Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alonso Alvarado, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH STEATOSIS and POST TREATMENT ACUTE EXACERBATION OF HEPATITIS B
See full prescribing information for complete Boxed Warning.
LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH STEATOSIS and POST TREATMENT ACUTE EXACERBATION OF HEPATITIS B: Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs, including tenofovir disoproxil fumarate, a component of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate, in combination with other antiretrovirals.
Elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarateis not approved for the treatment of chronic hepatitis B virus (HBV) infection and the safety and efficacy of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate have not been established in patients coinfected with HBV and HIV-1. Severe acute exacerbations of hepatitis B have been reported in patients who are coinfected with HBV and human immunodeficiency virus-1 (HIV-1) and have discontinued emtricitabineor tenofovir disoproxil fumarate, which are components of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who are coinfected with HIV-1 and HBV and discontinue elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate. If appropriate, initiation of anti-hepatitis B therapy may be warranted
|
Overview
Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate is a atiretroviral that is FDA approved for the treatment of HIV-1 infection in adults who are antiretroviral treatment-naïve. There is a Black Box Warning for this drug as shown here. Common adverse reactions include diarrhea, nausea, proteinuria.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Dosage Information
The recommended dosage of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate is one tablet taken orally once daily with food.
Dosage Adjustment in Patients with Renal Impairment
Initiation of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate in patients with estimated creatinine clearance below 70 mL per minute is not recommended. Because elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate is a fixed-dose combination tablet, elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate should be discontinued if estimated creatinine clearance declines below 50 mL per min during treatment with elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate as dose interval adjustment required for emtricitabine and tenofovir disoproxil fumarate (DF) cannot be achieved.
Dosage in Patients with Hepatic Impairment
No dosage adjustment of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate is required in patients with mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment. No pharmacokinetic or safety data are available regarding the use of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate in patients with severe hepatic impairment (Child-Pugh Class C). Therefore, elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate is not recommended for use in patients with severe hepatic impairment.
Testing Prior to Initiation of Elvitegravir, Cobicistat, Emtricitabine, and Tenofovir Disoproxil Fumarate
Prior to initiation of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate, patients should be tested for hepatitis B infection and estimated creatinine clearance, urine glucose and urine protein should be documented in all patients.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate in pediatric patients.
Contraindications
Coadministration of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate is contraindicated with drugs that are highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events. These drugs and other contraindicated drugs (which may lead to reduced efficacy of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate and possible resistance) are listed in Table 1

Warnings
|
WARNING: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH STEATOSIS and POST TREATMENT ACUTE EXACERBATION OF HEPATITIS B
See full prescribing information for complete Boxed Warning.
LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH STEATOSIS and POST TREATMENT ACUTE EXACERBATION OF HEPATITIS B: Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs, including tenofovir disoproxil fumarate, a component of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate, in combination with other antiretrovirals.
Elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarateis not approved for the treatment of chronic hepatitis B virus (HBV) infection and the safety and efficacy of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate have not been established in patients coinfected with HBV and HIV-1. Severe acute exacerbations of hepatitis B have been reported in patients who are coinfected with HBV and human immunodeficiency virus-1 (HIV-1) and have discontinued emtricitabineor tenofovir disoproxil fumarate, which are components of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who are coinfected with HIV-1 and HBV and discontinue elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate. If appropriate, initiation of anti-hepatitis B therapy may be warranted
|
Lactic Acidosis/Severe Hepatomegaly with Steatosis
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs, including tenofovir DF, a component of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate , in combination with other antiretrovirals. A majority of these cases have been in women. Obesity and prolonged nucleoside exposure may be risk factors. Particular caution should be exercised when administering nucleoside analogs to any patient with known risk factors for liver disease; however, cases have also been reported in patients with no known risk factors. Treatment with elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate should be suspended in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
Patients Coinfected with HIV-1 and HBV
It is recommended that all patients with HIV-1 be tested for the presence of chronic hepatitis B virus (HBV) before initiating antiretroviral therapy. Elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate is not approved for the treatment of chronic HBV infection and the safety and efficacy of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate have not been established in patients coinfected with HBV and HIV-1. Severe acute exacerbations of hepatitis B have been reported in patients who are coinfected with HBV and HIV-1 and have discontinued emtricitabine or tenofovir DF, two of the components of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate. In some patients infected with HBV and treated with emtricitabine, the exacerbations of hepatitis B were associated with liver decompensation and liver failure. Patients who are coinfected with HIV-1 and HBV should be closely monitored with both clinical and laboratory follow-up for at least several months after stopping treatment with elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate. If appropriate, initiation of anti-hepatitis B therapy may be warranted.
New Onset or Worsening Renal Impairment
Renal impairment, including cases of acute renal failure and Fanconi syndrome (renal tubular injury with severe hypophosphatemia), has been reported with the use of tenofovir DF, a component of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate, and with the use of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate.
In the clinical trials of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate over 96 weeks, 10 (1.4%) subjects in the elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate group (N=701) and 2 (0.3%) subjects in the combined comparator groups (N = 707) discontinued study drug due to a renal adverse reaction. Of these discontinuations, 8 in the elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate group and 1 in the combined comparator groups occurred during the first 48 week. Four (0.6%) of the subjects who received elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate developed laboratory findings consistent with proximal renal tubular dysfunction leading to discontinuation of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate compared to none in the comparator groups. Two of these four subjects had renal impairment (i.e. estimated creatinine clearance less than 70 mL per minute) at baseline. The laboratory findings in these 4 subjects with evidence of proximal tubulopathy improved but did not completely resolve in all subjects upon discontinuation of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate. Renal replacement therapy was not required for these subjects.
Estimated creatinine clearance, urine glucose and urine protein should be documented in all patients prior to initiating therapy. Initiation of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate in patients with estimated creatinine clearance below 70 mL per minute is not recommended.
Elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate should be avoided with concurrent or recent use of a nephrotoxic agent (e.g., high-dose or multiple non-steroidal anti-inflammatory drugs (NSAIDs)). Cases of acute renal failure after initiation of high dose or multiple NSAIDs have been reported in HIV-infected patients with risk factors for renal dysfunction who appeared stable on tenofovir DF. Some patients required hospitalization and renal replacement therapy. Alternatives to NSAIDs should be considered, if needed, in patients at risk for renal dysfunction.
Persistent or worsening bone pain, pain in extremities, fractures and/or muscular pain or weakness may be manifestations of proximal renal tubulopathy and should prompt an evaluation of renal function in at-risk patients.
Routine monitoring of estimated creatinine clearance, urine glucose, and urine protein should be performed during elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate therapy in all patients. Additionally, serum phosphorus should be measured in patients at risk for renal impairment. Although cobicistat (a component of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate) may cause modest increases in serum creatinine and modest declines in estimated creatinine clearance without affecting renal glomerular function, patients who experience a confirmed increase in serum creatinine of greater than 0.4 mg per dL from baseline should be closely monitored for renal safety.
The emtricitabine and tenofovir DF components of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate are primarily excreted by the kidney. elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate should be discontinued if estimated creatinine clearance declines below 50 mL per minute as dose interval adjustment required for emtricitabine and tenofovir DF cannot be achieved with the fixed-dose combination tablet.
