Certolizumab pegol: Difference between revisions
No edit summary |
No edit summary |
||
| (9 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag= | |authorTag={{VP}}; {{Ammu}} | ||
|genericName=certolizumab pegol | |||
{{VP}} | |aOrAn=an | ||
|drugClass=[[immunosupressant]] | |||
|indicationType=treatment | |||
|indication=[[crohn's disease]], [[rheumatoid arthritis]], [[psoriatic arthritis]] and [[ankylosing spondylitis]] | |||
|genericName= | |hasBlackBoxWarning=Yes | ||
|adverseReactions=[[arthralgia]] ,[[urinary tract infections]] and [[upper respiratory infection]] | |||
|blackBoxWarningTitle=WARNING: SERIOUS INFECTIONS AND MALIGNANCY | |||
|blackBoxWarningBody=SERIOUS INFECTIONS | |||
|aOrAn= | * Patients treated with certolizumab are at increased risk for developing serious infections that may lead to hospitalization or death. Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids. | ||
* Certolizumab should be discontinued if a patient develops a serious infection or sepsis. | |||
* Reported infections include: | |||
:* Active tuberculosis, including reactivation of latent tuberculosis. Patients with tuberculosis have frequently presented with disseminated or extrapulmonary disease. :* Patients should be tested for latent tuberculosis before certolizumab use and during therapy. Treatment for latent infection should be initiated prior to certolizumab use. | |||
|drugClass= | :* Invasive fungal infections, including histoplasmosis, coccidioidomycosis, candidiasis, aspergillosis, blastomycosis, and pneumocystosis. Patients with histoplasmosis or other invasive fungal infections may present with disseminated, rather than localized disease. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. Empiric anti-fungal therapy should be considered in patients at risk for invasive fungal infections who develop severe systemic illness. | ||
:* Bacterial, viral and other infections due to opportunistic pathogens, including Legionella and Listeria. | |||
[[ | :* The risks and benefits of treatment with certolizumab should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection. | ||
* Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with certolizumab, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy. | |||
|indication= | MALIGNANCY | ||
* Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF blockers, of which certolizumab is a member. Certolizumab is not indicated for use in pediatric patients. | |||
[[crohn's disease]], [[rheumatoid arthritis]], [[psoriatic arthritis]] | |fdaLIADAdult=====Indications==== | ||
=====Crohn's Disease===== | |||
|hasBlackBoxWarning= | * Certolizumab is indicated for reducing signs and symptoms of [[Crohn's disease]] and maintaining clinical response in adult patients with moderately to severely active disease who have had an inadequate response to conventional therapy. | ||
=====Rheumatoid Arthritis===== | |||
Yes | * Certolizumab is indicated for the treatment of adults with moderately to severely active [[rheumatoid arthritis]] (RA). | ||
=====Psoriatic Arthritis===== | |||
|adverseReactions= | * Certolizumab is indicated for the treatment of adult patients with active [[psoriatic arthritis]] (PsA). | ||
=====Ankylosing Spondylitis===== | |||
[[ | * Certolizumab is indicated for the treatment of adults with active [[ankylosing spondylitis]] (AS). | ||
=====Storage===== | |||
* Certolizumab is administered by subcutaneous injection. Injection sites should be rotated and injections should not be given into areas where the [[skin]] is tender, bruised, red or hard. When a 400 mg dose is needed (given as two subcutaneous injections of 200 mg), injections should occur at separate sites in the [[thigh]] or [[abdomen]]. | |||
* The solution should be carefully inspected visually for particulate matter and discoloration prior to administration. The solution should be a clear colorless to yellow liquid, essentially free from particulates and should not be used if cloudy or if foreign particulate matter is present. Certolizumab does not contain preservatives; therefore, unused portions of drug remaining in the syringe or vial should be discarded. | |||
|blackBoxWarningTitle= | |||
WARNING | |||
|blackBoxWarningBody= | |||
* | |||
* | |||
*Reported infections include: | |||
:*Active tuberculosis, including reactivation of latent tuberculosis. Patients with tuberculosis have frequently presented with disseminated or extrapulmonary disease. Patients should be tested for latent tuberculosis before | |||
:*Invasive fungal infections, including histoplasmosis, coccidioidomycosis, candidiasis, aspergillosis, blastomycosis, and pneumocystosis. Patients with histoplasmosis or other invasive fungal infections may present with disseminated, rather than localized disease. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. Empiric anti-fungal therapy should be considered in patients at risk for invasive fungal infections who develop severe systemic illness. | |||
:*Bacterial, viral and other infections due to opportunistic pathogens, including Legionella and Listeria. | |||
*The risks and benefits of treatment with | |||
*Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with | |||
=====Crohn's Disease===== | =====Crohn's Disease===== | ||
* The recommended initial adult dose of certolizumab is 400 mg (given as two [[subcutaneous]] injections of 200 mg) initially, and at Weeks 2 and 4. In patients who obtain a clinical response, the recommended maintenance regimen is 400 mg every four weeks. | |||
*The recommended initial adult dose of | |||
=====Rheumatoid Arthritis===== | =====Rheumatoid Arthritis===== | ||
* The recommended dose of certolizumab for adult patients with [[rheumatoid arthritis]] is 400 mg (given as two subcutaneous injections of 200 mg) initially and at Weeks 2 and 4, followed by 200 mg every other week. For maintenance dosing, certolizumab 400 mg every 4 weeks can be considered. | |||
*The recommended dose of | |||
=====Psoriatic Arthritis===== | =====Psoriatic Arthritis===== | ||
* The recommended dose of certolizumab for adult patients with [[psoriatic arthritis]] is 400 mg (given as 2 subcutaneous injections of 200 mg each) initially and at week 2 and 4, followed by 200 mg every other week. For maintenance dosing, certolizumab 400 mg every 4 weeks can be considered. | |||
*The recommended dose of | |||
=====Ankylosing Spondylitis===== | =====Ankylosing Spondylitis===== | ||
* The recommended dose of certolizumab for adult patients with [[ankylosing spondylitis]] is 400 mg (given as 2 [[subcutaneous]] injections of 200 mg each) initially and at weeks 2 and 4, followed by 200 mg every 2 weeks or 400 mg every 4 weeks. | |||
*The recommended dose of | =====Preparation and Administration of Certolizumab Using the Lyophilized Powder for Injection===== | ||
* Certolizumab Lyophilized powder should be prepared and administered by a health care professional. certolizumab is provided in a package that contains everything required to reconstitute and inject the drug. Step-by-step preparation and administration instructions are provided below. | |||
=====Preparation and Storage===== | |||
* Certolizumab should be brought to room temperature before reconstituting. | |||
* Use appropriate aseptic technique when preparing and administering certolizumab. | |||
Reconstitute the vial(s) of certolizumab with 1 mL of Sterile Water for Injection, USP using the 20-gauge needle provided. | |||
* Gently swirl each vial of certolizumab without shaking, assuring that all of the powder comes in contact with the Sterile Water for Injection. | |||
* Leave the vial(s) undisturbed to fully reconstitute, which may take approximately 30 minutes. | |||
===== | * The final reconstituted solution contains 200 mg/mL and should be clear to opalescent, colorless to pale yellow liquid essentially free from particulates. | ||
Once reconstituted, certolizumab can be stored in the vials for up to 24 hours between 2° to 8° C (36° to 46° F) prior to injection. Do not freeze. | |||
* | =====Administration===== | ||
* Prior to injecting, reconstituted certolizumab should be at room temperature but do not leave reconstituted certolizumab at room temperature for more than two hours prior to administration. | |||
* | * Withdraw the reconstituted solution into a separate syringe for each vial using a new 20-gauge needle for each vial so that each syringe contains 1 mL of certolizumab (200 mg of certolizumab pegol). | ||
* Replace the 20-gauge needle(s) on the syringes with a 23-gauge(s) for administration. | |||
* | * Inject the full contents of the syringe(s) subcutaneously into [[thigh]] or [[abdomen]]. Where a 400 mg dose is required, two injections are required, therefore, separate sites should be used for each 200 mg injection. | ||
=====Preparation and Administration of Certolizumab Using the Prefilled Syringe===== | |||
* | * After proper training in subcutaneous injection technique, a patient may self-inject with the certolizumab Prefilled Syringe if a physician determines that it is appropriate. | ||
* Patients using the Certolizumab Prefilled Syringe should be instructed to inject the full amount in the syringe (1 mL), according to the directions provided in the Instructions for Use booklet. | |||
=====Monitoring to Assess Safety===== | |||
* Before initiation of therapy with certolizumab, all patients must be evaluated for both active and inactive (latent) [[tuberculosis]] infection. The possibility of undetected [[latent tuberculosis]] should be considered in patients who have immigrated from or traveled to countries with a high prevalence of [[tuberculosis]] or had close contact with a person with active [[tuberculosis]]. Appropriate screening tests (e.g. tuberculin skin test and chest x-ray) should be performed in all patients. | |||
==== | =====Concomitant Medications===== | ||
* Certolizumab may be used as monotherapy or concomitantly with non-biological disease modifying [[anti-rheumatic drugs]] (DMARDs). | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | * The use of certolizumab in combination with biological DMARDs or other [[tumor necrosis factor]] (TNF) blocker therapy is not recommended. | ||
====Dosage==== | |||
* For Injection: Lyophilized Powder for Reconstitution | |||
* Sterile, white, lyophilized powder for reconstitution and then subcutaneous administration. Each single-use vial provides approximately 200 mg of certolizumab pegolA. | |||
* Injection: Prefilled Syringe | |||
* A single-use, 1 mL prefilled glass syringe with a fixed 25 gauge ½ inch thin wall needle, providing 200 mg per 1 mL of certolizumab pegol. | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | <!--Non–Guideline-Supported Use (Adult)--> | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
