Fenoprofen
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
|
Overview
Fenoprofen is an NSAID that is FDA approved for the treatment of analgesia, rheumatoid arthritis and osteoarthritis. There is a Black Box Warning for this drug as shown here. Common adverse reactions include edema, anemia, increased liver function test.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Analgesia
- For the treatment of mild to moderate pain, the recommended dosage is 200 mg given orally every 4 to 6 hours, as needed.
Rheumatoid Arthritis and Osteoarthritis
- For the relief of rheumatoid arthritis or osteoarthritis the recommended dose is 300 mg to 600 mg given orally, 3 or 4 times a day. The dose should be tailored to the needs of the patient and may be increased or decreased depending on the severity of the symptoms. Dosage adjustments may be made after initiation of drug therapy or during exacerbations of the disease. Total daily dosage should not exceed 3200 mg.
- Fenoprofen calcium may be administered with meals or with milk. Although the total amount absorbed is not affected, peak blood levels are delayed and diminished.
- Patients with rheumatoid arthritis generally seem to require larger doses of fenoprofen calcium than do those with osteoarthritis. The smallest dose that yields acceptable control should be employed.
- Although improvement may be seen in a few days in many patients, an additional 2 to 3 weeks may be required to gauge the full benefits of therapy.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Fenoprofen in adult patients.
Non–Guideline-Supported Use
Migraine
- Fenoprofen 200 milligrams (mg) or 600 mg 3 times daily.[1]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Fenoprofen in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Fenoprofen in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Fenoprofen in pediatric patients.
Contraindications
- Fenoprofen calcium tablets are contraindicated in patients with known hypersensitivity to fenoprofen calcium.
- Fenoprofen should not be given to patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs. Severe, rarely fatal, anaphylactic-like reactions to NSAIDs have been reported in such patients.
- Fenoprofen is contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery.
- Fenoprofen is contraindicated in patients with a history of significantly impaired renal function.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
|
- Cardiovascular Effects
- Cardiovascular Thrombotic Events
- Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular (CV) thrombotic events, myocardial infarction, and stroke, which can be fatal. All NSAIDs, both COX-2 selective and nonselective, may give a similar risk. Patients with known CV disease or risk factors for CV disease may be at greater risk. To minimize the potential risk for an adverse CV event in patients treated with an NSAID, the lowest effective dose should be used for the shortest duration possible. Physicians and patients should remain alert for the development of such events, even in the absence of previous CV symptoms. Patients should be informed about the signs and/or symptoms of serious CV events and the steps to take if they occur.
- There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use. The concurrent use of aspirin and an NSAID does increase the risk of serious GI events.
- Two large, controlled, clinical trials of a COX-2 selective NSAID for the treatment of pain in the first 10 to 14 days following CABG surgery found an increased incidence of myocardial infarction and stroke.
- Hypertension
- NSAIDs, including fenoprofen, can lead to onset of new hypertension or worsening of preexisting hypertension, either of which may contribute to the increased incidence of CV events. Patients taking thiazides or loop diuretics may have impaired response to these therapies when taking NSAIDs. NSAIDs, including fenoprofen, should be used with caution in patients with hypertension. Blood pressure (BP) should be monitored closely during the initiation of NSAID treatment and throughout the course of therapy.
- Congestive Heart Failure and Edema
- Fluid retention and edema have been observed in some patients taking NSAIDs. Fenoprofen should be used with caution in patients with fluid retention, compromised cardiac function or heart failure. The possibility of renal involvement should be considered.
- Gastrointestinal Effects
- Risk of Ulceration, Bleeding, and Perforation
- NSAIDs, including fenoprofen, can cause serious gastrointestinal (GI) adverse events including inflammation, bleeding, ulceration, and perforation of the stomach, small intestine, or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Only one in five patients, who develop a serious upper GI adverse event on NSAID therapy, is symptomatic. Upper GI ulcers, gross bleeding, or perforation caused by NSAIDs occur in approximately 1% of patients treated for 3 to 6 months, and in about 2 to 4% of patients treated for one year. These trends continue with longer duration of use, increasing the likelihood of developing a serious GI event at some time during the course of therapy. However, even short-term therapy is not without risk.
