Aceclofenac: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
|drugClass=[[Non Steroidal Anti-inflamatory Drug]] ([[NSAID]]) | |drugClass=[[Non Steroidal Anti-inflamatory Drug]] ([[NSAID]]) | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=[[rheumatoid arthritis]], [[ankylosing spondylitis]], [[osteoarthritis]] and [[periarthritis]] of scapulohumerous, [[lumbago]], [[ischiadynia]], pain caused by nonaticular rheutism | |indication=[[rheumatoid arthritis]], [[ankylosing spondylitis]], [[osteoarthritis]] and [[periarthritis]] of scapulohumerous, [[lumbago]], [[ischiadynia]], pain caused by nonaticular rheutism | ||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | |blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | ||
|fdaLIADAdult=The recommended dose is 200 mg daily, taken as one dose (every 24 hours). However, the dose and dose frequency of Aceclofenac can be modified under the supervison of physician or pharmacist. | |fdaLIADAdult=The recommended dose is 200 mg daily, taken as one dose (every 24 hours). However, the dose and dose frequency of Aceclofenac can be modified under the supervison of physician or pharmacist. | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Aceclofenac in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Aceclofenac in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Aceclofenac in adult patients. | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Aceclofenac in adult patients. | ||
|fdaLIADPed=The dosage and indication is not established yet for children with less than 6 years old. | |fdaLIADPed=The dosage and indication is not established yet for children with less than 6 years old. | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Aceclofenac in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Aceclofenac in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Aceclofenac in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Aceclofenac in pediatric patients. | ||
|contraindications=Patients with allergy to these drugs or other analogues (diclofenac). Patients with asthma. Like NSAIDS, acetylsalicylic acid and other drugs which inhibit prostagladin-synthesis may precipitate attacks of asthma, acute rhinitis or urticaria. Patients with active peptic ulcer. | |contraindications=Patients with allergy to these drugs or other analogues (diclofenac). Patients with asthma. Like NSAIDS, acetylsalicylic acid and other drugs which inhibit prostagladin-synthesis may precipitate attacks of asthma, acute rhinitis or urticaria. Patients with active peptic ulcer. | ||
|clinicalTrials=The majority of side effects observed have been reversible and of a minor nature and include gastro-intestinal disorders ([[dyspepsia]], [[abdominal pain]], [[nausea]]), [[rash]], [[ruber]], [[urticaria]], symptoms of [[enuresis]], [[headache]], [[dizziness]], and [[drowsiness]]. To report suspected adverse reactions, call 1-800-FDA-1088. | |clinicalTrials=The majority of side effects observed have been reversible and of a minor nature and include gastro-intestinal disorders ([[dyspepsia]], [[abdominal pain]], [[nausea]]), [[rash]], [[ruber]], [[urticaria]], symptoms of [[enuresis]], [[headache]], [[dizziness]], and [[drowsiness]]. To report suspected adverse reactions, call 1-800-FDA-1088. | ||
|drugInteractions=There has been no drug interactions reported, but close monitoring of patients on combination with [[lithium]] and [[digoxin]], oral [[anti diabetic agents]], [[anticoagulants]], [[diuretics]], and other [[analgesics]]. | |||
|drugInteractions=There has been no drug interactions reported, but close monitoring of patients on combination with [[lithium]] and [[digoxin]], oral [[anti diabetic agents]], [[anticoagulants]], [[diuretics]], and other [[analgesics]]. | |||
|useInPregnancyFDA=Since there is no information on the safe use of CLANZA CR during pregnancy and lactation, the use of Aclofenac should therefore be avoided in pregnancy and lactation. | |useInPregnancyFDA=Since there is no information on the safe use of CLANZA CR during pregnancy and lactation, the use of Aclofenac should therefore be avoided in pregnancy and lactation. | ||
|useInNursing=Since there is no information on the safe use of CLANZA CR during pregnancy and lactation, the use of CLANZA CR should therefore be avoided in pregnancy and lactation. | |useInNursing=Since there is no information on the safe use of CLANZA CR during pregnancy and lactation, the use of CLANZA CR should therefore be avoided in pregnancy and lactation. | ||
|useInPed=The dosage and indication is not established yet for children with less than 6 years old. | |useInPed=The dosage and indication is not established yet for children with less than 6 years old. | ||