|offLabelAdultNoGuideSupport= | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Pediatric Indications and Dosage--> | <!--Pediatric Indications and Dosage--> | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed=There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
|fdaLIADPed= | |||
There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | <!--Off-Label Use and Dosage (Pediatric)--> | ||
<!--Guideline-Supported Use (Pediatric)--> | <!--Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
|offLabelPedGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | <!--Non–Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
|offLabelPedNoGuideSupport= | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | <!--Contraindications--> | ||
|warnings======Risk of Serious Infections===== | |||
* Patients treated with certolizumab are at an increased risk for developing serious infections involving various [[organ]] systems and sites that may lead to hospitalization or death. | |||
* Opportunistic infections due to [[bacterial]], [[mycobacterial]], invasive fungal, viral, parasitic, or other opportunistic pathogens including [[aspergillosis]], [[blastomycosis]], [[candidiasis]], [[coccidioidomycosis]], [[histoplasmosis]], [[legionellosis]], [[listeriosis]], [[pneumocystosis]] and [[tuberculosis]] have been reported with [[TNF blockers]]. Patients have frequently presented with disseminated rather than localized [[disease]]. | |||
* Treatment with certolizumab should not be initiated in patients with an [[active infection]], including clinically important [[localized infections]]. Patients greater than 65 years of age, patients with co-morbid conditions, and/or patients taking concomitant immunosuppressants (e.g. [[corticosteroids]] or [[methotrexate]]) may be at a greater risk of infection. The risks and benefits of treatment should be considered prior to initiating therapy in patients: | |||
:* With chronic or recurrent [[infection]] | |||
:* Who have been exposed to [[tuberculosis]] | |||
:* With a history of an [[opportunistic infection]] | |||
|warnings | :* Who have resided or traveled in areas of endemic [[tuberculosis]] or endemic [[mycoses]], such as [[histoplasmosis]], [[coccidioidomycosis]], or [[blastomycosis]] | ||
:* With underlying conditions that may predispose them to [[infection]]. | |||
=====Tuberculosis===== | |||
* Cases of reactivation of tuberculosis or new [[tuberculosis infections]] have been observed in patients receiving certolizumab, including patients who have previously received treatment for latent or active [[tuberculosis]]. Patients should be evaluated for [[tuberculosis]] risk factors and tested for [[latent infection]] prior to initiating certolizumab and periodically during therapy. | |||
* Treatment of [[latent tuberculosis infection]] prior to therapy with TNF-blocking agents has been shown to reduce the risk of [[tuberculosis reactivation]] during therapy. Induration of 5 mm or greater with [[tuberculin skin testing]] should be considered a positive test result when assessing if treatment for [[latent tuberculosis]] is needed prior to initiating certolizumab, even for patients previously vaccinated with [[Bacille Calmette-Guerin]] (BCG). | |||
* [[Anti-tuberculosis therapy]] should also be considered prior to initiation of certolizumab in patients with a past history of latent or [[active tuberculosis]] in whom an adequate course of treatment cannot be confirmed, and for patients with a negative test for [[latent tuberculosis]] but having risk factors for [[tuberculosis infection]]. Consultation with a physician with expertise in the treatment of tuberculosis is recommended to aid in the decision of whether initiating anti-tuberculosis therapy is appropriate for an individual patient. | |||
* Tuberculosis should be strongly considered in patients who develop a new infection during certolizumab treatment, especially in patients who have previously or recently traveled to countries with a high prevalence of [[tuberculosis]], or who have had close contact with a person with [[active tuberculosis]]. | |||
=====Monitoring===== | |||
* Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with certolizumab, including the development of [[tuberculosis]] in patients who tested negative for [[latent tuberculosis infection]] prior to initiating therapy. Tests for [[latent tuberculosis]] infection may also be falsely negative while on therapy with certolizumab. | |||
:* | * certolizumab should be discontinued if a patient develops a serious infection or [[sepsis]]. | ||
:* | * A patient who develops a new infection during treatment with certolizumab should be closely monitored, undergo a prompt and complete diagnostic workup appropriate for an immunocompromised patient, and appropriate antimicrobial therapy should be initiated. | ||
:* | =====Invasive Fungal Infections===== | ||
:* | * For patients who reside or travel in regions where [[mycoses]] are endemic, invasive fungal infection should be suspected if they develop a serious systemic illness. Appropriate empiric antifungal therapy should be considered while a diagnostic workup is being performed. Antigen and antibody testing for [[histoplasmosis]] may be negative in some patients with active [[infection]]. When feasible, the decision to administer empiric antifungal therapy in these patients should be made in consultation with a physician with expertise in the diagnosis and treatment of invasive fungal infections and should take into account both the risk for severe fungal infection and risks of [[antifungal therapy]]. | ||
:* | =====Malignancies===== | ||
* In the controlled portions of clinical studies of some [[TNF blockers]], more cases of [[malignancies]] have been observed among patients receiving TNF blockers compared to control patients. During controlled and open-labeled portions of certolizumab studies of [[Crohn's disease]] and other diseases, [[malignancies]] (excluding non-melanoma [[skin cancer]]) were observed at a rate (95% confidence interval) of 0.5 (0.4, 0.7) per 100 patient-years among 4,650 certolizumab-treated patients versus a rate of 0.6 (0.1, 1.7) per 100 patient-years among 1,319 placebo-treated patients. The size of the control group and limited duration of the controlled portions of the studies precludes the ability to draw firm conclusions. | |||
* Malignancies, some fatal, have been reported among children, adolescents, and young adults who received treatment with TNF-blocking agents (initiation of therapy ≤ 18 years of age), of which certolizumab is a member. Approximately half the cases were lymphomas, including [[Hodgkin's]] and [[non-Hodgkin's lymphoma]]. The other cases represented a variety of different malignancies and included rare malignancies usually associated with [[immunosuppression]] and [[malignancies]] that are not usually observed in children and adolescents. The malignancies occurred after a median of 30 months of therapy (range 1 to 84 months). Most of the patients were receiving concomitant immunosuppressants. These cases were reported post-marketing and are derived from a variety of sources including registries and spontaneous post-marketing reports. Certolizumab is not indicated for use in pediatric patients. | |||
* In the controlled portions of clinical trials of all the [[TNF blockers]], more cases of [[lymphoma]] have been observed among patients receiving [[TNF blockers]] compared to control patients. In controlled studies of certolizumab for [[Crohn's disease]] and other investigational uses, there was one case of [[lymphoma]] among 2,657 certolizumab-treated patients and one case of [[Hodgkin's lymphoma]] among 1,319 placebo-treated patients. | |||
* In the certolizumab RA clinical trials (placebo-controlled and open label) a total of three cases of [[lymphoma]] were observed among 2,367 patients. This is approximately 2-fold higher than expected in the general population. Patients with RA, particularly those with highly active disease, are at a higher risk for the development of lymphoma. | |||
* Rates in clinical studies for certolizumab cannot be compared to the rates of clinical trials of other [[TNF blockers]] and may not predict the rates observed when certolizumab is used in a broader patient population. Patients with [[Crohn's disease]] that require chronic exposure to [[immunosuppressant]] therapies may be at higher risk than the general population for the development of [[lymphoma]], even in the absence of [[TNF blocker]] therapy. The potential role of [[TNF blocker]] therapy in the development of [[malignancies]] in adults is not known. | |||
* Postmarketing cases of hepatosplenic [[T-cell lymphoma]] (HSTCL), a rare type of [[T-cell lymphoma]] that has a very aggressive disease course and is usually fatal, have been reported in patients treated with TNF blockers, including certolizumab. The majority of reported TNF blocker cases occurred in adolescent and young adult males with [[Crohn's disease]] or [[ulcerative colitis]]. Almost all of these patients had received treatment with the [[immunosuppressants]] [[azathioprine]] and/or [[6-mercaptopurine]] (6-MP) concomitantly with a [[TNF blocker]] at or prior to diagnosis. It is uncertain whether the occurrence of HSTCL is related to use of a TNF blocker or a TNF blocker in combination with these other [[immunosuppressants]]. The potential risk of using a TNF blocker in combination with [[azathioprine]] or [[6-MP]] should be carefully considered. | |||
* Cases of acute and chronic [[leukemia]] have been reported in association with post-marketing TNF-blocker use in RA and other indications. Even in the absence of TNF-blocker therapy, patients with RA may be at a higher risk (approximately 2-fold) than the general population for the development of [[leukemia]]. | |||
* Periodic skin examinations are recommended for all patients, particularly those with risk factors for [[skin cancer]]. | |||
=====Heart Failure===== | |||
* Cases of worsening [[congestive heart failure]] (CHF) and new onset CHF have been reported with [[TNF blockers]], including certolizumab. Certolizumab has not been formally studied in patients with CHF; however, in clinical studies in patients with CHF with another [[TNF blocker]], worsening [[congestive heart failure]] (CHF) and increased mortality due to CHF were observed. Exercise caution in patients with [[heart failure]] and monitor them carefully. | |||
=====Hypersensitivity Reactions===== | |||
* The following symptoms that could be compatible with [[hypersensitivity reactions]] have been reported rarely following certolizumab administration to patients: [[angioedema]], [[dyspnea]], [[hypotension]], [[rash]], [[serum sickness]], and [[urticaria]]. Some of these reactions occurred after the first administration of certolizumab. If such reactions occur, discontinue further administration of certolizumab and institute appropriate therapy. There are no data on the risks of using certolizumab in patients who have experienced a severe [[hypersensitivity reaction]] towards another [[TNF blocker]]; in these patients caution is needed. | |||
