110 BPM it is called non-paroxysmal VT.
- A regular or slightly irregular (up to .03 seconds) rhythm.
- Abrupt onset and termination.
- AV dissociation.
- Atrial rate slower than ventricular rate.
- No relationship between atrial activity and ventricular activity.
- There can be VA conduction.
- The RP interval is > .11 seconds.
- Occurs in about 50% of cases.
- Uncommon when the ventricular rate is rapid (only 1/7 when the rate was>200).
- Capture beats.
- Occurs when a supraventricular impulse is conducted and captures the ventricle.
- They are rare.
- Fusion beats.
- Rare in VT at a rapid rate.
Differential Diagnosis of Tachycardia with Wide QRS Complex
- A regular tachycardia with a rate of 120 to 200 BPM with a QRS duration of 0.12 seconds or longer may be due to:
- Paroxysmal VT
- Supraventricular tachycardia with abnormally wide QRS
- Sinus tachycardia
- SA nodal reentrant tachycardia
- Paroxysmal atrial tachycardia
- Intraatrial reentrant tachycardia
- Atrial flutter with 2:1 conduction and occasional 1:1 conduction
- AV nodal reentrant tachycardia
- Automatic junctional tachycardia
- AV reentrant tachycardia using a bypass tract
Differential Diagnosis of Wide QRS Complexes
- Aberrant ventricular conduction
- Preexisting left or right bundle branch block
- Preexisting nonspecific IVCD
- Antegrade conduction through the bypass tract in patients with WPW
Clues to the Diagnosis of VT
- Morphology of Premature Beats During Sinus Rhythm:
- Previous EKG may show preexisting IVCD.
- If PVCs are present, and if the morphology of the arrhythmia is the same, then it is likely to be ventricular in origin.
- If there are PACs with aberrant conduction, then the origin of the arrhythmia may be supraventricular.
- Onset of the Tachycardia:
- Diagnosis of SVT made if the episode is initiated by a premature P wave.
- If the paroxysm begins with a QRS then the tachycardia may be either ventricular or junctional in origin.
- If the first QRS of the tachycardia is preceded by a sinus p wave with a PR interval shorter than that of the conducted sinus beats, the tachycardia is ventricular.
- AV Dissociation:
- Although is highly suggestive of VT, it may also be seen in junctional tachycardias with retrograde block.
- Morphology of the QRS Complexes and QRS Axis:
- 80 to 85% of aberrant beats have a RBBB pattern, but ectopic beats that arise from the LV have a similar morphology.
- The finding of a positive or negative QRS complex in all precordial leads is in favor of ventricular ectopy.
- A QRS duration of > .14 seconds (A Wellens criterion)
- Left axis deviation (A Wellens criterion)
- A monophasic or biphasic RBBB QRS complex in V1. But none of their patients with SVT had a preexisting RBBB. Therefore, this finding is of limited importance. (A Wellens criterion)
- Akhtar studied 150 patients with a wide complex tachycardia. The following were helpful in the diagnosis of VT:
- all patients with VT had a QRS duration > 120 msecond.
- QRS > .14 with a RBBB, QRS > .16 with LBBB.
- V1 - V6 all show a positive deflection.
- QRS axis between -90 and + 180 degrees.
- The QRS complexes have a LBBB but the QRS axis is rightward.
- In patients with preexisting bundle branch block, there is a change in the QRS pattern during the tachycardia.
- Capture beats:
- Rare, but one of the strongest pieces of evidence in favor of VT.
- Aberrancy rarely follows a beat of such short cycle length.
- Fusion beats:
- Rare but also strongly suggests VT.
- Vagal Stimulation:
- VT is not affected by vagal stimulation.
- May terminate reentrant arrhythmias
- Atrial pacing:
- A pacing wire is placed in the RA and the atrium is stimulated at a rate faster than the tachycardia.
- If ventricular capture occurs and the QRS is normal in duration, then one can exclude the possibility of aberrant conduction.