- NSAIDs should be prescribed with extreme caution in those with a prior history of ulcer disease or gastrointestinal bleeding. Patients with a prior history of peptic ulcer disease and/or gastrointestinal bleeding who use NSAIDs have a greater than 10-fold increased risk for developing a GI bleed compared to patients with neither of these risk factors. Other factors that increase the risk for GI bleeding in patients treated with NSAIDs include concomitant use of oral corticosteroids or anticoagulants, longer duration of NSAID therapy, smoking, use of alcohol, older age, and poor general health status. Most spontaneous reports of fatal GI events are in elderly or debilitated patients and therefore, special care should be taken in treating this population.
- To minimize the potential risk for an adverse GI event in patients treated with an NSAID, the lowest effective dose should be used for the shortest possible duration. Patients and physicians should remain alert for signs and symptoms of GI ulceration and bleeding during NSAID therapy and promptly initiate additional evaluation and treatment if a serious GI adverse event is suspected. This should include discontinuation of the NSAID until a serious GI adverse event is ruled out. For high risk patients, alternate therapies that do not involve NSAIDs should be considered.
- Renal Effects
- Long-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury. Renal toxicity has also been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients, administration of a non-steroidal anti-inflammatory drug may cause a dose-dependent reduction in prostaglandin formation and, secondarily, in renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics and ACE inhibitors, and the elderly. Discontinuation of NSAID therapy is usually followed by recovery to the pretreatment state.
- Advanced Renal Disease
- No information is available from controlled clinical studies regarding the use of fenoprofen in patients with advanced renal disease. Therefore, treatment with fenoprofen is not recommended in patients with advanced renal disease.
- Anaphylactoid Reactions
- As with other NSAIDs, anaphylactoid reactions may occur in patients without known prior exposure to fenoprofen. Fenoprofen should not be given to patients with the aspirin triad. This symptom complex typically occurs in asthmatic patients who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other NSAIDs. Emergency help should be sought in cases where an anaphylactoid reaction occurs.
- Skin Reactions
- NSAIDs, including fenoprofen, can cause serious skin adverse events such as exfoliative dermatitis, Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. These serious events may occur without warning. Patients should be informed about the signs and symptoms of serious skin manifestations and use of the drug should be discontinued at the first appearance of skin rash or any other sign of hypersensitivity.
- Pregnancy
- In late pregnancy, as with other NSAIDs, fenoprofen should be avoided because it may cause premature closure of the ductus arteriosus.
- Ocular
- Studies to date have not shown changes in the eyes attributable to the administration of fenoprofen. However, adverse ocular effects have been observed with other anti-inflammatory drugs. Eye examinations, therefore, should be performed if visual disturbances occur in patients taking fenoprofen.
- Central Nervous System
- Caution should be exercised by patients whose activities require alertness if they experience CNS side effects while taking fenoprofen.
- Hearing
- Since the safety of fenoprofen has not been established in patients with impaired hearing, these patients should have periodic tests of auditory function during prolonged therapy with fenoprofen.
Precautions
- General
- Fenoprofen cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may lead to disease exacerbation. Patients on prolonged corticosteroid therapy should have their therapy tapered slowly if a decision is made to discontinue corticosteroids.
- The pharmacological activity of fenoprofen reducing inflammation may diminish the utility of these diagnostic signs in detecting complications of presumed noninfectious, painful conditions.
- Hepatic Effects
- Borderline elevations of one or more liver tests may occur in up to 15% of patients taking NSAIDs including fenoprofen. These laboratory abnormalities may progress, may remain unchanged, or may be transient with continuing therapy. Notable elevations of ALT or AST (approximately three or more times the upper limit of normal) have been reported in approximately 1% of patients in clinical trials with NSAIDs. In addition, rare cases of severe hepatic reactions, including jaundice and fatal fulminant hepatitis, liver necrosis and hepatic failure, some of them with fatal outcomes have been reported.
- A patient with symptoms and/or signs suggesting liver dysfunction, or in whom an abnormal liver test has occurred, should be evaluated for evidence of the development of a more severe hepatic reaction while on therapy with fenoprofen. If clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, etc.), fenoprofen should be discontinued.