=====Hepatitis B Virus Reactivation===== | |||
* Use of [[TNF blockers]], including certolizumab, has been associated with reactivation of [[hepatitis B virus]] (HBV) in patients who are chronic carriers of this virus. In some instances, HBV reactivation occurring in conjunction with [[TNF blocker]] therapy has been fatal. The majority of reports have occurred in patients concomitantly receiving other medications that suppress the immune system, which may also contribute to HBV reactivation. | |||
* Test patients for [[HBV]] infection before initiating treatment with certolizumab. For patients who test positive for [[HBV]] infection, consultation with a physician with expertise in the treatment of [[hepatitis B]] is recommended. Adequate data are not available on the safety or efficacy of treating patients who are carriers of HBV with anti-viral therapy in conjunction with [[TNF blocker therapy]] to prevent HBV reactivation. Patients who are carriers of HBV and require treatment with certolizumab should be closely monitored for clinical and laboratory signs of active HBV infection throughout therapy and for several months following termination of therapy. | |||
* In patients who develop [[HBV reactivation]], discontinue certolizumab and initiate effective anti-viral therapy with appropriate supportive treatment. The safety of resuming [[TNF blocker]] therapy after HBV reactivation is controlled is not known. Therefore, exercise caution when considering resumption of certolizumab therapy in this situation and monitor patients closely. | |||
=====Neurologic Reactions===== | |||
* Use of [[TNF blockers]], of which certolizumab is a member, has been associated with rare cases of new onset or exacerbation of clinical symptoms and/or radiographic evidence of [[central nervous system]] demyelinating disease, including [[multiple sclerosis]], and with [[peripheral demyelinating disease]], including [[Guillain-Barré syndrome]] . Exercise caution in considering the use of certolizumab in patients with pre-existing or recent-onset central or [[peripheral nervous system]] demyelinating disorders. Rare cases of neurological disorders, including [[seizure disorder]], [[optic neuritis]], and [[peripheral neuropathy]] have been reported in patients treated with certolizumab. | |||
=====Hematological Reactions===== | |||
* Rare reports of [[pancytopenia]], including [[aplastic anemia]], have been reported with TNF blockers. Adverse reactions of the hematologic system, including medically significant [[cytopenia]] (e.g., [[leukopenia]], [[pancytopenia]], [[thrombocytopenia]]) have been infrequently reported with certolizumab. The causal relationship of these events to certolizumab remains unclear. | |||
* Although no high risk group has been identified, exercise caution in patients being treated with certolizumab who have ongoing, or a history of, significant hematologic abnormalities. Advise all patients to seek immediate medical attention if they develop signs and symptoms suggestive of [[blood dyscrasias]] or infection (e.g., persistent [[fever]], [[bruising]], [[bleeding]], [[pallor]]) while on certolizumab. Consider discontinuation of certolizumab therapy in patients with confirmed significant hematologic abnormalities. | |||
=====Use with Biological Disease-Modifying Antirheumatic Drugs (Biological DMARDs)===== | |||
* Serious infections were seen in clinical studies with concurrent use of [[anakinra]] (an [[interleukin-1 antagonist]]) and another [[TNF blocker]], [[etanercept]], with no added benefit compared to etanercept alone. A higher risk of serious infections was also observed in combination use of [[TNF blockers]] with [[abatacept]] and [[rituximab]]. Because of the nature of the adverse events seen with this combination therapy, similar toxicities may also result from the use of certolizumab in this combination. Therefore, the use of certolizumab in combination with other biological DMARDs is not recommended. | |||
=====Autoimmunity===== | |||
* Treatment with certolizumab may result in the formation of [[autoantibodies]] and rarely, in the development of a [[lupus]]-like syndrome. If a patient develops symptoms suggestive of a lupus-like syndrome following treatment with certolizumab, discontinue treatment. | |||
=====Immunizations===== | |||
* Patients treated with certolizumab may receive vaccinations, except for live or live attenuated vaccines. No data are available on the response to live vaccinations or the secondary transmission of infection by live vaccines in patients receiving certolizumab. | |||
* In a placebo-controlled clinical trial of patients with [[rheumatoid arthritis]], no difference was detected in antibody response to vaccine between certolizumab and placebo treatment groups when the [[pneumococcal polysaccharide vaccine]] and [[influenza vaccine]] were administered concurrently with certolizumab. Similar proportions of patients developed protective levels of [[anti-vaccine antibodies]] between certolizumab and placebo treatment groups; however patients receiving certolizumab and concomitant methotrexate had a lower humoral response compared with patients receiving certolizumab alone. The clinical significance of this is unknown. | |||
=====Immunosuppression===== | |||
* Since TNF mediates inflammation and modulates cellular immune responses, the possibility exists for [[TNF blockers]], including certolizumab, to affect host defenses against infections and malignancies. The impact of treatment with certolizumab on the development and course of malignancies, as well as active and/or chronic infections, is not fully understood. The safety and efficacy of certolizumab in patients with immunosuppression has not been formally evaluated. | |||
|clinicalTrials======Clinical Trials Experience===== | |||
* The most serious adverse reactions were: | |||
:* Serious [[Infections]] | |||
:* [[Malignancies]] | |||
:* [[Heart Failure]] | |||
* Because clinical studies are conducted under widely varying and controlled conditions, adverse reaction rates observed in clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug, and may not predict the rates observed in a broader patient population in clinical practice. | |||
* In premarketing controlled trials of all patient populations combined the most common adverse reactions (≥ 8%) were [[upper respiratory infections]] (18%), [[rash]] (9%) and [[urinary tract infections]] (8%). | |||
* Adverse Reactions Most Commonly Leading to Discontinuation of Treatment in Premarketing Controlled Trials | |||
* The proportion of patients with [[Crohn's disease]] who discontinued treatment due to adverse reactions in the controlled clinical studies was 8% for certolizumab and 7% for placebo. The most common adverse reactions leading to the discontinuation of certolizumab (for at least 2 patients and with a higher incidence than placebo) were [[abdominal pain]] (0.4% certolizumab, 0.2% placebo), [[diarrhea]] (0.4% certolizumab, 0% placebo), and [[intestinal obstruction]] (0.4% certolizumab, 0% placebo). | |||
* The proportion of patients with [[rheumatoid arthritis]] who discontinued treatment due to adverse reactions in the controlled clinical studies was 5% for certolizumab and 2.5% for placebo. The most common adverse reactions leading to discontinuation of certolizumab were [[tuberculosis infections]] (0.5%); and [[pyrexia]], [[urticaria]], [[pneumonia]], and [[rash]] (0.3%). | |||
|clinicalTrials= | |||
*Because clinical studies are conducted under widely varying and controlled conditions, adverse reaction rates observed in clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug, and may not predict the rates observed in a broader patient population in clinical practice. | |||
*In premarketing controlled trials of all patient populations combined the most common adverse reactions (≥ 8%) were upper | |||
*The proportion of patients with Crohn's disease who discontinued treatment due to adverse reactions in the controlled clinical studies was 8% for | |||
*The proportion of patients with rheumatoid arthritis who discontinued treatment due to adverse reactions in the controlled clinical studies was 5% for | |||
=====Controlled Studies with Crohn's Disease===== | =====Controlled Studies with Crohn's Disease===== | ||
* The data described below reflect exposure to certolizumab at 400 mg subcutaneous dosing in studies of patients with [[Crohn's disease]]. In the safety population in controlled studies, a total of 620 patients with [[Crohn's disease]] received certolizumab at a dose of 400 mg, and 614 subjects received placebo (including subjects randomized to placebo in Study CD2 following open label dosing of certolizumab at Weeks 0, 2, 4). In controlled and uncontrolled studies, 1,564 patients received certolizumab at some dose level, of whom 1,350 patients received 400 mg certolizumab. Approximately 55% of subjects were female, 45% were male, and 94% were Caucasian. The majority of patients in the active group were between the ages of 18 and 64. | |||
*The data described below reflect exposure to | * During controlled clinical studies, the proportion of patients with serious adverse reactions was 10% for certolizumab and 9% for placebo. The most common adverse reactions (occurring in ≥ 5% of certolizumab-treated patients, and with a higher incidence compared to placebo) in controlled clinical studies with certolizumab were upper respiratory infections (e.g. nasopharyngitis, laryngitis, viral infection) in 20% of certolizumab-treated patients and 13% of placebo-treated patients, [[urinary tract infections]] (e.g. [[bladder infection]], [[bacteriuria]], [[cystitis]]) in 7% of certolizumab-treated patients and in 6% of placebo-treated patients, and [[arthralgia]] (6% certolizumab, 4% placebo). | ||
*During controlled clinical studies, the proportion of patients with serious adverse reactions was 10% for | |||
=====Other Adverse Reactions===== | =====Other Adverse Reactions===== | ||
* The most commonly occurring adverse reactions in controlled trials of [[Crohn's disease]] were described above. Other serious or significant adverse reactions reported in controlled and uncontrolled studies in [[Crohn's disease]] and other diseases, occurring in patients receiving certolizumab at doses of 400 mg or other doses include: | |||
*The most commonly occurring adverse reactions in controlled trials of Crohn's disease were described above. Other serious or significant adverse reactions reported in controlled and uncontrolled studies in Crohn's disease and other diseases, occurring in patients receiving | * Blood and lymphatic system disorders: [[Anemia]], [[leukopenia]], [[lymphadenopathy]], [[pancytopenia]], and [[thrombophilia]]. | ||