- His bundle recording:
- In SVT, each QRS is preceded by a His bundle potential.
- In VT there is no preceding His deflection.
- The retrograde His deflection is usually obscured by the much larger QRS complex.
Differential Diagnosis of Wide QRS Complex Tachycardia
- The following favor the diagnosis of VT:
- AV dissociation
- RBBB with QRS > .14, or LBBB with QRS > .16
- QRS axis in RUQ between -90 and +180 degrees
- Positive QRS in all the precordial leads (V1-V6)
- LBBB with a rightward axis
- LBBB with the following QRS morphology
- R wave in V1 or V2 > 0.03 second
- any Q wave in V6
- Onset of the QRS to nadir of the S wave in V1 > 0.06 seconds
- Notching of the S wave in V1 or V2
- Capture beats, fusion beats
- QRS morphology identical to that of premature ventricular beats during sinus rhythm
Clinical Correlation
- Most patients with VT have organic heart disease.
- Post MI VT is associated with a doubling of the risk of death.
- This was an a risk factor independent of poor LV function.
- VT can be seen with reperfusion, but an accelerated idioventricular rhythm is more common.
- Digoxin intoxication is a common cause. Other antiarrhythmics, phenothiazines, TCAs, and pheochromocytoma may also cause this.
- Cardiac catheterization, DC countershock, following repair of congenital lesions, and the hereditary QT prolongation are all associated with VT.
Accelerated Idioventricular Rhythm
- EKG characteristics:
- Regular rhythm at a rate of 60 to 100 BPM.
- QRS complexes are abnormal and wide.
- The ventricular complexes are usually but not necessarily dissociated from the P waves.
- Ventricular capture and fusion beats are common.
Differential Diagnosis
- Because of its slower rate it may resemble NSR. Look for numerous fusion beats. The term accelerated isorhythmic ventricular rhythm has been suggested.
- Must be distinguished from junctional tachycardia with preexisting IVCDs. But in these patients there are no fusion or capture beats.
Clinical Correlation
- Seen in both AMIs and IMIs.
- Commonly seen following reperfusion.
- Usually occurs during sinus bradycardia.
- May also be caused by digitalis.
The Wolff-Parkinson-White Syndrome
History
First described more than 50 yrs ago by MGH physician Paul Dudley White in a series of 11 healthy young patients who had attacks of paroxysmal tachycardias in the presence of an EKG which showed a bundle branch block pattern with a short PR interval.
The EKG in WPW
- Two pathways between the atrium and the ventricle are present.
- There is a shortened PR interval
- PR less than .12 seconds
- in most cases it varies between .08 and .11 seconds
- A wide QRS with a delta wave.
- the QRS is .11 second or longer
- is inversely proportional to the PR (i.e. the shorter the PR, the longer the QRS secondary to greater preexcitation).
- the combination of the shortened PR interval and widened QRS is of normal duration
- The delta wave occurs as the ventricle is activated first via the accessory pathway (AP) and then normal activation follows down the normal pathway.
- the duration of the delta wave is .03 to .06 seconds
- The pattern of ventricular activation is determined by several factors:
- the location of the accessory pathway: The closer the accessory pathway to the SA node, the quicker the impulse will reach the atrial insertion site of the AP. In contrast, in those patients in whom the AP is located in the far lateral region of the LV, contribution to the AP during NSR may be minimal.
- the intra-atrial conduction time: Left atrial pathology will prolong the time necessary to reach the left sided AP, drugs can also prolong the time to reach a left-sided pathway.
- the conduction time over the accessory pathway: The conduction time over the AP depends on the length of the AP and velocity with which the impulse is conducted. Investigators have found that the accessory pathway may vary in length from 1 to 10 mm.
- the AV conduction time over the normal AV nodal-His-Purkinje pathway
- Secondary T wave changes:
- Because of the early asynchronous activation of the ventricle, the sequence of repolarization will be different leading to T wave changes.