Avoid Use with Other Antiretroviral Products
Elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate is indicated for use as a complete regimen for the treatment of HIV-1 infection and coadministration with other antiretroviral products is not recommended.
Elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate is not recommended for coadministration with the following: emtricitabine or tenofovir DF (ATRIPLA, COMPLERA, EMTRIVA, TRUVADA, VIREAD); products containing lamivudine (COMBIVIR, EPIVIR, EPIVIR-HBV, EPZICOM, TRIZIVIR) or adefovir dipivoxil (HEPSERA); ritonavir (NORVIR, KALETRA).
Bone Effects of Tenofovir DF
Bone Mineral Density
In clinical trials in HIV-1 infected adults, tenofovir DF (a component of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate) was associated with slightly greater decreases in bone mineral density (BMD) and increases in biochemical markers of bone metabolism, suggesting increased bone turnover relative to comparators. Serum parathyroid hormone levels and 1.25 Vitamin D levels were also higher in subjects receiving tenofovir DF. For additional information, see Adverse Reactions (6.1) and consult the VIREAD prescribing information.
The effects of tenofovir DF-associated changes in BMD and biochemical markers on long-term bone health and future fracture risk are unknown. Assessment of BMD should be considered for HIV-1 infected patients who have a history of pathologic bone fracture or other risk factors for osteoporosis or bone loss. Although the effect of supplementation with calcium and vitamin D was not studied, such supplementation may be beneficial in all patients. If bone abnormalities are suspected, then appropriate consultation should be obtained.
Mineralization Defects
Cases of osteomalacia associated with proximal renal tubulopathy, manifested as bone pain or pain in extremities and which may contribute to fractures, have been reported in association with the use of tenofovir DF. Arthralgias and muscle pain or weakness have also been reported in cases of proximal renal tubulopathy. Hypophosphatemia and osteomalacia secondary to proximal renal tubulopathy should be considered in patients at risk of renal dysfunction who present with persistent or worsening bone or muscle symptoms while receiving products containing tenofovir DF.
Fat Redistribution
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and "cushingoid appearance" have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia (PCP), or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves' disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution, however, the time to onset is more variable, and can occur many months after initiation of treatment.
Adverse Reactions
Clinical Trials Experience
The following adverse drug reactions are discussed in other sections of the labeling:
- Lactic Acidosis/Severe Hepatomegaly with Steatosis.
- Severe Acute Exacerbations of Hepatitis B.
- New Onset or Worsening Renal Impairment.
- Bone Effects of Tenofovir DF.
- Immune Reconstitution Syndrome.
Adverse Reactions from Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety assessment of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate is based on the Week 96 pooled data from 1408 subjects in two randomized, double-blind, active-controlled clinical trials, Study 102 and Study 103, in antiretroviral treatment-naïve HIV-1 infected adult subjects. A total of 701 subjects received elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate once daily for at least 96 weeks.
The proportion of subjects who discontinued treatment with elvitegravir 150 mg/cobicistat 150 mg/emtricitabine 200 mg/tenofovir DF 300 mg; efavirenz 600 mg/emtricitabine 200 mg/tenofovir DF 300 mg); or atazanavir (ATV) + ritonavir (RTV) + emtricitabine 200 mg/tenofovir DF 300 mg due to adverse events, regardless of severity, was 4.6%, 6.8% and 5.9%, respectively. Table 2 displays the frequency of adverse drug reactions greater than or equal to 5% of subjects in any treatment arm.

See Warnings and Precautions (5.3), for a discussion of renal adverse reactions from clinical trials experience with elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate.
Adverse Reactions from Clinical Trials of the Components of STRIBILD
Emtricitabine and Tenofovir Disoproxil Fumarate: In addition to the adverse reactions observed with elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate combination, the following adverse reactions occurred in at least 5% of treatment-experienced or treatment-naïve subjects receiving emtricitabine or tenofovir DF with other antiretroviral agents in other clinical trials: depression, abdominal pain, dyspepsia, vomiting, fever, pain, nasopharyngitis, pneumonia, sinusitis, upper respiratory tract infection, arthralgia, back pain, myalgia, paresthesia, peripheral neuropathy (including peripheral neuritis and neuropathy), anxiety, increased cough, and rhinitis.
Skin discoloration has been reported with higher frequency among emtricitabine-treated subjects; it was manifested by hyperpigmentation on the palms and/or soles and was generally mild and asymptomatic. The mechanism and clinical significance are unknown.
Laboratory Abnormalities
The frequency of laboratory abnormalities (Grades 3–4) occurring in at least 2% of subjects receiving elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate in Studies 102 and 103 are presented in Table 3.

In Study 103, BMD was assessed by DEXA in a non-random subset of 120 subjects. Mean percentage decreases in BMD from baseline to Week 96 in the elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate group (N = 47) were comparable to the ATV + RTV + TRUVADA group (N = 53) at the lumbar spine (-2.0% versus -3.5%, respectively) and at the hip (-3.2% versus -4.2%, respectively). In Studies 102 and 103, bone fractures occurred in 14 subjects (2.0%) in the elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate group, 8 subjects (2.3%) in the ATRIPLA group, and 14 subjects (3.9%) in the ATV + RTV + TRUVADA group. These findings were consistent with data from an earlier 144-week trial of treatment-naïve subjects receiving tenofovir DF + lamivudine + efavirenz.
Proteinuria (all grades) occurred in 46% of subjects receiving elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate, 38% of subjects receiving ATRIPLA, and 37% of subjects receiving ATV + RTV + TRUVADA.
The cobicistat component of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate has been shown to increase serum creatinine and decrease estimated creatinine clearance due to inhibition of tubular secretion of creatinine without affecting renal glomerular function. In Studies 102 and 103, increases in serum creatinine and decreases in estimated creatinine clearance occurred early in treatment with elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate, after which they stabilized. Table 4 displays the mean changes in serum creatinine and eGFR levels at Week 96 and the percentage of subjects with elevations in serum creatinine (All Grades).

Emtricitabine or Tenofovir DF: In addition to the laboratory abnormalities observed with elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate , the following laboratory abnormalities have been previously reported in subjects treated with emtricitabine or tenofovir DF with other antiretroviral agents in other clinical trials: Grade 3 or 4 laboratory abnormalities of ALT (M: greater than 215 U per L; F: greater than 170 U per L), alkaline phosphatase (greater than 550 U per L), bilirubin (greater than 2.5 × ULN), serum glucose (less than 40 or greater than 250 mg per dL), glycosuria (greater than or equal to 3+), neutrophils (less than 750 per mm3), fasting cholesterol (greater than 240 mg per dL), and fasting triglycerides (greater than 750 mg per dL).
Serum Lipids: In the clinical trials of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate, a similar percentage of subjects receiving elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate, ATRIPLA, and ATV + RTV + TRUVADA were on lipid lowering agents at baseline (11%, 11%, and 12%, respectively). While receiving study drug through Week 96, an additional 8% of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate subjects were started on lipid lowering agents, compared to 9% of ATRIPLA and 8% of ATV + RTV + TRUVADA subjects.
Changes from baseline in total cholesterol, HDL-cholesterol, LDL-cholesterol, and triglycerides are presented in Table 5.