|overdose=There are no human data available on the consequences of Aceclofenac overdosage. If overdosage is observed, therapeutic measures should be taken according to symptoms; supportive and symptomatic treatment should be given for complications such as [[hypotension]], [[gastrointestinal irritation]], respiratory [[depression]], and [[convulsions]]. | |overdose=There are no human data available on the consequences of Aceclofenac overdosage. If overdosage is observed, therapeutic measures should be taken according to symptoms; supportive and symptomatic treatment should be given for complications such as [[hypotension]], [[gastrointestinal irritation]], respiratory [[depression]], and [[convulsions]]. | ||
|drugBox={{Drugbox2 | |drugBox={{Drugbox2 | ||
| Line 89: | Line 83: | ||
|packLabel=[[file:Aceclofenac Appearance.png|none|400px]] | |packLabel=[[file:Aceclofenac Appearance.png|none|400px]] | ||
|alcohol=Alcohol-Aceclofenac interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Aceclofenac interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
|brandNames=*[[Acent]] | |||
*[[Acenal]] | |||
*[[Acelofan]] | |||
*[[Acelom]] | |||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
Revision as of 16:48, 16 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Aceclofenac is a Non Steroidal Anti-inflamatory Drug (NSAID) that is FDA approved for the treatment of rheumatoid arthritis, ankylosing spondylitis, osteoarthritis and periarthritis of scapulohumerous, lumbago, ischiadynia, pain caused by nonaticular rheutism. Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
The recommended dose is 200 mg daily, taken as one dose (every 24 hours). However, the dose and dose frequency of Aceclofenac can be modified under the supervison of physician or pharmacist.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Aceclofenac in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Aceclofenac in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
The dosage and indication is not established yet for children with less than 6 years old.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Aceclofenac in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Aceclofenac in pediatric patients.
Contraindications
Patients with allergy to these drugs or other analogues (diclofenac). Patients with asthma. Like NSAIDS, acetylsalicylic acid and other drugs which inhibit prostagladin-synthesis may precipitate attacks of asthma, acute rhinitis or urticaria. Patients with active peptic ulcer.
Warnings
There is limited information regarding Aceclofenac Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
The majority of side effects observed have been reversible and of a minor nature and include gastro-intestinal disorders (dyspepsia, abdominal pain, nausea), rash, ruber, urticaria, symptoms of enuresis, headache, dizziness, and drowsiness. To report suspected adverse reactions, call 1-800-FDA-1088.
Postmarketing Experience
There is limited information regarding Aceclofenac Postmarketing Experience in the drug label.
Drug Interactions
There has been no drug interactions reported, but close monitoring of patients on combination with lithium and digoxin, oral anti diabetic agents, anticoagulants, diuretics, and other analgesics.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
Since there is no information on the safe use of CLANZA CR during pregnancy and lactation, the use of Aclofenac should therefore be avoided in pregnancy and lactation.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Aceclofenac in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Aceclofenac during labor and delivery.
Nursing Mothers
Since there is no information on the safe use of CLANZA CR during pregnancy and lactation, the use of CLANZA CR should therefore be avoided in pregnancy and lactation.
Pediatric Use
The dosage and indication is not established yet for children with less than 6 years old.
Geriatic Use
There is no FDA guidance on the use of Aceclofenac in geriatric settings.
Gender
There is no FDA guidance on the use of Aceclofenac with respect to specific gender populations.
Race
There is no FDA guidance on the use of Aceclofenac with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Aceclofenac in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Aceclofenac in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Aceclofenac in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Aceclofenac in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Aceclofenac Administration in the drug label.
Monitoring
There is limited information regarding Aceclofenac Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Aceclofenac and IV administrations.
Overdosage
There are no human data available on the consequences of Aceclofenac overdosage. If overdosage is observed, therapeutic measures should be taken according to symptoms; supportive and symptomatic treatment should be given for complications such as hypotension, gastrointestinal irritation, respiratory depression, and convulsions.