- the T wave polarity is opposite in direction to the delta wave
- Concealed bypass tracts:
- If the accessory pathway's contribution to ventricular activation is minimal because of the coincidental arrival of the excitation wavefront over the normal pathway, then this should not be called a concealed accessory pathway.
- Concealed accessory pathways are those that conduct in a retrograde fashion (ventriculoatrial) only.
- Antegrade conduction in these patients is absent because the refractory period of the AP in the antegrade direction is longer than the sinus cycle length.
- when a recurrent tachycardia occurs in association with such concealed bypass, the conduction is called concealed WPW syndrome
- are usually located on the left side of the cardiac chambers
- consider this if during the tachycardia there is a negative P wave in lead V1, if there is a P wave after the QRS complex
- Findings are intermittent in 1/2 the cases
Incidence
- Somewhere between .1 and 3 per 1000 EKGs.
- May be underdiagnosed given the presence of left sided APs which may be silent during NSR.
- The incidence of tachyarrhythmias in patients with WPW has been estimated to be somewhere between 12% and 80%.
- WPW may be the most prevalent cause of paroxysmal regular supraventricular tachycardia One series found that 57% of patients c psvt were subsequently found to have WPW on the resting NSR EKG. If the attack occurs before the age of 21, then 73% of the patients had WPW. In patients > 21 years old, then WPW was found in 48% of patients with PSVT. (Wellens et al 1981).
EKG Classification
- Type A:
- Prominent R wave in lead V1 and V2.
- It has been found at EP studies that these patients have early activation of the LV.
- Generally V1 shows either a notched R wave or RS or Rsr' deflection
- Mimics a posterior MI, RVH
- Type B:
- Prominent S wave deflection in the right precordial leads, and upright R waves in the lateral precordial leads.
- EP studies have showed that this form of WPW is due to early activation of the lateral aspect of the right ventricle
- This form is more common.
- May resemble an abnormal Q wave in the right precordial leads and be mistaken for an anterior MI
- In both type A and B there may be abnormal q waves in leads 2,3,F.
Oversimplification
Histological studies have found that the AP fibers may insert in the septum and not the free wall as above. The location of AP may be impossible to determine in NSR, as it can be complicated by the existence of more than one AP in some patients, the coexistence of congenital lesions, the occasional superimposition of the P wave on the initial portion of the delta wave, and differences in the activation depending on whether the AP is epicardially or endocardially located.
Associated Cardiovascular Abnormalities
- Type B is found in 5% to 25% of the reported cases of Ebstein's dz. Suspect this if there is Type B WPW with RBBB.
- Also been found in patients with corrected transposition of the great arteries, tricuspid atresia, endocardial fibroelastosis, MVP, cardiomyopathies (hypertrophic obstructive and congestive).
Clinical Manifestations
- The most common form of paroxysmal tachycardia in these patients is a circus movement tachycardia (CMT) incorporating the AP.
- The CMT utilizes the following structures: the AV node, the His-Purkinje system, the ventricular myocardium (from the terminal portion of the His system to the ventricular end of the AP), the AP itself, and the atrial myocardium itself from the atrial insertion of the AP to the AV node.
- This circuit can conduct in both directions:
- Type I A CMT:
- This is the usual form of the CMT in patients with WPW.
- Is antegrade through the AV node, VA conduction through the AP.
- The QRS complex during the tachycardia shows either normal intraventricular conduction or typical bundle branch block configuration.
- Sx: Palpitations (97%), dyspnea (57%), anginal pain (56%), perspiration (55%), fatigue (41%), anxiety (30%), dizziness (30%),polyuria (26%).
- this is also called orthodromic reentrant tachycardia
- there is no delta wave
- the rate is 140 to 250 bpm
- it is faster than the rate of tachycardia due to reentry in the AV node
- often triggered by a PAC
- Type I B CMT:
- anterograde down the accessory pathway, retrograde down the AVN-His pathway.