* Cardiac disorders: [[Angina pectoris]], [[arrhythmias]], [[atrial fibrillation]], [[cardiac failure]], [[hypertensive heart disease]], [[myocardial infarction]], [[myocardial ischemia]], [[pericardial effusion]], [[pericarditis]], [[stroke]] and [[transient ischemic attack]]. | |||
* Eye disorders: [[Optic neuritis]], [[retinal hemorrhage]], and [[uveitis]]. | |||
* General disorders and administration site conditions: [[Bleeding]] and [[injection site reactions]]. | |||
Anemia, leukopenia, lymphadenopathy, pancytopenia, and thrombophilia. | * Hepatobiliary disorders: [[Elevated liver enzymes]] and [[hepatitis]]. | ||
* Immune system disorders: [[Alopecia totalis]]. | |||
* Psychiatric disorders: [[Anxiety]], [[bipolar disorder]], and [[suicide attempt]]. | |||
* Renal and urinary disorders: [[Nephrotic syndrome]] and [[renal failure]]. | |||
Angina pectoris, arrhythmias, atrial fibrillation, cardiac failure, hypertensive heart disease, myocardial infarction, myocardial ischemia, pericardial effusion, pericarditis, stroke and transient ischemic attack. | * Reproductive system and breast disorders: [[Menstrual disorder]] | ||
* Skin and subcutaneous tissue disorders: [[Dermatitis]], [[erythema nodosum]], and [[urticaria]]. | |||
* Vascular disorders: [[Thrombophlebitis]], [[vasculitis]]. | |||
* Controlled Studies with [[Rheumatoid Arthritis]]. | |||
Optic neuritis, retinal hemorrhage, and uveitis. | * Certolizumab was studied primarily in placebo-controlled trials and in long-term follow-up studies. The data described below reflect the exposure to certolizumab in 2,367 RA patients, including 2,030 exposed for at least 6 months, 1,663 exposed for at least one year and 282 for at least 2 years; and 1,774 in adequate and well-controlled studies. In placebo-controlled studies, the population had a median age of 53 years at entry; approximately 80% were females, 93% were Caucasian and all patients were suffering from active [[rheumatoid arthritis]], with a median disease duration of 6.2 years. Most patients received the recommended dose of certolizumab or higher. | ||
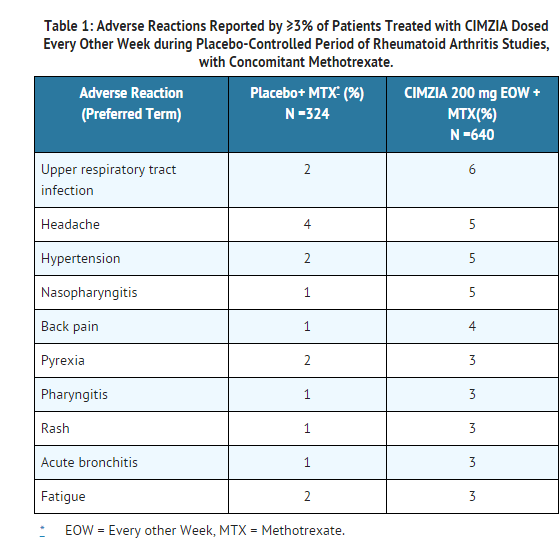
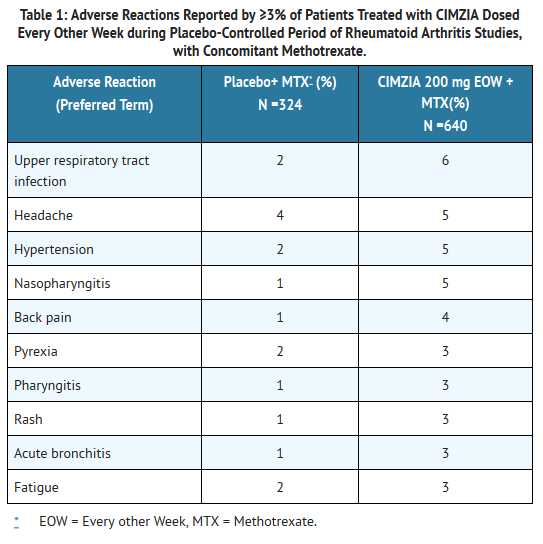
* Table 1 summarizes the reactions reported at a rate of at least 3% in patients treated with certolizumab 200 mg every other week compared to placebo (saline formulation), given concomitantly with methotrexate. | |||
: [[File:Cimia 01 Advr eff.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
* Hypertensive adverse reactions were observed more frequently in patients receiving CIMZIA than in controls. These adverse reactions occurred more frequently among patients with a baseline history of hypertension and among patients receiving concomitant [[corticosteroids]] and [[non-steroidal anti-inflammatory drugs]]. | |||
Bleeding and injection site reactions. | * Patients receiving certolizumab 400 mg as monotherapy every 4 weeks in [[rheumatoid arthritis]] controlled clinical trials had similar adverse reactions to those patients receiving certolizumab 200 mg every other week. | ||
Elevated liver enzymes and hepatitis. | |||
Alopecia totalis. | |||
Anxiety, bipolar disorder, and suicide attempt. | |||
Nephrotic syndrome and renal failure. | |||
Menstrual disorder | |||
Dermatitis, erythema nodosum, and urticaria. | |||
Thrombophlebitis, vasculitis. | |||
* | |||
*Table 1 summarizes the reactions reported at a rate of at least 3% in patients treated with | |||
*Hypertensive adverse reactions were observed more frequently in patients receiving CIMZIA than in controls. These adverse reactions occurred more frequently among patients with a baseline history of hypertension and among patients receiving concomitant corticosteroids and non-steroidal anti-inflammatory drugs. | |||
*Patients receiving | |||
=====Other Adverse Reactions===== | =====Other Adverse Reactions===== | ||
* Other infrequent adverse reactions (occurring in less than 3% of RA patients) were similar to those seen in [[Crohn's disease]] patients. | |||
*Other infrequent adverse reactions (occurring in less than 3% of RA patients) were similar to those seen in Crohn's disease patients. | |||
=====Psoriatic Arthritis Clinical Study===== | =====Psoriatic Arthritis Clinical Study===== | ||
* Certolizumab has been studied in 409 patients with [[psoriatic arthritis]] (PsA) in a placebo-controlled trial. The safety profile for patients with PsA treated with certolizumab was similar to the safety profile seen in patients with RA and previous experience with certolizumab. | |||
* | |||
=====Ankylosing Spondylitis Clinical Study===== | =====Ankylosing Spondylitis Clinical Study===== | ||
* Certolizumab has been studied in 325 patients with axial [[spondyloarthritis]] of whom the majority had [[ankylosing spondylitis]] (AS) in a placebo-controlled study (AS-1). The safety profile for patients in study AS-1 treated with certolizumab was similar to the safety profile seen in patients with RA. | |||
* | =====Infections===== | ||
* The incidence of infections in controlled studies in [[Crohn's disease]] was 38% for certolizumab-treated patients and 30% for placebo-treated patients. The infections consisted primarily of [upper respiratory infections]] (20% for certolizumab, 13% for placebo). The incidence of serious infections during the controlled clinical studies was 3% per patient-year for certolizumab-treated patients and 1% for placebo-treated patients. Serious infections observed included bacterial and viral infections, [[pneumonia]], and [[pyelonephritis]]. | |||
* The incidence of new cases of infections in controlled clinical studies in rheumatoid arthritis was 0.91 per patient-year for all certolizumab-treated patients and 0.72 per patient-year for placebo-treated patients. The infections consisted primarily of [[upper respiratory tract infections]], [[herpes infections]], [[urinary tract infections]], and [[lower respiratory tract infections]]. In the controlled [[rheumatoid arthritis]] studies, there were more new cases of serious infection adverse reactions in the certolizumab treatment groups, compared to the placebo groups (0.06 per patient-year for all certolizumab doses vs. 0.02 per patient-year for placebo). Rates of serious infections in the 200 mg every other week dose group were 0.06 per patient-year and in the 400 mg every 4 weeks dose group were 0.04 per patient-year. Serious infections included [[tuberculosis]], [[pneumonia]], [[cellulitis]], and [[pyelonephritis]]. In the placebo group, no serious infection occurred in more than one subject. There is no evidence of increased risk of infections with continued exposure over time. | |||
=====Tuberculosis and Opportunistic Infections===== | |||
* In completed and ongoing global clinical studies in all indications including 5,118 certolizumab-treated patients, the overall rate of [[tuberculosis]] is approximately 0.61 per 100 patient-years across all indications. | |||
* The majority of cases occurred in countries with high endemic rates of TB. Reports include cases of miliary, lymphatic, peritoneal, as well as [[pulmonary TB]]. The median time to onset of TB for all patients exposed to certolizumab across all indications was 345 days. In the studies with certolizumab in RA, there were 36 cases of TB among 2,367 exposed patients, including some fatal cases. Rare cases of opportunistic infections have also been reported in these clinical trials. | |||
=====Malignancies===== | |||
* In clinical studies of certolizumab, the overall incidence rate of [[malignancies]] was similar for certolizumab-treated and control patients. For some [[TNF blockers]], more cases of malignancies have been observed among patients receiving those TNF blockers compared to control patients. | |||
=====Heart Failure===== | |||
* In placebo-controlled and open-label rheumatoid arthritis studies, cases of new or worsening heart failure have been reported for certolizumab-treated patients. The majority of these cases were mild to moderate and occurred during the first year of exposure. | |||
=====Autoantibodies===== | |||
* In clinical studies in [[Crohn's disease]], 4% of patients treated with certolizumab and 2% of patients treated with placebo that had negative baseline ANA titers developed positive titers during the studies. One of the 1,564 [[Crohn's disease]] patients treated with certolizumab developed symptoms of a [[lupus]]-like syndrome. | |||
* In clinical trials of [[TNF blockers]], including certolizumab, in patients with RA, some patients have developed ANA. Four patients out of 2,367 patients treated with certolizumab in RA clinical studies developed clinical signs suggestive of a lupus-like syndrome. The impact of long-term treatment with certolizumab on the development of [[autoimmune diseases]] is unknown. | |||
=====Immunogenicity===== | |||
* Patients were tested at multiple time points for [[antibodies]] to certolizumab pegol during Studies CD1 and CD2. The overall percentage of antibody positive patients was 8% in patients continuously exposed to certolizumab, approximately 6% were neutralizing in vitro. No apparent correlation of antibody development to adverse events or efficacy was observed. Patients treated with concomitant immunosuppressants had a lower rate of antibody development than patients not taking immunosuppressants at baseline (3% and 11%, respectively). The following adverse events were reported in Crohn's disease patients who were antibody-positive (N = 100) at an incidence at least 3% higher compared to antibody-negative patients (N = 1,242): [[abdominal pain]], [[arthralgia]], [[edema]] peripheral, [[erythema nodosum]], [[injection site erythema]], [[injection site pain]], [[pain]] in extremity, and [[upper respiratory tract infection]]. | |||
* The overall percentage of patients with [[antibodies]] to certolizumab pegol detectable on at least one occasion was 7% (105 of 1,509) in the [[rheumatoid arthritis]] placebo-controlled trials. Approximately one third (3%, 39 of 1,509) of these patients had antibodies with neutralizing activity in vitro. Patients treated with concomitant immunosuppressants (MTX) had a lower rate of antibody development than patients not taking immunosuppressants at baseline. Patients treated with concomitant immunosuppressant therapy (MTX) in RA-I, RA-II, RA-III had a lower rate of neutralizing antibody formation overall than patients treated with certolizumab monotherapy in RA-IV (2% vs. 8%). Both the loading dose of 400 mg every other week at Weeks 0, 2 and 4 and concomitant use of MTX were associated with reduced immunogenicity. | |||