Postmarketing Experience
Because postmarketing reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The following adverse reactions have been identified during post approval use of tenofovir DF. No additional postmarketing adverse reactions specific for emtricitabine have been identified.
- Immune System Disorders: allergic reaction, including angioedema
- Metabolism and Nutrition Disorders: lactic acidosis, hypokalemia, hypophosphatemia
- Respiratory, Thoracic, and Mediastinal Disorders: dyspnea
- Gastrointestinal Disorders: pancreatitis, increased amylase, abdominal pain
- Hepatobiliary Disorders: hepatic steatosis, hepatitis, increased liver enzymes (most commonly AST, ALT, GGT)
- Skin and Subcutaneous Tissue Disorders: rash
- Musculoskeletal and Connective Tissue Disorders: rhabdomyolysis, osteomalacia (manifested as bone pain and which may contribute to fractures), muscular weakness, myopathy
- Renal and Urinary Disorders: acute renal failure, renal failure, acute tubular necrosis, Fanconi syndrome, proximal renal tubulopathy, interstitial nephritis (including acute cases), nephrogenic diabetes insipidus, renal insufficiency, increased creatinine, proteinuria, polyuria
- General Disorders and Administration Site Conditions: asthenia
The following adverse reactions, listed under the body system headings above, may occur as a consequence of proximal renal tubulopathy: rhabdomyolysis, osteomalacia, hypokalemia, muscular weakness, myopathy, hypophosphatemia.
Drug Interactions
Other Antiretroviral Medications
Elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate is a complete regimen for the treatment of HIV-1 infection; therefore, elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate should not be administered with other antiretroviral medications for treatment of HIV-1 infection. Complete information regarding potential drug-drug interactions with other antiretroviral medications is not provided.
Potential for Elvitegravir, Cobicistat, Emtricitabine, and Tenofovir Disoproxil Fumarate to Affect Other Drugs
Cobicistat, is an inhibitor of CYP3A and CYP2D6 and an inhibitor of the following transporters: p-glycoprotein (P-gp), BCRP, OATP1B1 and OATP1B3. Thus, coadministration of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate with drugs that are primarily metabolized by CYP3A or CYP2D6, or are substrates of P-gp, BCRP, OATP1B1 or OATP1B3 may result in increased plasma concentrations of such drugs. Elvitegravir is a modest inducer of CYP2C9 and may decrease the plasma concentrations of CYP2C9 substrates.
Potential for Other Drugs to Affect One or More Components of Elvitegravir, Cobicistat, Emtricitabine, and Tenofovir Disoproxil Fumarate
Elvitegravir and cobicistat, components of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate , are metabolized by CYP3A. Cobicistat is also metabolized, to a minor extent, by CYP2D6.
Drugs that induce CYP3A activity are expected to increase the clearance of elvitegravir and cobicistat, resulting in decreased plasma concentration of cobicistat and elvitegravir, which may lead to loss of therapeutic effect of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate and development of resistance (see Table 6).
Coadministration of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate with other drugs that inhibit CYP3A may decrease the clearance and increase the plasma concentration of cobicistat (see Table 6).
Drugs Affecting Renal Function
Because emtricitabine and tenofovir, components of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate are primarily excreted by the kidneys by a combination of glomerular filtration and active tubular secretion, coadministration of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate with drugs that reduce renal function or compete for active tubular secretion may increase concentrations of emtricitabine, tenofovir, and other renally eliminated drugs and this may increase the risk of adverse reactions. Some examples of drugs that are eliminated by active tubular secretion include, but are not limited to acyclovir, cidofovir, ganciclovir, valacyclovir, valganciclovir, aminoglycosides (e.g. gentamicin), and high-dose or multiple NSAIDs.
Established and Other Potentially Significant Interactions
Table 6 provides a listing of established or potentially clinically significant drug interactions. The drug interactions described are based on studies conducted with either elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate, the components of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate as individual agents and/or in combination, or are predicted drug interactions that may occur with elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate. The table includes potentially significant interactions but is not all inclusive.

Drugs without Clinically Significant Interactions with Elvitegravir, Cobicistat, Emtricitabine, and Tenofovir Disoproxil Fumarate
Based on drug interaction studies conducted with the components of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate, no clinically significant drug interactions have been either observed or are expected when elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate is combined with the following drugs: entecavir, famciclovir, H2 receptor antagonists, methadone, proton pump inhibitors and ribavirin.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Antiretroviral Pregnancy Registry: To monitor fetal outcomes of pregnant women exposed to elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate, an Antiretroviral Pregnancy Registry has been established. Healthcare providers are encouraged to register patients by calling 1-800-258-4263.
Animal Data
Elvitegravir: Studies in animals have shown no evidence of teratogenicity or an effect on reproductive function. In offspring from rat and rabbit dams treated with elvitegravir during pregnancy, there were no toxicologically significant effects on developmental endpoints. The exposures (AUC) at the embryo-fetal No Observed Adverse Effects Levels (NOAELs) in rats and rabbits were respectively 23 and 0.2 times higher than the exposure in humans at the recommended daily dose of 150 mg.
Cobicistat: Studies in animals have shown no evidence of teratogenicity or an effect on reproductive function. In offspring from rat and rabbit dams treated with cobicistat during pregnancy, there were no toxicologically significant effects on developmental endpoints. The exposures (AUC) at the embryo-fetal NOAELs in rats and rabbits were respectively 1.8 and 4.3 times higher than the exposure in humans at the recommended daily dose of 150 mg.
Emtricitabine: The incidence of fetal variations and malformations was not increased in embryo-fetal toxicity studies performed with emtricitabine in mice at exposures (AUC) approximately 60 times higher and in rabbits at approximately 120 times higher than human exposures at the recommended daily dose.
Tenofovir DF: Reproduction studies have been performed in rats and rabbits at doses up to 14 and 19 times the human dose based on body surface area comparisons and revealed no evidence of impaired fertility or harm to the fetus due to tenofovir.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate during labor and delivery.
Nursing Mothers
The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV. Studies in rats have demonstrated that elvitegravir, cobicistat, and tenofovir are secreted in milk. It is not known whether elvitegravir or cobicistat is excreted in human milk.
In humans, samples of breast milk obtained from five HIV-1 infected mothers show that emtricitabine is secreted in human milk. Breastfeeding infants whose mothers are being treated with emtricitabine may be at risk for developing viral resistance to emtricitabine. Other emtricitabine-associated risks in infants breastfed by mothers being treated with emtricitabine are unknown.
Samples of breast milk obtained from five HIV-1 infected mothers show that tenofovir is secreted in human milk. Tenofovir-associated risks, including the risk of viral resistance to tenofovir, in infants breastfed by mothers being treated with tenofovir disoproxil fumarate are unknown. Because of both the potential for HIV transmission and the potential for serious adverse reactions in nursing infants, mothers should be instructed not to breastfeed if they are receiving elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate.
Pediatric Use
Safety and effectiveness of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate in pediatric patients less than 18 years of age have not been established
Geriatic Use
Clinical studies of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, caution should be exercised in the administration of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate in elderly patients, keeping in mind the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate with respect to specific racial populations.