- the QRS is widened
- this form is rarer
- Type II CMT (intra-AV nodal):
- anterograde pathway is an AV nodal slow pathway, the retrograde pathway is an AV nodal fast pathway.
- No evidence of ventricular pre-excitation during the tachycardia.
- Type III CMT (uses two accessory pathways):
- Conducts anterograde down one accessory pathway and retrograde up a second accessory pathway.
- These patients can also experience atrial tachycardias and ventricular tachycardias.
- Type I A CMT:
Atrial Fibrillation in WPW
Can cause life-threatening ventricular rates due to the exclusive AV conduction over the accessory pathway.
- reduces cardiac output.
- may degenerate into VF, particularly in those with multiple bypass tracts.
- The only marker identified for degeneration into VF in the literature was the occurrence of RR intervals equal to or less than 205 msec during the a fib
- Seen in 78 of 256 of Wellen's patients with WPW. Reported incidence is 20 to 35% in other studies.
- The degree of ventricular preexcitation observed in the EKG during NSR bears no relationship whatsoever to the risk of developing life-threatening ventricular rates during the a.fib.
- The QRS complexes are wide and bizarre as a result of preexcitation.
- The ventricular rate is 220 to 360 beats per minute due to the short effective refractory period of the accessory pathway.
- It is often mistaken for VT.
- If the atrial rate in atrial fibrillation is greater than 200 BPM then suspect this. The rhythm will also be grossly irregular if it is due to atrial fibrillation. Such a rapid rate would be unusual if it were due to conduction by way of the normal AV conduction system.
Electrophysiologic Studies in WPW
The purpose of these studies is to:
- determine the location and number of accessory pathways.
- determine the mechanism and pathway of the tachycardia.
- to establish the diagnosis in those patients c a questionable resting EKG.
- to evaluate the efficacy of drugs.
- to select patients for surgery and to evaluate them postoperatively.
Diagnosis And Treatment
Circus Movement Tachycardias:
- Rapid heart rate is most likely due to CMT or atrial fibrillation.
- Palpitations are usually regular during a CMT, irregularly with atrial fib.
- Some patients report that their attacks of CMT may be broken by vagal maneuvers.
- A 24 hr holter should be done in those patients with a suspicion of WPW to assess the mode of initiation, and to determine the type (CMT vs afib).
- If the patients quality of life is affected and the holter is negative, then EP studies should be done.
- If there is an inability to induce CMT at EP then it is very unlikely that this is the arrhythmia that the patient is experiencing outside the hospital. Inability to induce CMT obviously does not exclude that the patient is experiencing afib.
- If the patient presents in a rapid rhythm, you should first try vagal maneuvers to terminate a CMT.
- The most likely arrhythmia on a statistical basis is a CMT with the AP conducting in a retrograde fashion. If the AP is incorporated in a retrograde fashion then there are often retrograde P waves apparent following the QRS, with a PR interval longer than the RP'interval.
- If the patient is known to have WPW and has a regular tachycardia thought to be a CMT, then verapamil should be the first drug used. If verapamil is not available, then use propranolol IV. Both terminate the CMT by prolonging the refractory period at the AV node.
- Digitalis has been used, however some patients may dev afib with this, and dig may abbreviate the refractory period of the AP resulting in higher ventricular rates during the afib. Therefore the use of dig is not recommended.
- If drugs affecting the AV node are not effective, then drugs that affect the accessory pathway are used such as procainamide or disopyramide.
- If the above fail to terminate the tachycardia or if the patient is tolerating the tachycardia poorly, then the patient should be paced out of it or cardioverted. Pacing is safer in patients on multiple drugs.
Atrial Fibrillation
- Patients can experience high rates during afib because of conduction over the accessory pathway which can have a very short refractory period.
- Mean ventricular rates in these patients range from 160 to 300 BPM.
- During these attacks there is not only the risk of hemodynamic compromise but also a risk of degenerate into VF.
- As a rule dig should be avoided in these patients.