* Antibody formation was associated with lowered drug plasma concentration and reduced efficacy. In patients receiving the recommended certolizumab dosage of 200 mg every other week with concomitant MTX, the ACR20 response was lower among antibody positive patients than among antibody-negative patients (Study RA-I, 48% versus 60%; Study RA-II 35% versus 59%, respectively). In Study RA-III, too few patients developed antibodies to allow for meaningful analysis of ACR20 response by antibody status. In Study RA-IV (monotherapy), the ACR20 response was 33% versus 56%, antibody-positive versus antibody-negative status, respectively. No association was seen between antibody development and the development of adverse events. | |||
* The data reflect the percentage of patients whose test results were considered positive for antibodies to certolizumab pegol in an ELISA, and are highly dependent on the sensitivity and specificity of the assay. The observed incidence of antibody (including neutralizing antibody) positivity in an assay is highly dependent on several factors, including assay sensitivity and specificity, assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to certolizumab pegol with the incidence of antibodies to other products may be misleading. | |||
=====Hypersensitivity Reactions===== | |||
* The following symptoms that could be compatible with [[hypersensitivity reactions]] have been reported rarely following certolizumab administration to patients: [[angioedema]], [[dermatitis allergic]], [[dizziness]] (postural), [[dyspnea]], hot flush, [[hypotension]], [[injection site reactions]], malaise, [[pyrexia]], [[rash]], [[serum sickness]], and (vasovagal) [[syncope]]. | |||
|postmarketing======Postmarketing Experience===== | |||
The following adverse reactions have been identified during post-approval use of CIMZIA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate reliably their frequency or establish a causal relationship to drug exposure. | |||
* Vascular disorder: systemic vasculitis has been identified during post-approval use of [[TNF blockers]]. | |||
* [[Skin]]: case of severe [[skin]] reactions, including [[Stevens-Johnson syndrome]], [[toxic epidermal necrolysis]], [[erythema multiforme]], and new or worsening [[psoriasis]] (all sub-types including pustular and palmoplantar) have been identified during post-approval use of [[TNF blockers]]. | |||
* Immune System Disorders: [[Sarcoidosis]] | |||
|drugInteractions======Use with Anakinra, Abatacept, Rituximab, and Natalizumab===== | |||
* An increased risk of serious infections has been seen in clinical studies of other TNF-blocking agents used in combination with [[anakinra]] or [[abatacept]], with no added benefit. Formal drug interaction studies have not been performed with [[rituximab]] or [[natalizumab]]. Because of the nature of the adverse events seen with these combinations with TNF blocker therapy, similar toxicities may also result from the use of certolizumab in these combinations. There is not enough information to assess the safety and efficacy of such combination therapy. Therefore, the use of certolizumab in combination with [[anakinra]], [[abatacept]], [[rituximab]], or [[natalizumab]] is not recommended. | |||
=====Live Vaccines===== | |||
* Avoid use of live (including attenuated) vaccines concurrently with certolizumab. | |||
=====Laboratory Tests===== | |||
* Interference with certain coagulation assays has been detected in patients treated with CIMZIA. Certolizumab pegol may cause erroneously elevated activated [[partial thromboplastin time]] (aPTT) assay results in patients without coagulation abnormalities. This effect has been observed with the PTT-Lupus Anticoagulant (LA) test and Standard Target [[Activated Partial Thromboplastin time]] (STA-PTT) Automate tests from Diagnostica Stago, and the HemosIL APTT-SP liquid and HemosIL lyophilized silica tests from Instrumentation Laboratories. Other aPTT assays may be affected as well. Interference with thrombin time (TT) and prothrombin time (PT) assays has not been observed. There is no evidence that certolizumab therapy has an effect on in vivo coagulation. | |||
|FDAPregCat=B | |||
|useInPregnancyFDA======Risk Summary===== | |||
* Adequate and well-controlled studies with certolizumab have not been conducted in [[pregnant]] women. | |||
* Certolizumab pegol plasma concentrations obtained from 10 women treated with certolizumab during pregnancy and their newborn infants demonstrated low placental transfer of certolizumab pegol. Certolizumab may be eliminated at a slower rate in exposed [[infants]] than in adult patients. No fetal harm was observed in animal reproduction studies. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. | |||
* There is a [[pregnancy]] exposure registry that monitors [[pregnancy]] outcomes in women exposed to certolizumab during [[pregnancy]]. To enroll, healthcare providers or patients can call 1-877-311-8972. | |||
=====Human Data===== | |||
* In an independent clinical study conducted in 10 pregnant women with [[Crohn´s disease]] treated with certolizumab, certolizumab pegol concentrations were measured in maternal blood as well as in cord and infant blood (n=12) at the day of birth. The last dose of certolizumab (400 mg for every mother) was given on average 19 days prior to delivery (range 5-42 days). Plasma certolizumab pegol concentrations were <0.41 –1.66 μg/mL in cord blood, <0.41 – 1.58 μg/mL in infant blood, and 1.87–59.57 μg/mL in maternal blood. Plasma certolizumab pegol concentrations were lower (by at least 75%) in the infants than in mothers suggesting low placental transfer of certolizumab pegol. In one infant, the plasma certolizumab pegol concentration declined from 1.02 to 0.84 μg /mL over 4 weeks suggesting that certolizumab may be eliminated at a slower rate in infants than adults. | |||
=====Animal Data===== | |||
* Because certolizumab pegol does not cross-react with mouse or rat TNFα, reproduction studies were performed in rats using a rodent anti-murine TNFα pegylated Fab' fragment (cTN3 PF) similar to certolizumab pegol. Reproduction studies have been performed in rats at doses up to 100 mg/kg and have revealed no evidence of impaired fertility or harm to the fetus due to cTN3 PF. | |||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
|useInPregnancyAUS= | |||
* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | ||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInLaborDelivery= | |useInNursing=* It is not known whether certolizumab pegol is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from certolizumab, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother. | ||
There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |useInPed=* Safety and effectiveness in pediatric patients have not been established. Due to its inhibition of [[TNFα]], certolizumab administered during [[pregnancy]] could affect immune responses in the in utero-exposed newborn and infant. Although certolizumab pegol levels were low in 12 infants exposed to certolizumab in utero, the clinical significance of these low levels is unknown. Additional data available from one exposed infant suggests that certolizumab may be eliminated at a slower rate in infants than in adults. The safety of administering live or live-attenuated vaccines in exposed infants is unknown. Risks and benefits should be considered prior to vaccinating (live or live-attenuated) exposed infants. | ||
|useInGeri=* Clinical studies of certolizumab did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. Population pharmacokinetic analyses of patients enrolled in certolizumab clinical studies concluded that there was no apparent difference in drug concentration regardless of age. Because there is a higher incidence of infections in the elderly population in general, use caution when treating the elderly with certolizumab. | |||
|useInNursing= | |useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | ||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInPed= | |useInHepaticImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | ||
|useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
|useInGeri= | |||
|useInGender= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential= | |||
There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp= | |||
There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* [[Oral]] | |||
| | * [[Intravenous]] | ||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
<!--IV Compatibility--> | <!--IV Compatibility--> | ||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
|IVCompat= | |||
<!--Overdosage--> | <!--Overdosage--> | ||
|overdose=* The maximum tolerated dose of certolizumab pegol has not been established. Doses of up to 800 mg subcutaneous and 20 mg/kg intravenous have been administered without evidence of dose-limiting toxicities. In cases of overdosage, it is recommended that patients be monitored closely for any adverse reactions or effects, and appropriate symptomatic treatment instituted immediately. | |||
|drugBox={{Drugbox2 | |||
| Verifiedfields = changed | |||
| verifiedrevid = 460025906 | |||
| | <!--Monoclonal antibody data--> | ||
| type = mab | |||
| mab_type = Fab' | |||
| source = zu/o | |||
| target = [[TNF alpha]] | |||
=== | <!--Clinical data--> | ||
| tradename = Cimzia | |||
| Drugs.com = {{drugs.com|CDI|certolizumab_pegol}} | |||
| MedlinePlus = a608041 | |||
| pregnancy_AU = | |||
| pregnancy_US = B | |||
| pregnancy_category = | |||
| legal_AU = | |||
| legal_CA = | |||
| legal_UK = | |||
| legal_US = Rx-only | |||
| legal_status = | |||
| routes_of_administration = Subcutaneous | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = NA | |||
==== | <!--Pharmacokinetic data--> | ||
| bioavailability = | |||
| protein_bound = | |||
| metabolism = | |||
| elimination_half-life = | |||
| excretion = | |||
<!--Identifiers--> | |||
| CAS_number_Ref = {{cascite|changed|??}} | |||
| CAS_number = 428863-50-7 | |||
| ATC_prefix = L04 | |||
| ATC_suffix = AB05 | |||
| PubChem = | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = | |||
| UNII_Ref = {{fdacite|changed|FDA}} | |||
| UNII = UMD07X179E | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D03441 | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ChEMBL = 1201831 | |||
==== | <!--Chemical data--> | ||
| C=2115 | H=3252 | N=556 | O=673 | S=16 | |||
* | | molecular_weight = 47.75 [[kDa]] | ||
}} | |||
|mechAction=* Certolizumab (certolizumab pegol) is a TNF blocker. Certolizumab is a recombinant, humanized antibody Fab' fragment, with specificity for human [[tumor necrosis factor alpha]] (TNFα), conjugated to an approximately 40kDa [[polyethylene glycol]] (PEG2MAL40K). The Fab' fragment is manufactured in E. coli and is subsequently subjected to purification and conjugation to PEG2MAL40K, to generate certolizumab pegol. The Fab' fragment is composed of a light chain with 214 amino acids and a heavy chain with 229 amino acids. The molecular weight of certolizumab pegol is approximately 91 kiloDaltons. | |||