- Hematological Effects
- Anemia is sometimes seen in patients receiving NSAIDs, including fenoprofen. This may be due to fluid retention, occult or gross GI blood loss, or an incompletely described effect upon erythropoiesis. Patients on long-term treatment with NSAIDs, including fenoprofen, should have their hemoglobin or hematocrit checked if they exhibit any signs or symptoms of anemia. NSAIDs inhibit platelet aggregation and have been shown to prolong bleeding time in some patients. Unlike aspirin, their effect on platelet function is quantitatively less, of shorter duration, and reversible. Patients receiving fenoprofen who may be adversely affected by alterations in platelet function, such as those with coagulation disorders or patients receiving anticoagulants, should be carefully monitored.
- Preexisting Asthma
- Patients with asthma may have aspirin-sensitive asthma. The use of aspirin in patients with aspirin-sensitive asthma has been associated with severe bronchospasm which can be fatal. Since cross reactivity, including bronchospasm, between aspirin and other non-steroidal anti-inflammatory drugs has been reported in such aspirin-sensitive patients, fenoprofen should not be administered to patients with this form of aspirin sensitivity and should be used with caution in patients with preexisting asthma.
Adverse Reactions
Clinical Trials Experience
- During clinical studies for rheumatoid arthritis, osteoarthritis or mild to moderate pain and studies of pharmacokinetics, complaints were compiled from a checklist of potential adverse reactions and the following data emerged. These encompass observations in 6,786 patients, including 188 observed for at least 52 weeks. For comparison, data are also presented from complaints received from the 266 patients who received placebo in these same trials. During short-term studies for analgesia, the incidence of adverse reactions was markedly lower than that seen in longer-term studies.
INCIDENCE GREATER THAN 1%
- Probable Causal Relationship
Digestive System
During clinical trials with fenoprofen calcium, the most common adverse reactions were gastrointestinal in nature and occurred in about 20% of patients receiving fenoprofen as compared to 16% of patients receiving placebo. In descending order of frequency, these reactions included dyspepsia (10.3% fenoprofen vs. 2.3% placebo), nausea (7.7% vs. 7.1%), constipation (7% vs. 1.5%), vomiting (2.6% vs. 1.9%), abdominal pain (2% vs. 1.1%) and diarrhea (1.8% vs. 4.1%). The drug was discontinued because of adverse gastrointestinal reactions in less than 2% of patients during premarketing studies.
Nervous System
The most frequent adverse neurologic reactions were headache (8.7% vs. 7.5%) and somnolence (8.5% vs. 6.4%). Dizziness (6.5% vs. 5.6%), tremor (2.2% vs. 0.4%) and confusion (1.4% vs. none) were noted less frequently.
Fenoprofen was discontinued in less than 0.5% of patients because of these side effects during premarketing studies.
Skin and Appendages
Increased sweating (4.6% vs. 0.4%), pruritus (4.2% vs. 0.8%) and rash (3.7% vs. 0.4%) were reported. Fenoprofen was discontinued in about 1% of patients because of an adverse effect related to the skin during premarketing studies.
Special Senses
Tinnitus (4.5% vs. 0.4%), blurred vision (2.2% vs. none), and decreased hearing (1.6% vs. none) were reported. Fenoprofen was discontinued in less than 0.5% of patients because of adverse effects related to the special senses during premarketing studies.
Cardiovascular
Palpitations (2.5% vs. 0.4%). Fenoprofen was discontinued in about 0.5% of patients because of adverse cardiovascular reactions during premarketing studies.
Miscellaneous
Nervousness (5.7% vs. 1.5%), asthenia (5.4% vs. 0.4%), peripheral edema (5.0% vs. 0.4%), dyspnea (2.8% vs. none), fatigue (1.7% vs. 1.5%), upper respiratory infection (1.5% vs. 5.6%) and nasopharyngitis (1.2 % vs. none).