Renal Impairment
Initiation of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate in patients with estimated creatinine clearance below 70 mL per min is not recommended. Because elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate is a fixed-dose combination tablet, elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate should be discontinued if estimated creatinine clearance declines below 50 mL per min during treatment with elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate as dose interval adjustment required for emtricitabine and tenofovir DF cannot be achieved
Hepatic Impairment
No dose adjustment of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate is required in patients with mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment. No pharmacokinetic or safety data are available regarding the use of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate in patients with severe hepatic impairment (Child-Pugh Class C). Therefore, elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate is not recommended for use in patients with severe hepatic impairment
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
Elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate is not approved for the treatment of chronic hepatitis B virus (HBV) infection. Severe acute exacerbations of hepatitis B have been reported in patients coinfected with HIV-1 and HBV who have discontinued emtricitabine or tenofovir disoproxil fumarate, two of the components of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate. Hepatic function should be monitored closely in these patients. If appropriate, initiation of anti-hepatitis B therapy may be warranted.
IV Compatibility
There is limited information regarding the compatibility of Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate and IV administrations.
Overdosage
No data are available on overdose of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate in patients. If overdose occurs the patient must be monitored for evidence of toxicity. Treatment of overdose with elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate consists of general supportive measures including monitoring of vital signs as well as observation of the clinical status of the patient.
Elvitegravir: Limited clinical experience is available at doses higher than the therapeutic dose of elvitegravir. In one study, boosted elvitegravir equivalent to 2 times the therapeutic dose of 150 mg once daily for 10 days was administered to 42 healthy subjects. No severe adverse reactions were reported. The effects of higher doses are not known. As elvitegravir is highly bound to plasma proteins, it is unlikely that it will be significantly removed by hemodialysis or peritoneal dialysis.
Cobicistat: Limited clinical experience is available at doses higher than the therapeutic dose of cobicistat. In two studies, a single dose of cobicistat 400 mg (2.7 times the dose in elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate) was administered to a total of 60 healthy subjects. No severe adverse reactions were reported. The effects of higher doses are not known. As cobicistat is highly bound to plasma proteins, it is unlikely that it will be significantly removed by hemodialysis or peritoneal dialysis.
Emtricitabine: Limited clinical experience is available at doses higher than the therapeutic dose of emtricitabine. In one clinical pharmacology study, single doses of emtricitabine 1200 mg (6 times the dose in elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate) were administered to 11 subjects. No severe adverse reactions were reported. The effects of higher doses are not known.
Hemodialysis treatment removes approximately 30% of the emtricitabine dose over a 3 hour dialysis period starting within 1.5 hours of emtricitabine dosing (blood flow rate of 400 mL per minute and a dialysate flow rate of 600 mL per minute). It is not known whether emtricitabine can be removed by peritoneal dialysis.
Tenofovir DF: Limited clinical experience at doses higher than the therapeutic dose of tenofovir disoproxil fumarate 300 mg is available. In one study, 600 mg tenofovir DF (2 times the dosage in elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate) was administered to 8 subjects orally for 28 days, and no severe adverse reactions were reported. The effects of higher doses are not known. Tenofovir is efficiently removed by hemodialysis with an extraction coefficient of approximately 54%. Following a single 300 mg dose of tenofovir disoproxil fumarate, a 4-hour hemodialysis session removed approximately 10% of the administered tenofovir dose.
Pharmacology
There is limited information regarding Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate Mechanism of Action in the drug label.
Structure
There is limited information regarding Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate Structure in the drug label.
Pharmacodynamics
There is limited information regarding Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate Clinical Studies in the drug label.
How Supplied
There is limited information regarding Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate How Supplied in the drug label.
Storage
There is limited information regarding Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
|mechAction=Elvitegravir: Elvitegravir inhibits the strand transfer activity of HIV-1 integrase (integrase strand transfer inhibitor; INSTI), an HIV-1 encoded enzyme that is required for viral replication. Inhibition of integrase prevents the integration of HIV-1 DNA into host genomic DNA, blocking the formation of the HIV-1 provirus and propagation of the viral infection. Elvitegravir does not inhibit human topoisomerases I or II. Cobicistat: Cobicistat is a selective, mechanism-based inhibitor of cytochromes P450 of the CYP3A subfamily. Inhibition of CYP3A-mediated metabolism by cobicistat enhances the systemic exposure of CYP3A substrates, such as elvitegravir, where bioavailability is limited and half-life is shortened by CYP3A-dependent metabolism. Emtricitabine: Emtricitabine, a synthetic nucleoside analog of cytidine, is phosphorylated by cellular enzymes to form emtricitabine 5'-triphosphate. Emtricitabine 5'-triphosphate inhibits the activity of the HIV-1 RT by competing with the natural substrate deoxycytidine 5'-triphosphate and by being incorporated into nascent viral DNA which results in chain termination. Emtricitabine 5'-triphosphate is a weak inhibitor of mammalian DNA polymerases α, β, ε, and mitochondrial DNA polymerase γ. Tenofovir DF: Tenofovir DF is an acyclic nucleoside phosphonate diester analog of adenosine monophosphate. Tenofovir DF requires initial diester hydrolysis for conversion to tenofovir and subsequent phosphorylations by cellular enzymes to form tenofovir diphosphate. Tenofovir diphosphate inhibits the activity of HIV-1 RT by competing with the natural substrate deoxyadenosine 5'-triphosphate and, after incorporation into DNA, by DNA chain termination. Tenofovir diphosphate is a weak inhibitor of mammalian DNA polymerases α, β, and mitochondrial DNA polymerase γ.
Antiviral Activity in Cell Culture
Elvitegravir, Cobicistat, Emtricitabine, and Tenofovir DF: The triple combination of elvitegravir, emtricitabine, and tenofovir was not antagonistic in cell culture combination antiviral activity assays and was not affected by the addition of cobicistat. Elvitegravir: The antiviral activity of elvitegravir against laboratory and clinical isolates of HIV-1 was assessed in T lymphoblastoid cell lines, monocyte/macrophage cells, and primary peripheral blood lymphocytes. The 50% effective concentrations (EC50) ranged from 0.02 to 1.7 nM. Elvitegravir displayed antiviral activity in cell culture against HIV-1 clades A, B, C, D, E, F, G, and O (EC50 values ranged from 0.1 to 1.3 nM) and activity against HIV-2 (EC50 value of 0.53 nM). Elvitegravir did not show inhibition of replication of HBV or HCV in cell culture. Cobicistat: Cobicistat has no detectable antiviral activity in cell culture against HIV-1, HBV, or HCV and does not antagonize the antiviral activity of elvitegravir, emtricitabine, or tenofovir. Emtricitabine: The antiviral activity of emtricitabine against laboratory and clinical isolates of HIV-1 was assessed in T lymphoblastoid cell lines, the MAGI-CCR5 cell line, and primary peripheral blood mononuclear cells. The EC50 values for emtricitabine were in the range of 0.0013–0.64 micromolar. Emtricitabine displayed antiviral activity in cell culture against HIV-1 clades A, B, C, D, E, F, and G (EC50 values ranged from 0.007–0.075 micromolar) and showed strain specific activity against HIV-2 (EC50 values ranged from 0.007–1.5 micromolar). Tenofovir DF: The antiviral activity of tenofovir against laboratory and clinical isolates of HIV-1 was assessed in T lymphoblastoid cell lines, primary monocyte/]]macrophage]] cells and peripheral blood lymphocytes. The EC50 values for tenofovir were in the range of 0.04–8.5 micromolar. Tenofovir displayed antiviral activity in cell culture against HIV-1 clades A, B, C, D, E, F, G, and O (EC50 values ranged from 0.5–2.2 micromolar) and showed strain specific activity against HIV-2 (EC50 values ranged from 1.6–5.5 micromolar).