* Certolizumab is supplied as either a sterile, white, lyophilized powder for solution or as a sterile, solution in a single-use prefilled 1 mL glass syringe for subcutaneous injection. After reconstitution of the lyophilized powder with 1 mL sterile Water for Injection, USP, the resulting pH is approximately 5.2. Each single-use vial provides approximately 200 mg certolizumab pegol, 0.9 mg lactic acid, 0.1 mg polysorbate, and 100 mg sucrose. | |||
* Each single-use prefilled syringe of certolizumab delivers 200 mg in 1 mL of solution with a pH of approximately 4.7 for subcutaneous use. Each 1 mL syringe of certolizumab contains certolizumab pegol (200 mg), sodium acetate (1.36 mg), sodium chloride (7.31 mg), and Water for Injection, USP. | |||
* Certolizumab is a clear to opalescent solution that is colorless to pale yellow and essentially free from particulates. No preservatives are present. | |||
* Certolizumab pegol binds to human TNFα with a KD of 90pM. [[TNFα]] is a key pro-inflammatory [[cytokine]] with a central role in inflammatory processes. Certolizumab pegol selectively neutralizes TNFα (IC90 of 4 ng/mL for inhibition of human TNFα in the in vitro L929 murine [[fibrosarcoma]] cytotoxicity assay) but does not neutralize [[lymphotoxin α]] (TNFβ). Certolizumab pegol cross-reacts poorly with TNF from rodents and rabbits, therefore in vivo efficacy was evaluated using animal models in which human TNFα was the physiologically active molecule. | |||
* Certolizumab pegol was shown to neutralize membrane-associated and soluble human TNFα in a dose-dependent manner. Incubation of monocytes with certolizumab pegol resulted in a dose-dependent inhibition of LPS-induced TNFα and IL-1β production in human monocytes. | |||
* Certolizumab pegol does not contain a fragment crystallizable (Fc) region, which is normally present in a complete [[antibody]], and therefore does not fix complement or cause antibody-dependent cell-mediated cytotoxicity in vitro. It does not induce apoptosis in vitro in human peripheral blood-derived [[monocytes]] or [[lymphocytes]], nor does certolizumab pegol induce [[neutrophil]] degranulation. | |||
* A [[tissue]] reactivity study was carried out ex vivo to evaluate potential cross-reactivity of certolizumab pegol with cryosections of normal [[human]] tissues. Certolizumab pegol showed no reactivity with a designated standard panel of normal human [[tissues]]. | |||
<!--Structure--> | <!--Structure--> | ||
|structure=* Certolizumab (certolizumab pegol) is a TNF blocker. certolizumab is a recombinant, humanized antibody Fab' fragment, with specificity for human [[tumor necrosis factor alpha ]](TNFα), conjugated to an approximately 40kDa polyethylene glycol (PEG2MAL40K). The Fab' fragment is manufactured in E. coli and is subsequently subjected to purification and conjugation to PEG2MAL40K, to generate certolizumab pegol. The Fab' fragment is composed of a light chain with 214 amino acids and a heavy chain with 229 amino acids. The molecular weight of certolizumab pegol is approximately 91 kiloDaltons. | |||
|structure= | * Certolizumab is supplied as either a sterile, white, lyophilized powder for solution or as a sterile, solution in a single-use prefilled 1 mL glass syringe for subcutaneous injection. After reconstitution of the lyophilized powder with 1 mL sterile Water for Injection, USP, the resulting pH is approximately 5.2. Each single-use vial provides approximately 200 mg certolizumab pegol, 0.9 mg lactic acid, 0.1 mg polysorbate, and 100 mg sucrose. | ||
* Each single-use prefilled syringe of certolizumab delivers 200 mg in 1 mL of solution with a pH of approximately 4.7 for subcutaneous use. Each 1 mL syringe of certolizumab contains certolizumab pegol (200 mg), sodium acetate (1.36 mg), sodium chloride (7.31 mg), and Water for Injection, USP. | |||
* | * certolizumab is a clear to opalescent solution that is colorless to pale yellow and essentially free from particulates. No preservatives are present. | ||
: [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
|PD=* Certolizumab pegol binds to human TNFα with a KD of 90pM. TNFα is a key pro-inflammatory cytokine with a central role in inflammatory processes. Certolizumab pegol selectively neutralizes TNFα (IC90 of 4 ng/mL for inhibition of human TNFα in the in vitro L929 murine [[fibrosarcoma]] cytotoxicity assay) but does not neutralize lymphotoxin α (TNFβ). Certolizumab pegol cross-reacts poorly with TNF from rodents and rabbits, therefore in vivo efficacy was evaluated using animal models in which human TNFα was the physiologically active molecule. | |||
|PD= | * Certolizumab pegol was shown to neutralize membrane-associated and soluble human TNFα in a dose-dependent manner. Incubation of monocytes with certolizumab pegol resulted in a dose-dependent inhibition of LPS-induced TNFα and IL-1β production in human monocytes. | ||
* Certolizumab pegol does not contain a fragment crystallizable (Fc) region, which is normally present in a complete antibody, and therefore does not fix complement or cause antibody-dependent cell-mediated cytotoxicity in vitro. It does not induce apoptosis in vitro in human peripheral blood-derived [[monocytes]] or [[lymphocytes]], nor does certolizumab pegol induce [[neutrophil degranulation]]. | |||
* A tissue reactivity study was carried out ex vivo to evaluate potential cross-reactivity of certolizumab pegol with cryosections of normal human tissues. Certolizumab pegol showed no reactivity with a designated standard panel of normal human tissues. | |||
|PK======Absorption===== | |||
* A total of 126 healthy subjects received doses of up to 800 mg certolizumab pegol subcutaneously (sc) and up to 10 mg/kg intravenously (IV) in four pharmacokinetic studies. Data from these studies demonstrate that single intravenous and subcutaneous doses of certolizumab pegol have predictable dose-related plasma concentrations with a linear relationship between the dose administered and the maximum plasma concentration (Cmax), and the Area Under the certolizumab pegol plasma concentration versus time Curve (AUC). A mean Cmax of approximately 43 to 49 mcg/mL occurred at Week 5 during the initial loading dose period using the recommended dose regimen for the treatment of patients with rheumatoid arthritis (400 mg sc at Weeks 0, 2 and 4 followed by 200 mg every other week). | |||
* Certolizumab pegol plasma concentrations were broadly dose-proportional and pharmacokinetics observed in patients with rheumatoid arthritis and [[Crohn's disease]] were consistent with those seen in healthy subjects. | |||
|PK= | * Following subcutaneous administration, peak plasma concentrations of certolizumab pegol were attained between 54 and 171 hours post-injection. Certolizumab pegol has bioavailability (F) of approximately 80% (ranging from 76% to 88%) following subcutaneous administration compared to intravenous administration. | ||
=====Distribution===== | |||
* The steady state volume of distribution (Vss) was estimated as 6 to 8 L in the population pharmacokinetic analysis for patients with [[Crohn's disease]] and patients with [[rheumatoid arthritis]]. | |||
=====Metabolism===== | |||
* The metabolism of certolizumab pegol has not been studied in human subjects. Data from animals indicate that once cleaved from the Fab' fragment the PEG moiety is mainly excreted in urine without further metabolism. | |||
=====Elimination===== | |||
* PEGylation, the covalent attachment of PEG polymers to peptides, delays the metabolism and elimination of these entities from the circulation by a variety of mechanisms, including decreased renal clearance, proteolysis, and immunogenicity. | |||
* Accordingly, certolizumab pegol is an antibody Fab' fragment conjugated with PEG in order to extend the terminal plasma elimination half-life (t1/2) of the Fab'. The terminal elimination phase half-life (t1/2) was approximately 14 days for all doses tested. The clearance following IV administration to healthy subjects ranged from 9.21 mL/h to 14.38 mL/h. The clearance following sc dosing was estimated 17 mL/h in the Crohn's disease population PK analysis with an inter-subject variability of 38% (CV) and an inter-occasion variability of 16%. Similarly, the clearance following sc dosing was estimated as 21.0 mL/h in the RA population PK analysis, with an inter-subject variability of 30.8% (%CV) and inter-occasion variability 22.0%. The route of elimination of certolizumab pegol has not been studied in human subjects. Studies in animals indicate that the major route of elimination of the PEG component is via urinary excretion. | |||
=====Special Populations===== | |||
* Population pharmacokinetic analysis was conducted on data from patients with rheumatoid arthritis and patients with Crohn's disease, to evaluate the effect of age, race, gender, methotrexate use, concomitant medication, creatinine clearance and presence of anti-certolizumab antibodies on pharmacokinetics of certolizumab pegol. | |||
* Only bodyweight and presence of anti-certolizumab antibodies significantly affected certolizumab pegol pharmacokinetics. Pharmacokinetic exposure was inversely related to body weight but pharmacodynamic exposure-response analysis showed that no additional therapeutic benefit would be expected from a weight-adjusted dose regimen. The presence of anti-certolizumab antibodies was associated with a 3.6-fold increase in clearance. | |||
* Age: Pharmacokinetics of certolizumab pegol was not different in elderly compared to young adults. | |||
* Gender: Pharmacokinetics of certolizumab pegol was similar in male and female subjects. | |||
* Renal Impairment: Specific clinical studies have not been performed to assess the effect of renal impairment on the pharmacokinetics of certolizumab. The pharmacokinetics of the PEG (polyethylene glycol) fraction of certolizumab pegol is expected to be dependent on renal function but has not been assessed in renal impairment. There are insufficient data to provide a dosing recommendation in moderate and severe renal impairment. | |||
* Race: A specific clinical study showed no difference in pharmacokinetics between Caucasian and Japanese subjects. | |||
=====Drug Interaction Studies===== | |||
* Methotrexate pharmacokinetics is not altered by concomitant administration with certolizumab in patients with rheumatoid arthritis. The effect of methotrexate on certolizumab pharmacokinetics was not studied. However, methotrexate-treated patients have lower incidence of antibodies to certolizumab. Thus, therapeutic plasma levels are more likely to be sustained when certolizumab is administered with methotrexate in patients with rheumatoid arthritis. | |||
* Formal drug-drug interaction studies have not been conducted with certolizumab upon concomitant administration with [[corticosteroids]], [[nonsteroidal anti-inflammatory drugs]], [[analgesics]] or [[immunosuppressants]]. | |||