INCIDENCE LESS THAN 1%
- Probable Causal Relationship
- The following adverse reactions, occurring in less than 1% of patients, were reported in controlled clinical trials and voluntary reports made since fenoprofen was initially marketed. The probability of a causal relationship exists between fenoprofen and these adverse reactions:
Digestive System
Gastritis, peptic ulcer with/without perforation, gastrointestinal hemorrhage, anorexia, flatulence, dry mouth and blood in the stool. Increases in alkaline phosphatase, LDH, SGOT, jaundice and cholestatic hepatitis were observed.
Genitourinary Tract
Renal failure, dysuria, cystitis, hematuria, oliguria, azotemia, anuria, interstitial nephritis, nephrosis and papillary necrosis.
Hypersensitivity
Angioedema (angioneurotic edema).
Hematologic
Purpura, bruising, hemorrhage, thrombocytopenia, hemolytic anemia, aplastic anemia, agranulocytosis and pancytopenia.
Miscellaneous
Anaphylaxis, urticaria, malaise, insomnia and tachycardia.
INCIDENCE LESS THAN 1%
- Causal Relationship Unknown
- Other reactions, reported either in clinical trials or spontaneously, occurred in circumstances in which a causal relationship could not be established. However, with these rarely reported reactions, the possibility of such a relationship cannot be excluded. Therefore, these observations are listed to alert the physician.
Skin and Appendages
Exfoliative dermatitis, toxic epidermal necrolysis, Stevens-Johnson Syndrome and alopecia.
Digestive System
Aphthous ulcerations of the buccal mucosa, metallic taste, and pancreatitis.
Cardiovascular
Atrial fibrillation, pulmonary edema, electrocardiographic changes, and supraventricular tachycardia.
Nervous System
Depression, disorientation, seizures, and trigeminal neuralgia.
Special Senses
Burning tongue, diplopia, and optic neuritis.
Miscellaneous
Personality change, lymphadenopathy, mastodynia, and fever.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Fenoprofen in the drug label.
Drug Interactions
- ACE Inhibitors
- Reports suggest that NSAIDs may diminish the antihypertensive effect of ACE inhibitors. This interaction should be given consideration in patients taking NSAIDs concomitantly with ACE inhibitors.
- Aspirin
- The coadministration of aspirin decreases the biologic half-life of fenoprofen because of an increase in metabolic clearance that results in a greater amount of hydroxylated fenoprofen in the urine. Although the mechanism of interaction between fenoprofen and aspirin is not totally known, enzyme induction and displacement of fenoprofen from plasma albumin binding sites are possibilities. As with other NSAIDs, concomitant administration of fenoprofen calcium and aspirin is not generally recommended because of the potential of increased adverse effects.
- Diuretics
- Clinical studies, as well as post marketing observations, have shown that fenoprofen can reduce the natriuretic effect of furosemide and thiazides in some patients. This response has been attributed to inhibition of renal prostaglandin synthesis. During concomitant therapy with NSAIDs, the patient should be observed closely for signs of renal failure, as well as to assure diuretic efficacy.
- Lithium
- NSAIDs have produced an elevation of plasma lithium levels and a reduction in renal lithium clearance. The mean minimum lithium concentration increased 15% and the renal clearance was decreased by approximately 20%. These effects have been attributed to inhibition of renal prostaglandin synthesis by the NSAID. Thus, when NSAIDs and lithium are administered concurrently, subjects should be observed carefully for signs of lithium toxicity.
- Methotrexate
- NSAIDs have been reported to competitively inhibit methotrexate accumulation in rabbit kidney slices. This may indicate that they could enhance the toxicity of methotrexate. Caution should be used when NSAIDs are administered concomitantly with methotrexate.
- Warfarin
- The effects of warfarin and NSAIDs on GI bleeding are synergistic, such that users of both drugs together have a risk of serious GI bleeding higher than users of either drug alone.
- Phenobarbital
- 8Chronic administration of phenobarbital, a known enzyme inducer, may be associated with a decrease in the plasma half-life of fenoprofen. When phenobarbital is added to or withdrawn from treatment, dosage adjustment of fenoprofen may be required.