Resistance
In Cell Culture
- Elvitegravir: HIV-1 isolates with reduced susceptibility to elvitegravir have been selected in cell culture. Reduced susceptibility to elvitegravir was associated with the primary integrase substitutions T66A/I, E92G/Q, S147G, and Q148R. Additional integrase substitutions observed in cell culture selection included D10E, S17N, H51Y, F121Y, S153F/Y, E157Q, D232N, R263K, and V281M.
- Emtricitabine and Tenofovir DF: HIV-1 isolates with reduced susceptibility to emtricitabine or tenofovir have been selected in cell culture. Reduced susceptibility to emtricitabine was associated with M184V/I substitutions in HIV-1 RT. HIV-1 isolates selected by tenofovir expressed a K65R substitution in HIV-1 RT and showed a 2–4 fold reduction in susceptibility to tenofovir.
In Clinical Studies
In Treatment-Naïve HIV-1-Infected Adult Subjects: In Studies 102 and 103 [see Clinical Studies (14)], the development of one or more primary substitutions associated with resistance to elvitegravir (T66A/I/K, E92G/Q, T97A, S147G, Q148H/K/R, and N155H), emtricitabine, and/or tenofovir was observed in 53% (16/30) of the elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate-treatment failure subjects with evaluable genotypic resistance data who received at least 8 weeks of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate and had HIV-1 RNA greater than 400 copies per mL at Week 96 or at the time of early study drug discontinuation. The most common substitutions that emerged were M184V/I (N=15) in HIV-1 RT and the primary elvitegravir resistance-associated substitutions, E92Q (N=9), N155H (N=5), Q148R (N=3), and T66I (N=2) in integrase; K65R in RT was also detected (N=5). In virus isolates harboring the observed primary elvitegravir resistance substitutions, additional substitutions in integrase were detected including H51Y, L68I/V, G70R, I73V, G140C, S153A and E157Q. All subjects with evaluable data for RT and IN and who developed integrase substitutions associated with elvitegravir resistance (N=13) also developed the M184I/V RT substitutions, conferring reduced susceptibility to both elvitegravir and emtricitabine. In phenotypic analyses, HIV-1 isolates expressing M184V/I RT substitutions showed reduced susceptibility to emtricitabine (42- to greater than 152-fold); those expressing the primary elvitegravir resistance-associated integrase substitutions showed reduced susceptibility to elvitegravir (6- to greater than 198-fold); and those expressing the K65R RT substitution showed reduced susceptibility to tenofovir (0.8- to 1.6-fold), compared to wild-type reference HIV-1.
Cross Resistance
Elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate-treatment failure subject isolates exhibited varying degrees of cross resistance within the INSTI and NRTI drug classes depending on the specific substitutions observed. These isolates remained susceptible to all NNRTIs and protease inhibitors.
- Elvitegravir: Cross-resistance has been observed among INSTIs. Elvitegravir-resistant viruses showed varying degrees of cross-resistance in cell culture to raltegravir depending on the type and number of substitutions in HIV-1 integrase. Of the primary elvitegravir resistance-associated substitutions tested (T66A/I/K, E92G/Q, T97A, S147G, Q148H/K/R, and N155H), all but three (T66I, E92G, and S147G) conferred greater than 1.5-fold reduced susceptibility to raltegravir (above the biological cutoff for raltegravir) when introduced individually into a wild-type virus by site-directed mutagenesis. Of the primary raltegravir resistance-associated substitutions (Y143C/H/R, Q148H/K/R, and N155H), all but Y143C/H conferred greater than 2.5-fold reductions in susceptibility to elvitegravir (above the biological cutoff for elvitegravir).
- Emtricitabine: Cross-resistance has been observed among NRTIs. Emtricitabine-resistant isolates harboring an M184V/I substitution in HIV-1 RT were cross-resistant to lamivudine. HIV-1 isolates containing the K65R RT substitution, selected in vivo by abacavir, didanosine, and tenofovir, demonstrated reduced susceptibility to inhibition by emtricitabine.
- Tenofovir DF: Cross-resistance has been observed among NRTIs. The K65R substitution in HIV-1 RT selected by tenofovir is also selected in some HIV-1-infected patients treated with abacavir or didanosine. HIV-1 isolates with the K65R substitution also showed reduced susceptibility to emtricitabine and lamivudine. Therefore, cross-resistance among these NRTIs may occur in patients whose virus harbors the K65R substitution. HIV-1 isolates from patients (N=20) whose HIV-1 expressed a mean of 3 zidovudine-associated RT amino acid substitutions (M41L, D67N, K70R, L210W, T215Y/F, or K219Q/E/N) showed a 3.1-fold decrease in the susceptibility to tenofovir. Subjects whose virus expressed an L74V RT substitution without zidovudine resistance-associated substitutions (N=8) had reduced response to tenofovir. Limited data are available for patients whose virus expressed a Y115F substitution (N=3), Q151M substitution (N=2), or T69 insertion (N=4) in HIV-1 RT, all of whom had a reduced response in clinical trials.
|structure=Elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate is a fixed-dose combination tablet containing elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate for oral administration. Elvitegravir is an HIV-1 integrase strand transfer inhibitor.
Cobicistat is a mechanism-based inhibitor of cytochrome P450 (CYP) enzymes of the CYP3A family. Emtricitabine is a synthetic nucleoside analog of cytidine. EMTRIVA is the brand name for emtricitabine. Tenofovir DF is converted in vivo to tenofovir, an acyclic nucleoside phosphonate (nucleotide) analog of adenosine 5'-monophosphate. VIREAD is the brand name for tenofovir DF.
Each tablet contains 150 mg of elvitegravir, 150 mg of cobicistat, 200 mg of emtricitabine, and 300 mg of tenofovir DF (equivalent to 245 mg of tenofovir disoproxil). The tablets include the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, silicon dioxide, croscarmellose sodium, hydroxypropyl cellulose, sodium lauryl sulfate, and magnesium stearate. The tablets are film-coated with a coating material containing indigo carmine (FD&C Blue #2) aluminum lake, polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide, and yellow iron oxide.
Elvitegravir: The chemical name of elvitegravir is 6-(3-Chloro-2-fluorobenzyl)-1-[(2S)-1-hydroxy-3-methylbutan-2-yl]-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid.
It has a molecular formula of C23H23ClFNO5 and a molecular weight of 447.9. It has the following structural formula:
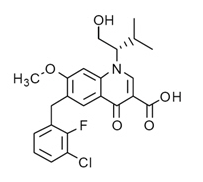
Elvitegravir is a white to pale yellow powder with a solubility of less than 0.3 micrograms per mL in water at 20 °C.