| | |nonClinToxic======Carcinogenesis, Mutagenesis, and Impairment of Fertility===== | ||
* Long-term animal studies of certolizumab have not been conducted to assess its carcinogenic potential. Certolizumab pegol was not genotoxic in the Ames test, the human peripheral blood lymphocytes chromosomal aberration assay, or the mouse bone marrow micronucleus assay. | |||
* | * Since certolizumab pegol does not cross-react with mouse or rat TNFα, reproduction studies were performed in rats using a rodent anti-murine TNFα pegylated Fab fragment (cTN3 PF), similar to certolizumab pegol. The cTN3 PF had no effects on the fertility and general reproductive performance of male and female rats at intravenous doses up 100 mg/kg, administered twice weekly. | ||
|clinicalStudies======Crohn's Disease===== | |||
* The efficacy and safety of certolizumab were assessed in two double-blind, randomized, placebo-controlled studies in patients aged 18 years and older with moderately to severely active [[Crohn's disease]], as defined by a [[Crohn's Disease]] Activity Index (CDAI1) of 220 to 450 points, inclusive. certolizumab was administered subcutaneously at a dose of 400 mg in both studies. Stable concomitant medications for Crohn's disease were permitted. | |||
=====Study CD1===== | |||
| | * Study CD1 was a randomized placebo-controlled study in 662 patients with active Crohn's disease. Certolizumab or placebo was administered at Weeks 0, 2, and 4 and then every four weeks to Week 24. Assessments were done at Weeks 6 and 26. Clinical response was defined as at least a 100-point reduction in CDAI score compared to baseline, and clinical remission was defined as an absolute CDAI score of 150 points or lower. | ||
* The results for Study CD1 are provided in Table 2. At Week 6, the proportion of clinical responders was statistically significantly greater for certolizumab-treated patients compared to controls. The difference in clinical remission rates was not statistically significant at Week 6. The difference in the proportion of patients who were in clinical response at both Weeks 6 and 26 was also statistically significant, demonstrating maintenance of clinical response. | |||
: [[File:Cimia 2 Clinical studies.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
|howSupplied=* Refrigerate intact carton between 2 to 8 °C (36 to 46 °F) | |||
|storage=* Do not freeze. Do not separate contents of carton prior to use. Do not use beyond expiration date, which is located on the drug label and carton. Protect solution from light. | |||
|packLabel=[[File:Cimia 04.jpg|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
|alcohol= | [[File:Cimiia 05.jpg|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
[[File:Cimia Pamphlet.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |fdaPatientInfo======Patient Counseling===== | ||
* Advise patients of the potential risks and benefits of certolizumab therapy. Be sure that patients receive the Medication Guide and allow them time to read it prior to starting certolizumab therapy and to review it periodically. Any questions resulting from the patient's reading of the Medication Guide should be discussed. Because caution should be exercised in prescribing certolizumab to patients with clinically important active infections, advise patients of the importance of informing their health care providers about all aspects of their health. | |||
=====Immunosuppression===== | |||
* Inform patients that certolizumab may lower the ability of the immune system to fight infections. Instruct patients of the importance of contacting their doctor if they develop any symptoms of infection, including tuberculosis and reactivation of hepatitis B virus infections. | |||
* Counsel patients about the possible risk of [[lymphoma]] and other [[malignancies]] while receiving certolizumab. | |||
=====Allergic Reactions===== | |||
* Advise patients to seek immediate medical attention if they experience any symptoms of severe allergic reactions. The prefilled syringe components do not contain any latex or dry natural rubber. | |||
=====Other Medical Conditions===== | |||
* Advise patients to report any signs of new or worsening medical conditions such as heart disease, neurological disease, or autoimmune disorders. Advise patients to report promptly any symptoms suggestive of a [[cytopenia]] such as bruising, bleeding, or persistent fever. | |||
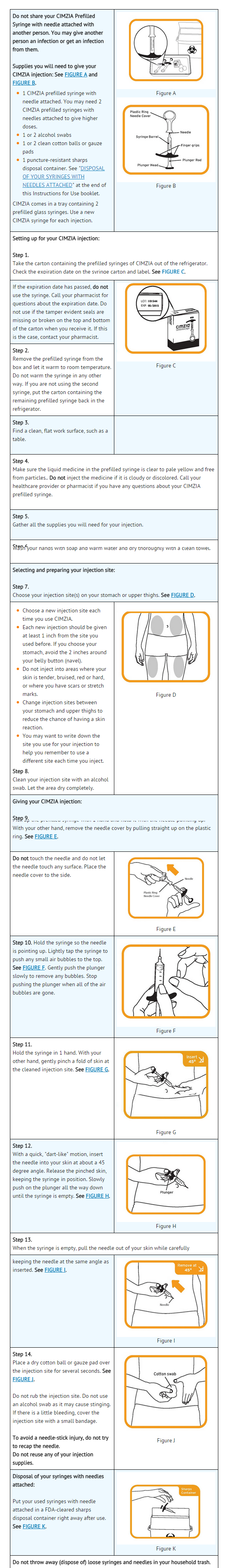
=====Instruction on Prefilled Syringe Self-Injection Technique===== | |||
* After proper training by a qualified healthcare professional in subcutaneous injection technique, a patient may self inject with certolizumab using the Prefilled Syringe if a healthcare provider determines that it is appropriate. A patient's ability to administer certolizumab subcutaneous injections should be checked to ensure correct administration. Suitable sites for injection include the [[thigh]] or [[abdomen]]. Certolizumab should be injected when the liquid is at room temperature. | |||
* Full injection instructions are provided in the Instructions for Use booklet for the Prefilled Syringe, packaged in each certolizumab Prefilled Syringe kit. | |||
* To avoid needle-stick injury, patients and healthcare providers should not attempt to place the needle cover back on the syringe or otherwise recap the needle. Be sure to properly dispose of needles and syringes in a puncture-proof container, and instruct patients and caregivers in proper syringe and needle disposal technique. Actively discourage any reuse of the injection materials. | |||
: [[File:Cimizia Patient counselling info.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames=* CIMZIA®<ref>{{Cite web | title =CIMZIA- certolizumab pegol | url = http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b4c2c9dc-a0bb-4d64-a667-a67ebe88392d}}</ref> | |||
|brandNames= | |||
* | |||
<!--Look-Alike Drug Names--> | <!--Look-Alike Drug Names--> | ||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
{{PillImage | |||
|fileName=No image.jpg | |||
}} | |||
{{LabelImage | |||
|fileName={{PAGENAME}}11.png | |||
}} | |||
{{LabelImage | |||
|fileName={{PAGENAME}}11.png | |||
}} | |||
<!--Pill Image--> | |||
<!--Label Display Image--> | <!--Label Display Image--> | ||
<!--Category--> | <!--Category--> | ||
[[Category:Drug]] | [[Category:Drug]] | ||
[[Category:Biotechnology]] | |||
Latest revision as of 12:22, 28 April 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]; Ammu Susheela, M.D. [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: SERIOUS INFECTIONS AND MALIGNANCY
See full prescribing information for complete Boxed Warning.
SERIOUS INFECTIONS
MALIGNANCY
|
Overview
Certolizumab pegol is an immunosupressant that is FDA approved for the treatment of crohn's disease, rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. There is a Black Box Warning for this drug as shown here. Common adverse reactions include arthralgia ,urinary tract infections and upper respiratory infection.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
Crohn's Disease
- Certolizumab is indicated for reducing signs and symptoms of Crohn's disease and maintaining clinical response in adult patients with moderately to severely active disease who have had an inadequate response to conventional therapy.
Rheumatoid Arthritis
- Certolizumab is indicated for the treatment of adults with moderately to severely active rheumatoid arthritis (RA).
Psoriatic Arthritis
- Certolizumab is indicated for the treatment of adult patients with active psoriatic arthritis (PsA).
Ankylosing Spondylitis
- Certolizumab is indicated for the treatment of adults with active ankylosing spondylitis (AS).
Storage
- Certolizumab is administered by subcutaneous injection. Injection sites should be rotated and injections should not be given into areas where the skin is tender, bruised, red or hard. When a 400 mg dose is needed (given as two subcutaneous injections of 200 mg), injections should occur at separate sites in the thigh or abdomen.
- The solution should be carefully inspected visually for particulate matter and discoloration prior to administration. The solution should be a clear colorless to yellow liquid, essentially free from particulates and should not be used if cloudy or if foreign particulate matter is present. Certolizumab does not contain preservatives; therefore, unused portions of drug remaining in the syringe or vial should be discarded.
Crohn's Disease
- The recommended initial adult dose of certolizumab is 400 mg (given as two subcutaneous injections of 200 mg) initially, and at Weeks 2 and 4. In patients who obtain a clinical response, the recommended maintenance regimen is 400 mg every four weeks.
Rheumatoid Arthritis
- The recommended dose of certolizumab for adult patients with rheumatoid arthritis is 400 mg (given as two subcutaneous injections of 200 mg) initially and at Weeks 2 and 4, followed by 200 mg every other week. For maintenance dosing, certolizumab 400 mg every 4 weeks can be considered.
Psoriatic Arthritis
- The recommended dose of certolizumab for adult patients with psoriatic arthritis is 400 mg (given as 2 subcutaneous injections of 200 mg each) initially and at week 2 and 4, followed by 200 mg every other week. For maintenance dosing, certolizumab 400 mg every 4 weeks can be considered.
Ankylosing Spondylitis
- The recommended dose of certolizumab for adult patients with ankylosing spondylitis is 400 mg (given as 2 subcutaneous injections of 200 mg each) initially and at weeks 2 and 4, followed by 200 mg every 2 weeks or 400 mg every 4 weeks.
Preparation and Administration of Certolizumab Using the Lyophilized Powder for Injection
- Certolizumab Lyophilized powder should be prepared and administered by a health care professional. certolizumab is provided in a package that contains everything required to reconstitute and inject the drug. Step-by-step preparation and administration instructions are provided below.
Preparation and Storage
- Certolizumab should be brought to room temperature before reconstituting.
- Use appropriate aseptic technique when preparing and administering certolizumab.
Reconstitute the vial(s) of certolizumab with 1 mL of Sterile Water for Injection, USP using the 20-gauge needle provided.
- Gently swirl each vial of certolizumab without shaking, assuring that all of the powder comes in contact with the Sterile Water for Injection.
- Leave the vial(s) undisturbed to fully reconstitute, which may take approximately 30 minutes.
- The final reconstituted solution contains 200 mg/mL and should be clear to opalescent, colorless to pale yellow liquid essentially free from particulates.
Once reconstituted, certolizumab can be stored in the vials for up to 24 hours between 2° to 8° C (36° to 46° F) prior to injection. Do not freeze.