- Plasma Protein Binding
- In vitro studies have shown that fenoprofen, because of its affinity for albumin, may displace from their binding sites other drugs that are also albumin bound and this may lead to drug interactions. Theoretically, fenoprofen could likewise be displaced. Patients receiving hydantoins, sulfonamides or sulfonylureas should be observed for increased activity of these drugs and, therefore, signs of toxicity from these drugs.
Use in Specific Populations
Pregnancy
- Pregnancy Category C
- Teratogenic Effects
- Reproductive studies conducted in rats and rabbits have not demonstrated evidence of developmental abnormalities. However, animal reproduction studies are not always predictive of human response. There are no adequate and well controlled studies in pregnant women. Fenoprofen should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Nonteratogenic Effects
- Because of the known effects of non-steroidal anti-inflammatory drugs on the fetal cardiovascular system (closure of ductus arteriosus), use during pregnancy (particularly late pregnancy) should be avoided.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Fenoprofen in women who are pregnant.
Labor and Delivery
- In rat studies with NSAIDs, as with other drugs known to inhibit prostaglandin synthesis, an increased incidence of dystocia, delayed parturition, and decreased pup survival occurred. The effects of fenoprofen on labor and delivery in pregnant women are unknown.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from fenoprofen, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness in pediatric patients below the age of 18 have not been established.
Geriatic Use
As with any NSAIDs, caution should be exercised in treating the elderly (65 years and older).
Gender
There is no FDA guidance on the use of Fenoprofen with respect to specific gender populations.
Race
There is no FDA guidance on the use of Fenoprofen with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Fenoprofen in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Fenoprofen in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Fenoprofen in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Fenoprofen in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Fenoprofen in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Fenoprofen in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Symptoms of overdose appear within several hours and generally involve the gastrointestinal and central nervous systems. They include dyspepsia, nausea, vomiting, abdominal pain, dizziness, headache, ataxia, tinnitus, tremor, drowsiness and confusion. Hyperpyrexia, tachycardia, hypotension and acute renal failure may occur rarely following overdose. Respiratory depression and metabolic acidosis have also been reported following overdose with certain NSAIDs.
Management
- To obtain up-to-date information about the treatment of overdose, a good resource is your certified Regional Poison Control Center. Telephone numbers of certified poison control centers are listed in the Physicians' Desk Reference (PDR). In managing overdosage, consider the possibility of multiple drug overdoses, interaction among drugs and unusual drug kinetics in your patient.
- Protect the patient's airway and support ventilation and perfusion. Meticulously monitor and maintain, within acceptable limits, the patient's vital signs, blood gases, serum electrolytes, etc. Absorption of drugs from the gastrointestinal tract may be decreased by giving activated charcoal, which, in many cases, is more effective than emesis or lavage; consider charcoal instead of or in addition to gastric emptying. Repeated doses of charcoal over time may hasten elimination of some drugs that have been absorbed. Safeguard the patient's airway when employing gastric emptying or charcoal.
- Alkalinization of the urine, forced diuresis, peritoneal dialysis, hemodialysis and charcoal hemoperfusion do not enhance systemic drug elimination.
Chronic Overdose
There is limited information regarding Chronic Overdose of Fenoprofen in the drug label.
Pharmacology
| |
Fenoprofen
| |
| Systematic (IUPAC) name | |
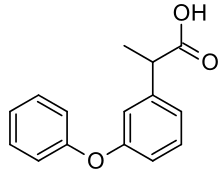
| 2-(3-phenoxyphenyl)propanoic acid | |
| Identifiers | |
| CAS number | |
| ATC code | M01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 242.26986 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Major urinary metabolites are fenoprofen glucuronide and 4′-hydroxyfenoprofen glucuronide. |
| Half life | 3 hours |
| Excretion | Renal (~90%) |
| Therapeutic considerations | |
| Pregnancy cat. |
D(US) C |
| Legal status |
POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral |
Mechanism of Action
- Fenoprofen calcium is a non-steroidal, anti-inflammatory, antiarthritic drug that also possesses analgesic and antipyretic activities. Its exact mode of action is unknown, but it is thought that prostaglandin synthetase inhibition is involved. Fenoprofen has been shown to inhibit prostaglandin synthetase isolated from bovine seminal vesicles. Reproduction studies in rats have shown fenoprofen to be associated with prolonged labor and difficult parturition when given during late pregnancy. Evidence suggests that this may be due to decreased uterine contractility resulting from the inhibition of prostaglandin synthesis. Its action is not mediated through the adrenal gland.