Cobicistat: The chemical name for cobicistat is 1,3-thiazol-5-ylmethyl [(2R,5R)-5-{[(2S)-2-[(methyl{[2-(propan-2-yl)-1,3-thiazol-4-yl]methyl}carbamoyl)amino]-4-(morpholin-4-yl)butanoyl]amino}-1,6-diphenylhexan-2-yl]carbamate. It has a molecular formula of C40H53N7O5S2 and a molecular weight of 776.0. It has the following structural formula:

Cobicistat is adsorbed onto silicon dioxide. Cobicistat on silicon dioxide is a white to pale yellow solid with a solubility of 0.1 mg per mL in water at 20 °C.
Emtricitabine: The chemical name of emtricitabine is 5-fluoro-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Emtricitabine is the (-)enantiomer of a thio analog of cytidine, which differs from other cytidine analogs in that it has a fluorine in the 5-position.
It has a molecular formula of C8H10FN3O3S and a molecular weight of 247.25. It has the following structural formula:
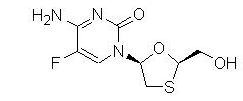
Emtricitabine is a white to off-white crystalline powder with a solubility of approximately 112 mg per mL in water at 25 °C.
Tenofovir Disoproxil Fumarate: Tenofovir DF is a fumaric acid salt of the bis-isopropoxycarbonyloxymethyl ester derivative of tenofovir. The chemical name of tenofovir DF is 9-[(R)-2-([bis([(isopropoxycarbonyl)oxy]-methoxy)phosphinyl]methoxy)propyl]adenine fumarate (1:1). It has a molecular formula of C19H30N5O10P ∙ C4H4O4 and a molecular weight of 635.51. It has the following structural formula:
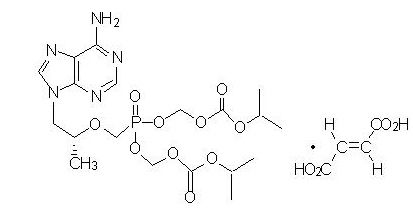
Tenofovir DF is a white to off-white crystalline powder with a solubility of 13.4 mg per mL in water at 25 °C. All dosages are expressed in terms of tenofovir DF except where otherwise noted. |PD======Effects on Electrocardiogram===== Thorough QT studies have been conducted for elvitegravir and cobicistat. The effect of the other two components, tenofovir and emtricitabine, or the combination regimen elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate on the QT interval is not known.
The effect of multiple doses of elvitegravir 125 and 250 mg (0.83 and 1.67 times the dose in elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate) (coadministered with 100 mg RTV to boost the blood levels of elvitegravir) on QTc interval was evaluated in a randomized, placebo- and active-controlled (moxifloxacin 400 mg) parallel group thorough QT study in 126 healthy subjects. In a study with demonstrated ability to detect small effects, the upper bound of the one-sided 95% confidence interval for the largest placebo adjusted, baseline-corrected QTc based on Fridericia's correction method (QTcF) was below 10 msec. In this study, there was no clinically relevant prolongation of the QTc interval.
The effect of a single dose of cobicistat 250 mg and 400 mg (1.67 and 2.67 times the dose in elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate) on QTc interval was evaluated in a randomized, placebo- and active-controlled (moxifloxacin 400 mg) four-period crossover thorough QT study in 48 healthy subjects. In a study with demonstrated ability to detect small effects, the upper bound of the one-sided 95% confidence interval for the largest placebo adjusted, baseline-corrected QTc based on individual correction method (QTc) was below 10 msec, the threshold for regulatory concern. Prolongation of the PR interval was noted in subjects receiving cobicistat in the same study. The maximum mean (95% upper confidence bound) difference in PR from placebo after baseline-correction was 9.5 (12.1) msec for 250 mg dose and 20.2 (22.8) for 400 mg dose cobicistat. Because the 150 mg cobicistat dose used in the elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate fixed-dose combination tablet is lower than the lowest dose studied in the thorough QT study, it is unlikely that treatment with elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate will result in clinically relevant PR prolongation. |PK======Absorption and Bioavailability===== Elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate: Following oral administration of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate with food in HIV-1 infected subjects, peak plasma concentrations were observed 4 hours post-dose for elvitegravir, 3 hours post-dose for cobicistat, 3 hours post-dose for emtricitabine, and 2 hours for tenofovir following the conversion of tenofovir DF (see Table 7 for additional pharmacokinetic parameters).

Effect of Food on Oral Absorption
Relative to fasting conditions, the administration of single dose elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate with a light meal (~373 kcal, 20% fat) increased the mean systemic exposure of elvitegravir and tenofovir by 34% and 24%, respectively. The alterations in mean systemic exposures of cobicistat and emtricitabine were not clinically significant.
Relative to fasting conditions, the administration of single dose elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate with a high fat meal (~ 800 kcal, 50% fat) increased the mean systemic exposure of elvitegravir and tenofovir by 87% and 23%, respectively. The alterations in mean systemic exposures of cobicistat and emtricitabine were not clinically significant.
elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate tablets should be taken with food.
Distribution
- Elvitegravir: Elvitegravir is 98–99% bound to human plasma proteins and binding is independent of drug concentration over the range of 1 ng per mL to 1.6 micrograms per mL. The mean blood-to-plasma ratio was 0.73.
- Cobicistat: Cobicistat is 97–98% bound to human plasma proteins and the mean blood-to-plasma ratio was approximately 0.5.
- Emtricitabine: In vitro binding of emtricitabine to human plasma proteins is less than 4% and is independent of drug concentration over the range of 0.02–200 micrograms per mL.
- Tenofovir Disoproxil Fumarate: In vitro binding of tenofovir to human plasma proteins is less than 0.7% and is independent of concentration over the range of 0.01–25 micrograms per mL.
Metabolism
- Elvitegravir: The majority of elvitegravir metabolism is mediated by CYP3A enzymes. Elvitegravir also undergoes glucuronidation via UGT1A1/3 enzymes.
- Cobicistat: Cobicistat is metabolized by CYP3A and to a minor extent by CYP2D6 enzymes and does not undergo glucuronidation.
- Emtricitabine and tenofovir are not significantly metabolized.
Elimination
Elvitegravir: The median terminal plasma half-life of elvitegravir following administration of STRIBILD is approximately 12.9 hours. After single dose administration of [14C] elvitegravir (coadministered with 100 mg RTV), 94.8% and 6.7% of the administered dose was excreted in feces and urine, respectively. Cobicistat: The median terminal plasma half-life of cobicistat following administration of STRIBILD is approximately 3.5 hours. With single dose administration of [14C] cobicistat after multiple dosing of cobicistat for six days, 86.2% and 8.2% of the administered dose was excreted in feces and urine, respectively. Emtricitabine and tenofovir are primarily excreted in the urine by a combination of glomerular filtration and active tubular secretion.
Special Populations
Patients with Renal Impairment
Elvitegravir and cobicistat: A study of the pharmacokinetics of cobicistat-boosted elvitegravir was performed in healthy subjects and subjects with severe renal impairment (estimated creatinine clearance less than 30 mL per min). No clinically relevant differences in elvitegravir or cobicistat pharmacokinetics were observed between healthy subjects and subjects with severe renal impairment. Emtricitabine and Tenofovir Disoproxil Fumarate: The pharmacokinetics of emtricitabine and tenofovir are altered in subjects with estimated creatinine clearance below 50 mL per min or with end stage renal disease requiring dialysis.