Pharmacology
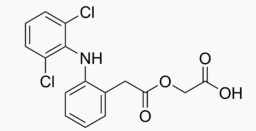
| |
Aceclofenac
| |
| Systematic (IUPAC) name | |
| 2-[2-[2-[(2,6-dichlorophenyl)amino]phenyl]acetyl]oxyacetic acid | |
| Identifiers | |
| CAS number | |
| ATC code | M01 M02AA25 (WHO) |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 354.18472 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | oral, topical |
Mechanism of Action
There is limited information regarding Aceclofenac Mechanism of Action in the drug label.
Structure

Pharmacodynamics
There is limited information regarding Aceclofenac Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Aceclofenac Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Aceclofenac Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Aceclofenac Clinical Studies in the drug label.
How Supplied
10 Blister Packs with 10 Tablets in each Blister Pack
Storage
Preserve in tight containers. Store at room temperature not exceeding 30oC. Three (3) years from manufacturing date. Do not exceed the expiry date for use printed on the box.
Images
Drug Images
{{#ask: Page Name::Aceclofenac |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Aceclofenac |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Aceclofenac Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Aceclofenac interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Aceclofenac Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Aceclofenac |Label Name=Aceclofenac Package.png
}}
| File:Aceclofenac.png | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C16H13Cl2NO4 |
| Molar mass | 354.18472 g/mol |
Aceclofenac is a non-steroidal anti-inflammatory drug.
How does it work?
Aceclofenac belongs to a group of medicines called non-steroidal anti-inflammatory drugs (NSAIDs). It works by blocking the action of a substance in the body called cyclo-oxygenase. Cyclo-oxygenase is involved in the production of various chemicals in the body, some of which are known as prostaglandins. Prostaglandins are produced in response to injury or certain diseases and would otherwise go on to cause pain, swelling and inflammation. Arthritic conditions are one example of this. Aceclofenac is used to relieve pain and inflammation in arthritic conditions. All the medicines in this group reduce inflammation caused by the body's own immune system and are effective pain killers. What is it used for?
A form of arthritis (ankylosing spondylitis)
Inflammatory disease of the joints
Osteoarthritis
Warning!
This medicine may cause dizziness. Avoid driving and operating machinery if affected.
Individuals receiving long-term treatment with this medicine should be regularly monitored (kidney and liver function tests, blood counts).
There is no experience with the use of this medicine in children.
Use with caution in
A type of life long inherited blood disease caused by a defect in the liver (hepatic porphyria)
Bleeding tendencies
Blood disorders
Crohn's disease
Decreased heart function
Elderly people
History of peptic ulcers
Inflammation of the bowel and back passage
Mildly decreased kidney function
People who have recently had major surgery
People with symptoms of stomach or intestinal disorders
Severely decreased liver function
Not to be used in
Active peptic ulcer
Bleeding from the stomach or intestines
Moderate to severely decreased kidney function
People in whom aspirin or other medicines in this class (NSAIDs), cause attacks of asthma, itchy rash (urticaria) or nasal inflammation (rhinitis)
Suspected peptic ulcer
This medicine should not be used if you are allergic to one or any of its ingredients. Please inform your doctor or pharmacist if you have previously experienced such an allergy. If you feel you have experienced an allergic reaction, stop using this medicine and inform your doctor or pharmacist immediately.
Pregnancy and Breastfeeding
Certain medicines should not be used during pregnancy or breastfeeding. However, other medicines may be safely used in pregnancy or breastfeeding providing the benefits to the mother outweigh the risks to the unborn baby. Always inform your doctor if you are pregnant or planning a pregnancy, before using any medicine.
The safety of this medicine for use during pregnancy has not been established. It is not recommended for use in pregnancy unless considered essential by your doctor. Seek medical advice from your doctor. It is not known if this medicine passes into breast milk. It is not recommended for use during breastfeeding unless considered essential by your doctor. Seek medical advice from your doctor. Label warnings
Aceclofenac
- Pages with script errors
- Pages with broken file links
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Aromatic amines
- Carboxylic acids