- Cardioversion is the tx of choice. If the patient is receiving drugs that promote asystole following electrical cardioversion (e.g. verapamil, beta-blockers, and probably amiodarone) then a temporary pacer should be positioned in the RV before the cardioversion.
- If the ventricular rate during the afib is not > 200, then you could try procainamide, disopyramide, or quinidine which may prolong the refractory period of the accessory pathway.
Drug Prophylaxis In The Patient With Proven Tachyarrhythmias
- Should receive prophylactic treatment if the arrhythmia is poorly tolerated.
- In Wellen's experience amiodarone is the most effective in preventing attacks of paroxysmal tachycardia in patients with WPW. Otherwise he recommends quinidine, disopyramide, procainamide or propranolol alone or in combination.
- For those patients with life-threatening rates during afib, Wellen's recommends amiodarone as prophylaxis which prolongs the AP refractory period. Quinidine is an alternative, but is less effective.
- These patients should undergo EP studies to assess the adequacy of treatment.
- These authors state that the refractory period of the AP can shorten in the presence of sympathetic stimulation and advocate the addition of a beta blocker.
Approach to the Patient with a Questionable EKG
- As mentioned previously, the diagnosis can be difficult in those patients with normal EKGs at rest.
- In some patients the diagnosis can be made with the following noninvasive procedures:
- CSP to increase the AV nodal delay therefore enhancing conduction over the accessory pathway.
- IV procainamide may cause QRS abnormality to disappear by prolonging conduction down the AP.
- β-blockers, verapamil, digoxin may also facilitate the conduction down the accessory pathway
Differential Diagnosis
Diagnosis of Hypertrophy, Bundle Branch Block and MI in the Presence of WPW are all obscured.
- Type A WPW
- RBBB
- RVH
- True posterior MI
- IMI
- Type B WPW
- LBBB
- Anterior MI
- IMI
- WPW and atrial flutter
- paroxysmal VT or flutter
Intractable Tachyarrhythmias in WPW
In patients with afib with rapid ventricular response then surgical interruption should be considered.
Variants of WPW
LGL: Lown-Ganong-Levine Syndrome
- there is a short PR, but no delta wave
- due to intranodal bypass tracts (i.e. there is conduction down James fibers)
- normal QRS duration
- PR less than 0.12 seconds
- normal P wave
Mahaim Type of Preexcitation
- nodoventricular, nodofascicular or fasciculoventricular connections
- the impulse may travel through the AV node normally and this may then be followed by premature conduction to the basal ventricular myocardium
- there is a delta wave with a normal PR interval
- is rarer than WPW or LGL
- in older patients there can be a prolonged conduction down the accessory pathway resulting in a normal PR interval in the presence of WPW which is tough to distinguish from Mahaim fibers.
EKG in Limb Lead Reversal
Shown below is an example of EKG's showing two 12 lead electrocardiograms from the same person taken one after another. The leads have been misplaced on the bottom recording. This is one of the lead reversals that causes surprisingly few QRS changes. The left arm and the left leg lead have been reversed.
Shown below is an example of EKG's showing two 12 lead electrocardiograms from the same person taken one after another. The leads have been displaced from the conventional positions on the top recording. This is an uncommon lead reversal where the right arm and left leg have been reversed. This is one of the switches that distorts the limb leads the most. Notice the precordial leads are unaffected.

Related Chapters
Sources
- Chou's Electrocardiography in Clinical Practice Third Edition.
- Http://en.ecgpedia.org/wiki/File:De-Formule_QTc.png
Additional Resources
- ECGpedia: Course for interpretation of ECG
- The whole ECG - A basic ECG primer
- 12-lead ECG library
- Simulation tool to demonstrate and study the relation between the electric activity of the heart and the ECG
- ECG information from Children's Hospital Heart Center, Seattle
- ECG Challenge from the ACC D2B Initiative
- National Heart, Lung, and Blood Institute, Diseases and Conditions Index
- A history of electrocardiography
- EKG Interpretations in infants and children