Structure
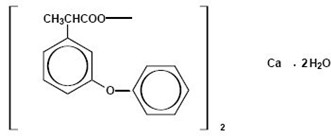
- Fenoprofen calcium is a non-steroidal, anti-inflammatory, antiarthritic drug. Chemically, fenoprofen calcium is an arylacetic acid derivative. The structural formula is as follows:
- Benzeneacetic acid, α-methyl-3-phenoxy-, calcium salt (2:1)-(±)-, dihydrate
- Fenoprofen calcium, USP is a white, crystalline powder, soluble in alcohol (95%) to the extent of approximately 15 mg/mL at 25°C, slightly soluble in water, and insoluble in benzene.
- The pKa of fenoprofen calcium is 4.5 at 25°C.
- Film-coated fenoprofen calcium tablets for oral administration are available containing fenoprofen calcium as the dihydrate equivalent to 600 mg of fenoprofen and the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, pregelatinized starch, sodium lauryl sulfate, titanium dioxide and FD&C Yellow No. 6 Aluminum Lake.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Fenoprofen in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Fenoprofen in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Fenoprofen in the drug label.
Clinical Studies
- Fenoprofen shows anti-inflammatory effects in rodents by inhibiting the development of redness and edema in acute inflammatory conditions and by reducing soft-tissue swelling and bone damage associated with chronic inflammation. It exhibits analgesic activity in rodents by inhibiting the writhing response caused by the introduction of an irritant into the peritoneal cavities of mice and by elevating pain thresholds that are related to pressure in edematous hindpaws of rats. In rats made febrile by the subcutaneous administration of brewer's yeast, fenoprofen produces antipyretic action. These effects are characteristic of non-steroidal, anti-inflammatory, antipyretic, analgesic drugs.
- The results in humans confirmed the anti-inflammatory and analgesic actions found in animals. The emergence and degree of erythemic response were measured in adult male volunteers exposed to ultraviolet irradiation. The effects of fenoprofen, aspirin and indomethacin were each compared with those of a placebo. All three drugs demonstrated antierythemic activity.
- In all patients with rheumatoid arthritis, the anti-inflammatory action of fenoprofen has been evidenced by relief of pain, increase in grip strength and reductions in joint swelling, duration of morning stiffness and disease activity (as assessed by both the investigator and the patient). The anti-inflammatory action of fenoprofen has also been evidenced by increased mobility (i.e., a decrease in the number of joints having limited motion).
- The use of fenoprofen in combination with gold salts or corticosteroids has been studied in patients with rheumatoid arthritis. The studies, however, were inadequate in demonstrating whether further improvement is obtained by adding fenoprofen to maintenance therapy with gold salts or steroids. Whether or not fenoprofen, used in conjunction with partially effective doses of a corticosteroid, has a "steroid-sparing" effect is unknown.
- In patients with osteoarthritis, the anti-inflammatory and analgesic effects of fenoprofen have been demonstrated by reduction in tenderness as a response to pressure and reductions in night pain, stiffness, swelling and overall disease activity (as assessed by both the patient and the investigator). These effects have also been demonstrated by relief of pain with motion and at rest and increased range of motion in involved joints.
- In patients with rheumatoid arthritis and osteoarthritis, clinical studies have shown fenoprofen to be comparable to aspirin in controlling the aforementioned measures of disease activity, but mild gastrointestinal reactions (nausea, dyspepsia) and tinnitus occurred less frequently in patients treated with fenoprofen than in aspirin-treated patients. It is not known whether fenoprofen calcium causes less peptic ulceration than does aspirin.
- In patients with pain, the analgesic action of fenoprofen has produced a reduction in pain intensity, an increase in pain relief, improvement in total analgesia scores, and a sustained analgesic effect.
