Bufexamac
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Topical, rectal |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
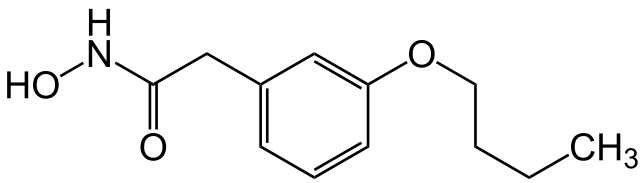
| Formula | C12H17NO3 |
| Molar mass | 223.268 g/mol |
| 3D model (JSmol) | |
| |
| |
|
WikiDoc Resources for Bufexamac |
|
Articles |
|---|
|
Most recent articles on Bufexamac |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Bufexamac at Clinical Trials.gov Clinical Trials on Bufexamac at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Bufexamac
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Bufexamac Discussion groups on Bufexamac Directions to Hospitals Treating Bufexamac Risk calculators and risk factors for Bufexamac
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Bufexamac |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Bufexamac is a drug used as an anti-inflammatory agent on the skin, as well as rectally. Common brand names include Paraderm and Parfenac. It was withdrawn in Europe because of allergic reactions.
Indications
Ointments and lotions containing bufexamac are used for the treatment of subacute and chronic eczema of the skin, including atopic eczema, as well as sunburn and other minor burns,[citation needed] and itching. Suppositories containing bufexamac in combination with local anaesthetics are used against haemorrhoids.[1]
Pharmacology
Bufexamac is thought to act by inhibiting the enzyme cyclooxygenase, which would make it a non-steroidal anti-inflammatory drug. Evidence on the mechanism of action is scarce.[2] Furthermore, bufexamac was identified as a specific inhibitor of class IIB histone deacetylases (HDAC6 and HDAC10),[3] which may contribute to its clinical efficacy.[citation needed]
Side effects
Bufexamac can cause severe contact dermatitis which is often hard to distinguish from the initial condition.[4] As a consequence, the European Medicines Agency recommended to withdraw the marketing approval in April 2010.[5]
References
- ↑ Dinnendahl, V, Fricke, U, ed. (2010). Arzneistoff-Profile (in German). 2 (23 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-9846-3.
- ↑ Gloor, Max; Thoma, Karl; Fluhr, Joachim (2000). Dermatologische Externatherapie: Unter besonderer Berücksichtigung der Magistralrezeptur (in German). Springer. p. 349. ISBN 3-540-67174-9.
- ↑ Bantscheff, Marcus; Hopf, Carsten; Savitski, Mikhail M; Dittmann, Antje; Grandi, Paola; Michon, Anne-Marie; Schlegl, Judith; Abraham, Yann; Becher, Isabelle; Bergamini, Giovanna; Boesche, Markus; Delling, Manja; Dümpelfeld, Birgit; Eberhard, Dirk; Huthmacher, Carola; Mathieson, Toby; Poeckel, Daniel; Reader, Valérie; Strunk, Katja; Sweetman, Gavain; Kruse, Ulrich; Neubauer, Gitte; Ramsden, Nigel G; Drewes, Gerard (2011). "Chemoproteomics profiling of HDAC inhibitors reveals selective targeting of HDAC complexes". Nature Biotechnology. 29 (3): 255–65. doi:10.1038/nbt.1759. PMID 21258344.
- ↑ "Bufexamac: Ein Ekzemtherapeutikum, das selbst häufig allergische Kontaktekzeme hervorruft". Deutsches Ärzteblatt (in German) (47). 2000.
- ↑ "European Medicines Agency recommends revocation of marketing authorisations for bufexamac" (PDF). European Medicines Agency. 2010-04-22.
- Pages with script errors
- CS1 maint: Multiple names: editors list
- CS1 maint: Unrecognized language
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles containing unverified chemical infoboxes
- All articles with unsourced statements
- Articles with unsourced statements from May 2010
- Articles with invalid date parameter in template
- Articles with unsourced statements from October 2011
- Anti-inflammatory agents
- Drug
- Phenol ethers