Aceclofenac
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Aceclofenac is a Non Steroidal Anti-inflamatory Drug (NSAID) that is FDA approved for the treatment of rheumatoid arthritis, ankylosing spondylitis, osteoarthritis and periarthritis of scapulohumerous, lumbago, ischiadynia, pain caused by nonaticular rheutism. Common adverse reactions include dyspepsia, abdominal pain, nausea, rash, ruber, urticaria, symptoms of enuresis, headache, dizziness, and drowsiness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- The recommended dose is 200 mg daily, taken as one dose (every 24 hours). However, the dose and dose frequency of Aceclofenac can be modified under the supervison of physician or pharmacist.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Aceclofenac in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Aceclofenac in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
The dosage and indication is not established yet for children with less than 6 years old.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Aceclofenac in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Aceclofenac in pediatric patients.
Contraindications
Aceclofenac is contraindicated in patients with:
- Patients with allergy to these drugs or other analogues (diclofenac).
- Patients with asthma.
- Like NSAIDS, acetylsalicylic acid and other drugs which inhibit prostagladin-synthesis may precipitate attacks of asthma, acute rhinitis or urticaria.
- Patients with active peptic ulcer.
Warnings
- Patients with symptoms indicative of gastro-intestinal disorders, with a history of gastric ulceration. Patients with severe hepatic impairment or cardiac or renal impairment. Patients under the medication of diuretics. Patients in recovery after surgical treatment.
Adverse Reactions
Clinical Trials Experience
- The majority of side effects observed have been reversible and of a minor nature and include gastro-intestinal disorders (dyspepsia, abdominal pain, nausea), rash, ruber, urticaria, symptoms of enuresis, headache, dizziness, and drowsiness. To report suspected adverse reactions, call 1-800-FDA-1088.
Postmarketing Experience
There is limited information regarding Aceclofenac Postmarketing Experience in the drug label.
Drug Interactions
- There has been no drug interactions reported, but close monitoring of patients on combination with lithium and digoxin, oral anti diabetic agents, anticoagulants, diuretics, and other analgesics.
Use in Specific Populations
Pregnancy
- Since there is no information on the safe use of Aceclofenac during pregnancy and lactation, the use of Aclofenac should therefore be avoided in pregnancy and lactation.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Aceclofenac in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Aceclofenac during labor and delivery.
Nursing Mothers
- Since there is no information on the safe use of Aceclofenac during pregnancy and lactation, the use of Aceclofenac should therefore be avoided in pregnancy and lactation.
Pediatric Use
- The dosage and indication is not established yet for children with less than 6 years old.
Geriatic Use
There is no FDA guidance on the use of Aceclofenac in geriatric settings.
Gender
There is no FDA guidance on the use of Aceclofenac with respect to specific gender populations.
Race
There is no FDA guidance on the use of Aceclofenac with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Aceclofenac in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Aceclofenac in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Aceclofenac in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Aceclofenac in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Aceclofenac Administration in the drug label.
Monitoring
There is limited information regarding Aceclofenac Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Aceclofenac and IV administrations.
Overdosage
- There are no human data available on the consequences of Aceclofenac overdosage. If overdosage is observed, therapeutic measures should be taken according to symptoms; supportive and symptomatic treatment should be given for complications such as hypotension, gastrointestinal irritation, respiratory depression, and convulsions.
Pharmacology
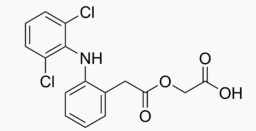
| |
Aceclofenac
| |
| Systematic (IUPAC) name | |
| 2-[2-[2-[(2,6-dichlorophenyl)amino]phenyl]acetyl]oxyacetic acid | |
| Identifiers | |
| CAS number | |
| ATC code | M01 M02AA25 (WHO) |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 354.18472 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | oral, topical |
Mechanism of Action
There is limited information regarding Aceclofenac Mechanism of Action in the drug label.
Structure

Pharmacodynamics
There is limited information regarding Aceclofenac Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Aceclofenac Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Aceclofenac Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Aceclofenac Clinical Studies in the drug label.
How Supplied
- 10 Blister Packs with 10 Tablets in each Blister Pack
Storage
- Preserve in tight containers. Store at room temperature not exceeding 30oC. Three (3) years from manufacturing date. Do not exceed the expiry date for use printed on the box.
Images
Drug Images
{{#ask: Page Name::Aceclofenac |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
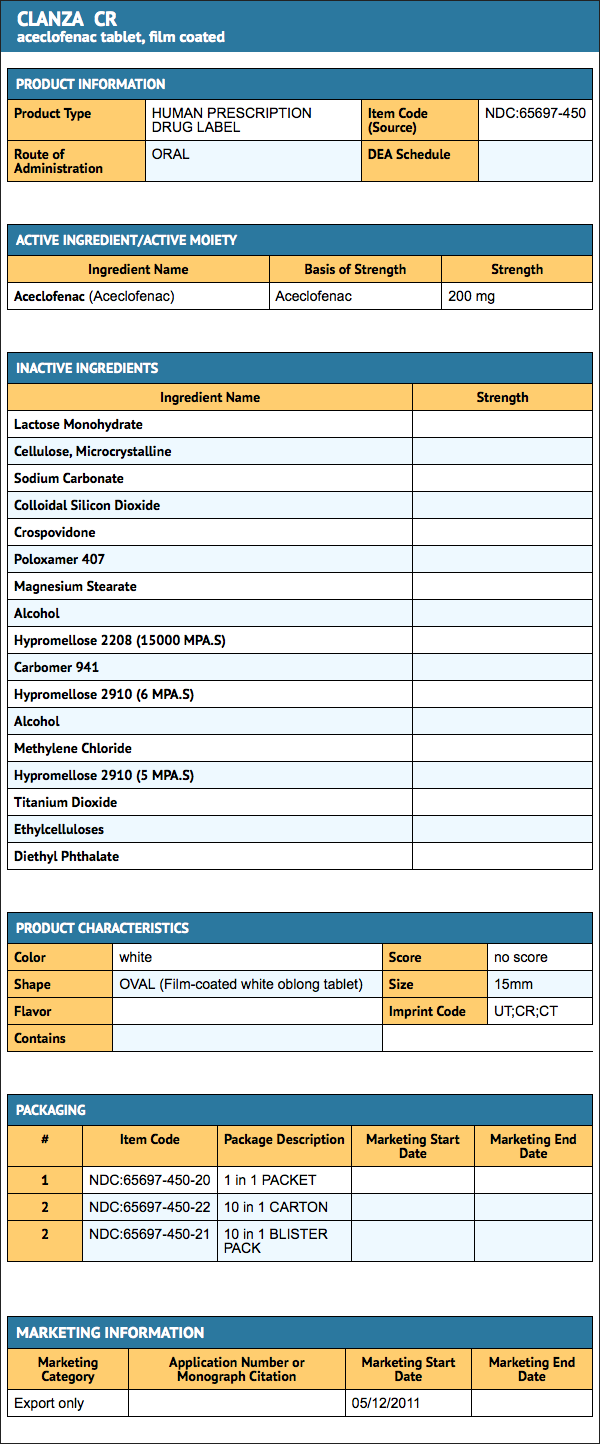
{{#ask: Label Page::Aceclofenac |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Aceclofenac Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Aceclofenac interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Aceclofenac Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Aceclofenac |Label Name=Aceclofenac Package.png
}}