Patients with Hepatic Impairment
- Elvitegravir and cobicistat: A study of the pharmacokinetics of cobicistat-boosted elvitegravir was performed in healthy subjects and subjects with moderate hepatic impairment. No clinically relevant differences in elvitegravir or cobicistat pharmacokinetics were observed between subjects with moderate hepatic impairment (Child-Pugh Class B) and healthy subjects. No dosage adjustment of elvitegravir or cobicistat is necessary for patients with mild to moderate hepatic impairment. The effect of severe hepatic impairment (Child-Pugh Class C) on the pharmacokinetics of elvitegravir or cobicistat has not been studied.
- Emtricitabine: The pharmacokinetics of emtricitabine has not been studied in subjects with hepatic impairment; however, emtricitabine is not significantly metabolized by liver enzymes, so the impact of liver impairment should be limited.
- Tenofovir Disoproxil Fumarate: The pharmacokinetics of tenofovir following a 300 mg dose of VIREAD has been studied in healthy subjects with moderate to severe hepatic impairment. No clinically relevant differences in tenofovir pharmacokinetics were observed between subjects with hepatic impairment and healthy subjects.
Hepatitis B and/or Hepatitis C Virus Co-infection
- Elvitegravir: Limited data from population pharmacokinetic analysis (N=24) indicated that hepatitis B and/or C virus infection had no clinically relevant effect on the exposure of cobicistat-boosted elvitegravir.
- Cobicistat: There were insufficient pharmacokinetic data in the clinical trials to determine the effect of hepatitis B and/or C virus infection on the pharmacokinetics of cobicistat.
- Emtricitabine and Tenofovir: Pharmacokinetics of emtricitabine and tenofovir DF have not been fully evaluated in subjects coinfected with hepatitis B and/or C virus.
Race
- Elvitegravir: Population pharmacokinetic analysis of elvitegravir in HIV-1 infected subjects indicated that race had no clinically relevant effect on the exposure of cobicistat-boosted elvitegravir.
- Cobicistat: Population pharmacokinetics analysis of cobicistat in HIV-1 infected subjects indicated that race had no clinically relevant effect on the exposure of COBI.
- Emtricitabine: No pharmacokinetic differences due to race have been identified following the administration of emtricitabine.
- Tenofovir Disoproxil Fumarate: There were insufficient numbers from racial and ethnic groups other than Caucasian to adequately determine potential pharmacokinetic differences among these populations following the administration of tenofovir.
Gender
No clinically relevant pharmacokinetic differences have been observed between men and women for cobicistat-boosted elvitegravir, emtricitabine and tenofovir DF.
Pediatric Patients
Emtricitabine has been studied in pediatric subjects from 3 months to 17 years of age. Tenofovir DF has been studied in pediatric subjects from 2 years to less than 18 years of age. The pharmacokinetics of elvitegravir or cobicistat in pediatric subjects have not been established.
Geriatric Patients
Pharmacokinetics of elvitegravir, cobicistat, emtricitabine and tenofovir have not been fully evaluated in elderly (65 years of age and older) patients.
Assessment of Drug Interactions
The drug-drug interaction studies described in Tables 8 and 9 were conducted with elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate, elvitegravir (coadministered with cobicistat or RTV), or cobicistat administered alone.
As elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate is indicated for use as a complete regimen for the treatment of HIV-1 infection and should not be administered with other antiretroviral medications, information regarding drug-drug interactions with other antiretrovirals agents is not provided.
The effects of coadministered drugs on the exposure of elvitegravir are shown in Table 8. The effects of elvitegravir or cobicistat on the exposure of coadministered drugs are shown in Table 9. For information regarding clinical recommendations, see Drug Interactions (7).


|nonClinToxic=Elvitegravir: Long-term carcinogenicity studies of elvitegravir were carried out in mice (104 weeks) and in rats for up to 88 weeks (males) and 90 weeks (females). No drug-related increases in tumor incidence were found in mice at doses up to 2000 mg per kg per day alone or in combination with 25 mg per kg per day RTV at exposures 3- and 14-fold, respectively, the human systemic exposure at the recommended daily dose of 150 mg. No drug-related increases in tumor incidence were found in rats at doses up to 2000 mg per kg per day at exposures 12- to 27-fold, respectively in male and female, the human systemic exposure.
- Elvitegravir was not genotoxic in the reverse mutation bacterial test (Ames test) and the rat micronucleus assay. In an in vitro chromosomal aberration test, elvitegravir was negative with metabolic activation; however, an equivocal response was observed without activation.
- Elvitegravir did not affect fertility in male and female rats at approximately 16- and 30-fold higher exposures (AUC), respectively, than in humans at the therapeutic 150 mg daily dose.
- Fertility was normal in the offspring of rats exposed daily from before birth (in utero) through sexual maturity at daily exposures (AUC) of approximately 18-fold higher than human exposures at the recommended 150 mg daily dose.
Cobicistat: In a long-term carcinogenicity study in mice, no drug-related increases in tumor incidence were observed at doses up to 50 and 100 mg/kg/day (males and females, respectively). Cobicistat exposures at these doses were approximately 7 (male) and 16 (females) times, respectively, the human systemic exposure at the therapeutic daily dose. In a long-term carcinogenicity study of cobicistat in rats, an increased incidence of follicular cell adenomas and/or carcinomas in the thyroid gland was observed at doses of 25 and 50 mg/kg/day in males, and at 30 mg/kg/day in females. The follicular cell findings are considered to be rat-specific, secondary to hepatic microsomal enzyme induction and thyroid hormone imbalance, and are not relevant for humans. At the highest doses tested in the rat carcinogenicity study, systemic exposures were approximately 2 times the human systemic exposure at the therapeutic daily dose.
- Cobicistat was not genotoxic in the reverse mutation bacterial test (Ames test), mouse lymphoma or rat micronucleus assays.
- Cobicistat did not affect fertility in male or female rats at daily exposures (AUC) approximately 4-fold higher than human exposures at the recommended 150 mg daily dose.
- Fertility was normal in the offspring of rats exposed daily from before birth (in utero) through sexual maturity at daily exposures (AUC) of approximately 1.2-fold higher than human exposures at the recommended 150 mg daily dose.
Emtricitabine: In long-term carcinogenicity studies of emtricitabine, no drug-related increases in tumor incidence were found in mice at doses up to 750 mg per kg per day (23 times the human systemic exposure at the therapeutic dose of 200 mg per day) or in rats at doses up to 600 mg per kg per day (28 times the human systemic exposure at the therapeutic dose).
- Emtricitabine was not genotoxic in the reverse mutation bacterial test (Ames test), mouse lymphoma or mouse micronucleus assays.
- Emtricitabine did not affect fertility in male rats at approximately 140-fold or in male and female mice at approximately 60-fold higher exposures (AUC) than in humans given the recommended 200 mg daily dose. Fertility was normal in the offspring of mice exposed daily from before birth (in utero) through sexual maturity at daily exposures (AUC) of approximately 60-fold higher than human exposures at the recommended 200 mg daily dose.
Tenofovir Disoproxil Fumarate: Long-term oral carcinogenicity studies of tenofovir DF in mice and rats were carried out at exposures up to approximately 10 times (mice) and 4 times (rats) those observed in humans at the therapeutic dose for HIV-1 infection. At the high dose in female mice, liver adenomas were increased at exposures 10 times of that in humans. In rats, the study was negative for carcinogenic findings at exposures up to 4 times that observed in humans at the therapeutic dose.