- Under fasting conditions, fenoprofen is rapidly absorbed and peak plasma levels of 50 mcg/mL are achieved within 2 hours after oral administration of 600 mg doses. Good dose proportionality was observed between 200 mg and 600 mg doses in fasting male volunteers. The plasma half-life is approximately 3 hours. About 90% of a single oral dose is eliminated within 24 hours as fenoprofen glucuronide and 4'-hydroxy-fenoprofen glucuronide, the major urinary metabolites of fenoprofen. Fenoprofen is highly bound (99%) to albumin.
- The concomitant administration of antacid (containing both aluminum and magnesium hydroxide) does not interfere with absorption of fenoprofen.
- There is less suppression of collagen-induced platelet aggregation with single doses of fenoprofen calcium than there is with aspirin.
How Supplied
- Fenoprofen calcium tablets, USP are available containing fenoprofen calcium, USP equivalent to 600 mg fenoprofen.
- The 600 mg tablet is an orange, film-coated, capsule-shaped, tablet debossed with M471 on one side of the tablet and scored on the other side. They are available as follows:
- NDC 0378-0471-01
- bottles of 100 tablets
- Store at 20° to 25°C (68° to 77°F).
- Protect from light.
- Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Storage
There is limited information regarding Fenoprofen Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Fenoprofen |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Fenoprofen |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients should be informed of the following information before initiating therapy with an NSAID and periodically during the course of ongoing therapy. Patients should also be encouraged to read the NSAID Medication Guide that accompanies each prescription dispensed.
- Fenoprofen, like other NSAIDs, may cause serious CV side effects, such as MI or stroke, which may result in hospitalization and even death. Although serious CV events can occur without warning symptoms, patients should be alert for the signs and symptoms of chest pain, shortness of breath, weakness, slurring of speech, and should ask for medical advice when observing any indicative sign or symptoms. Patients should be apprised of the importance of this follow-up.
- Fenoprofen, like other NSAIDs, can cause GI discomfort and, rarely, serious GI side effects, such as ulcers and bleeding, which may result in hospitalization and even death. Although serious GI tract ulcerations and bleeding can occur without warning symptoms, patients should be alert for the signs and symptoms of ulcerations and bleeding, and should ask for medical advice when observing any indicative sign or symptoms including epigastric pain, dyspepsia, melena, and hematemesis. Patients should be apprised of the importance of this follow-up.
- Fenoprofen, like other NSAIDs, can cause serious skin side effects such as exfoliative dermatitis, SJS, and TEN, which may result in hospitalization and even death. Although serious skin reactions may occur without warning, patients should be alert for the signs and symptoms of skin rash and blisters, fever, or other signs of hypersensitivity such as itching, and should ask for medical advice when observing any indicative signs or symptoms. Patients should be advised to stop the drug immediately if they develop any type of rash and contact their physicians as soon as possible.
- Patients should promptly report signs or symptoms of unexplained weight gain or edema to their physicians.
- Patients should be informed of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, pruritus, jaundice, right upper quadrant tenderness, and "flu-like" symptoms). If these occur, patients should be instructed to stop therapy and seek immediate medical therapy.
- Patients should be informed of the signs of an anaphylactoid reaction (e.g. difficulty breathing, swelling of the face or throat). If these occur, patients should be instructed to seek immediate emergency help.
- In late pregnancy, as with other NSAIDs, fenoprofen should be avoided because it may cause premature closure of the ductus arteriosus.
Precautions with Alcohol
- Alcohol-Fenoprofen interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- FENOPROFEN CALCIUM®[2]
Look-Alike Drug Names
There is limited information regarding Fenoprofen Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Diamond S, Solomon GD, Freitag FG, Mehta ND (1987). "Fenoprofen in the prophylaxis of migraine: a double-blind, placebo controlled study". Headache. 27 (5): 246–9. PMID 3298164.
- ↑ "FENOPROFEN CALCIUM fenoprofen calcium tablet, film coated".
{{#subobject:
|Page Name=Fenoprofen
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Fenoprofen |Label Name=Fenoprofen03.png
}}
{{#subobject:
|Label Page=Fenoprofen |Label Name=Fenoprofen04.png
}}