- Tenofovir DF was mutagenic in the in vitro mouse lymphoma assay and negative in an in vitro bacterial mutagenicity test (Ames test). In an in vivo mouse micronucleus assay, tenofovir DF was negative when administered to male mice.
- There were no effects on fertility, mating performance or early embryonic development when tenofovir DF was administered to male rats at a dose equivalent to 10 times the human dose based on body surface area comparisons for 28 days prior to mating and to female rats for 15 days prior to mating through day seven of gestation. There was, however, an alteration of the estrous cycle in female rats.
|clinicalStudies=The efficacy of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate is based on the analyses of 96-week data from two randomized, double-blind, active-controlled trials, Study 102 and Study 103, in treatment-naïve, HIV-1 infected subjects (N=1408, randomized and dosed) with baseline estimated creatinine clearance above 70 mL per min.
In Study 102, subjects were randomized in a 1:1 ratio to receive either elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate (N=348) once daily or ATRIPLA (efavirenz 600 mg/emtricitabine 200 mg/tenofovir DF 300 mg; N=352) once daily. The mean age was 38 years (range 18–67), 89% were male, 63% were White, 28% were Black, and 2% were Asian. Twenty-four percent of subjects identified as Hispanic/Latino. The mean baseline plasma HIV-1 RNA was 4.8 log10 copies per mL (range 2.6–6.5). The mean baseline CD4+ cell count was 386 cells per mm3 (range 3–1348) and 13% had CD4+ cell counts less than 200 cells per mm3. Thirty-three percent of subjects had baseline viral loads greater than 100,000 copies per mL.
In Study 103, subjects were randomized in a 1:1 ratio to receive either elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate (N=353) once daily or ATV 300 mg + RTV 100 mg + TRUVADA (emtricitabine 200 mg/tenofovir DF 300 mg) (N=355) once daily. The mean age was 38 years (range 19–72), 90% were male, 74% were White, 17% were Black, and 5% were Asian. Sixteen percent of subjects identified as Hispanic/Latino. The mean baseline plasma HIV-1 RNA was 4.8 log10 copies per mL (range 1.7–6.6). The mean baseline CD4+ cell count was 370 cells per mm3 (range 5–1132) and 13% had CD4+ cell count less than 200 cells per mm3. Forty-one percent of subjects had baseline viral loads greater than 100,000 copies per mL.
In both studies, subjects were stratified by baseline HIV-1 RNA (less than or equal to 100,000 copies per mL or greater than 100,000 copies per mL).
Treatment outcomes of Study 102 and Study 103 through 96 weeks are presented in Table 10.

In Study 102, the mean increase from baseline in CD4+ cell count at Week 96 was 278 cells per mm3 in the elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate-treated subjects and 247 cells per mm3 in the ATRIPLA -treated subjects. In Study 103, the mean increase from baseline in CD4+ cell count at Week 96 was 242 cells per mm3 in the elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate-treated subjects and 240 cells per mm3 in the ATV + RTV + tenofovir disoproxil fumarate-treated subjects. |howSupplied=STRIBILD tablets are green, capsule-shaped, film-coated, debossed with "GSI" on one side and the number "1" surrounded by a square box (figure) on the other side. Each bottle contains 30 tablets (NDC 61958-1201-1), a silica gel desiccant and closed with a child-resistant closure. |storage=Store at 25 °C (77 °F), excursions permitted to 15–30 °C (59–86 °F) (see USP Controlled Room Temperature).
- Keep container tightly closed.
- Dispense only in original container.
- Do not use if seal over bottle opening is broken or missing.
|fdaPatientInfo=A statement to patients and healthcare providers is included on the product's bottle label: ALERT: Find out about medicines that should NOT be taken with elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate. Patients should be advised that:
- Elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate may interact with many drugs; therefore, patients should be advised to report to their healthcare provider the use of any other prescription or non-prescription medication or herbal products including St. John's wort.
- Patients should remain under the care of a healthcare provider when using elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate.
- Patients should be informed that elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate is not a cure for HIV-1 infection. Patients should stay on continuous HIV therapy to control HIV-1 infection and decrease HIV-related illnesses. Patients should be told that sustained decreases in plasma HIV RNA have been associated with a reduced risk of progression to AIDS and death.
- Patients should avoid doing things that can spread HIV-1 infection to others.
- Do not share needles or other injection equipment.
- Do not share personal items that can have blood or body fluids on them, like toothbrushes and razor blades.
- Do not have any kind of sex without protection. Always practice safer sex by using a latex or polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or blood.
- Do not breastfeed. At least two of the drugs contained in elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate can be passed to the baby in breast milk. It is not known whether this could harm the baby. Also, mothers with HIV-1 should not breastfeed because HIV-1 can be passed to the baby in breast milk.
- It is important to take elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate on a regular dosing schedule with food and to avoid missing doses.
- Do not miss a dose of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate. If a patient misses a dose of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate, the patient should take the missed dose as soon as they remember. If it is almost time for the next dose of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate, the patient should not take the missed dose, but resume the usual dosing schedule. Inform the patient that he or she should not take more or less than the prescribed dose of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate at any one time.
- Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported. Advise patients that treatment with elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate should be suspended if they develop clinical symptoms suggestive of lactic acidosis or pronounced hepatotoxicity (including nausea, vomiting, unusual or unexpected stomach discomfort, and weakness).
- Instruct the patient that hepatitis B testing is recommended prior to initiating antiretroviral therapy. Severe acute exacerbations of hepatitis B have been reported in patients who are coinfected with HBV and HIV-1 and have discontinued emtricitabine, or tenofovir disoproxil fumarate. Elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate should not be discontinued without first informing their healthcare provider.
- Renal impairment, including cases of acute renal failure and Fanconi syndrome, has been reported in association with the use of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate. Elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate should be avoided with concurrent or recent use of a nephrotoxic agent (e.g., high dose or multiple NSAIDs).
- Elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate should not be coadministered with other antiretroviral products because it provides a complete treatment regimen and because of potential drug interactions .
- Elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate should not be administered in combination with ATRIPLA, COMPLERA, EMTRIVA, TRUVADA, or VIREAD; with drugs containing lamivudine, including COMBIVIR, EPIVIR or EPIVIR-HBV, EPZICOM, or TRIZIVIR; with drugs containing RTV or regimens containing RTV; or with HEPSERA.
- Decreases in bone mineral density have been observed with the use of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate. Assessment of bone mineral density (BMD) should be considered in patients who have a history of pathologic bone fracture or other risk factors for osteoporosis or bone loss.
- Redistribution or accumulation of body fat may occur in patients receiving antiretroviral therapy and that the cause and long-term health effects of these conditions are not known.
- In some patients with advanced HIV infection (AIDS), signs and symptoms of inflammation from previous infections may occur soon after anti-HIV treatment is started. It is believed that these symptoms are due to an improvement in the body's immune response, enabling the body to fight infections that may have been present with no obvious symptoms. Patients should be advised to inform their healthcare provider immediately of any symptoms of infection.
|alcohol=Alcohol-elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. |brandNames=Stribild }} {{#subobject:
|Label Page=Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate |Label Name=Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate Package.png
}}