Administration
- Prior to injecting, reconstituted certolizumab should be at room temperature but do not leave reconstituted certolizumab at room temperature for more than two hours prior to administration.
- Withdraw the reconstituted solution into a separate syringe for each vial using a new 20-gauge needle for each vial so that each syringe contains 1 mL of certolizumab (200 mg of certolizumab pegol).
- Replace the 20-gauge needle(s) on the syringes with a 23-gauge(s) for administration.
- Inject the full contents of the syringe(s) subcutaneously into thigh or abdomen. Where a 400 mg dose is required, two injections are required, therefore, separate sites should be used for each 200 mg injection.
Preparation and Administration of Certolizumab Using the Prefilled Syringe
- After proper training in subcutaneous injection technique, a patient may self-inject with the certolizumab Prefilled Syringe if a physician determines that it is appropriate.
- Patients using the Certolizumab Prefilled Syringe should be instructed to inject the full amount in the syringe (1 mL), according to the directions provided in the Instructions for Use booklet.
Monitoring to Assess Safety
- Before initiation of therapy with certolizumab, all patients must be evaluated for both active and inactive (latent) tuberculosis infection. The possibility of undetected latent tuberculosis should be considered in patients who have immigrated from or traveled to countries with a high prevalence of tuberculosis or had close contact with a person with active tuberculosis. Appropriate screening tests (e.g. tuberculin skin test and chest x-ray) should be performed in all patients.
Concomitant Medications
- Certolizumab may be used as monotherapy or concomitantly with non-biological disease modifying anti-rheumatic drugs (DMARDs).
- The use of certolizumab in combination with biological DMARDs or other tumor necrosis factor (TNF) blocker therapy is not recommended.
Dosage
- For Injection: Lyophilized Powder for Reconstitution
- Sterile, white, lyophilized powder for reconstitution and then subcutaneous administration. Each single-use vial provides approximately 200 mg of certolizumab pegolA.
- Injection: Prefilled Syringe
- A single-use, 1 mL prefilled glass syringe with a fixed 25 gauge ½ inch thin wall needle, providing 200 mg per 1 mL of certolizumab pegol.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Certolizumab pegol in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Certolizumab pegol in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Certolizumab pegol in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Certolizumab pegol in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Certolizumab pegol in pediatric patients.
Contraindications
There is limited information regarding Certolizumab pegol Contraindications in the drug label.
Warnings
|
WARNING: SERIOUS INFECTIONS AND MALIGNANCY
See full prescribing information for complete Boxed Warning.
SERIOUS INFECTIONS
MALIGNANCY
|
Risk of Serious Infections
- Patients treated with certolizumab are at an increased risk for developing serious infections involving various organ systems and sites that may lead to hospitalization or death.
- Opportunistic infections due to bacterial, mycobacterial, invasive fungal, viral, parasitic, or other opportunistic pathogens including aspergillosis, blastomycosis, candidiasis, coccidioidomycosis, histoplasmosis, legionellosis, listeriosis, pneumocystosis and tuberculosis have been reported with TNF blockers. Patients have frequently presented with disseminated rather than localized disease.
- Treatment with certolizumab should not be initiated in patients with an active infection, including clinically important localized infections. Patients greater than 65 years of age, patients with co-morbid conditions, and/or patients taking concomitant immunosuppressants (e.g. corticosteroids or methotrexate) may be at a greater risk of infection. The risks and benefits of treatment should be considered prior to initiating therapy in patients:
- With chronic or recurrent infection
- Who have been exposed to tuberculosis
- With a history of an opportunistic infection
- Who have resided or traveled in areas of endemic tuberculosis or endemic mycoses, such as histoplasmosis, coccidioidomycosis, or blastomycosis
- With underlying conditions that may predispose them to infection.
Tuberculosis
- Cases of reactivation of tuberculosis or new tuberculosis infections have been observed in patients receiving certolizumab, including patients who have previously received treatment for latent or active tuberculosis. Patients should be evaluated for tuberculosis risk factors and tested for latent infection prior to initiating certolizumab and periodically during therapy.
- Treatment of latent tuberculosis infection prior to therapy with TNF-blocking agents has been shown to reduce the risk of tuberculosis reactivation during therapy. Induration of 5 mm or greater with tuberculin skin testing should be considered a positive test result when assessing if treatment for latent tuberculosis is needed prior to initiating certolizumab, even for patients previously vaccinated with Bacille Calmette-Guerin (BCG).
- Anti-tuberculosis therapy should also be considered prior to initiation of certolizumab in patients with a past history of latent or active tuberculosis in whom an adequate course of treatment cannot be confirmed, and for patients with a negative test for latent tuberculosis but having risk factors for tuberculosis infection. Consultation with a physician with expertise in the treatment of tuberculosis is recommended to aid in the decision of whether initiating anti-tuberculosis therapy is appropriate for an individual patient.
- Tuberculosis should be strongly considered in patients who develop a new infection during certolizumab treatment, especially in patients who have previously or recently traveled to countries with a high prevalence of tuberculosis, or who have had close contact with a person with active tuberculosis.
Monitoring
- Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with certolizumab, including the development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy. Tests for latent tuberculosis infection may also be falsely negative while on therapy with certolizumab.
- certolizumab should be discontinued if a patient develops a serious infection or sepsis.
- A patient who develops a new infection during treatment with certolizumab should be closely monitored, undergo a prompt and complete diagnostic workup appropriate for an immunocompromised patient, and appropriate antimicrobial therapy should be initiated.
Invasive Fungal Infections
- For patients who reside or travel in regions where mycoses are endemic, invasive fungal infection should be suspected if they develop a serious systemic illness. Appropriate empiric antifungal therapy should be considered while a diagnostic workup is being performed. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. When feasible, the decision to administer empiric antifungal therapy in these patients should be made in consultation with a physician with expertise in the diagnosis and treatment of invasive fungal infections and should take into account both the risk for severe fungal infection and risks of antifungal therapy.
Malignancies
- In the controlled portions of clinical studies of some TNF blockers, more cases of malignancies have been observed among patients receiving TNF blockers compared to control patients. During controlled and open-labeled portions of certolizumab studies of Crohn's disease and other diseases, malignancies (excluding non-melanoma skin cancer) were observed at a rate (95% confidence interval) of 0.5 (0.4, 0.7) per 100 patient-years among 4,650 certolizumab-treated patients versus a rate of 0.6 (0.1, 1.7) per 100 patient-years among 1,319 placebo-treated patients. The size of the control group and limited duration of the controlled portions of the studies precludes the ability to draw firm conclusions.
- Malignancies, some fatal, have been reported among children, adolescents, and young adults who received treatment with TNF-blocking agents (initiation of therapy ≤ 18 years of age), of which certolizumab is a member. Approximately half the cases were lymphomas, including Hodgkin's and non-Hodgkin's lymphoma. The other cases represented a variety of different malignancies and included rare malignancies usually associated with immunosuppression and malignancies that are not usually observed in children and adolescents. The malignancies occurred after a median of 30 months of therapy (range 1 to 84 months). Most of the patients were receiving concomitant immunosuppressants. These cases were reported post-marketing and are derived from a variety of sources including registries and spontaneous post-marketing reports. Certolizumab is not indicated for use in pediatric patients.
- In the controlled portions of clinical trials of all the TNF blockers, more cases of lymphoma have been observed among patients receiving TNF blockers compared to control patients. In controlled studies of certolizumab for Crohn's disease and other investigational uses, there was one case of lymphoma among 2,657 certolizumab-treated patients and one case of Hodgkin's lymphoma among 1,319 placebo-treated patients.
- In the certolizumab RA clinical trials (placebo-controlled and open label) a total of three cases of lymphoma were observed among 2,367 patients. This is approximately 2-fold higher than expected in the general population. Patients with RA, particularly those with highly active disease, are at a higher risk for the development of lymphoma.
- Rates in clinical studies for certolizumab cannot be compared to the rates of clinical trials of other TNF blockers and may not predict the rates observed when certolizumab is used in a broader patient population. Patients with Crohn's disease that require chronic exposure to immunosuppressant therapies may be at higher risk than the general population for the development of lymphoma, even in the absence of TNF blocker therapy. The potential role of TNF blocker therapy in the development of malignancies in adults is not known.
- Postmarketing cases of hepatosplenic T-cell lymphoma (HSTCL), a rare type of T-cell lymphoma that has a very aggressive disease course and is usually fatal, have been reported in patients treated with TNF blockers, including certolizumab. The majority of reported TNF blocker cases occurred in adolescent and young adult males with Crohn's disease or ulcerative colitis. Almost all of these patients had received treatment with the immunosuppressants azathioprine and/or 6-mercaptopurine (6-MP) concomitantly with a TNF blocker at or prior to diagnosis. It is uncertain whether the occurrence of HSTCL is related to use of a TNF blocker or a TNF blocker in combination with these other immunosuppressants. The potential risk of using a TNF blocker in combination with azathioprine or 6-MP should be carefully considered.
- Cases of acute and chronic leukemia have been reported in association with post-marketing TNF-blocker use in RA and other indications. Even in the absence of TNF-blocker therapy, patients with RA may be at a higher risk (approximately 2-fold) than the general population for the development of leukemia.
- Periodic skin examinations are recommended for all patients, particularly those with risk factors for skin cancer.
Heart Failure
- Cases of worsening congestive heart failure (CHF) and new onset CHF have been reported with TNF blockers, including certolizumab. Certolizumab has not been formally studied in patients with CHF; however, in clinical studies in patients with CHF with another TNF blocker, worsening congestive heart failure (CHF) and increased mortality due to CHF were observed. Exercise caution in patients with heart failure and monitor them carefully.
Hypersensitivity Reactions
- The following symptoms that could be compatible with hypersensitivity reactions have been reported rarely following certolizumab administration to patients: angioedema, dyspnea, hypotension, rash, serum sickness, and urticaria. Some of these reactions occurred after the first administration of certolizumab. If such reactions occur, discontinue further administration of certolizumab and institute appropriate therapy. There are no data on the risks of using certolizumab in patients who have experienced a severe hypersensitivity reaction towards another TNF blocker; in these patients caution is needed.
Hepatitis B Virus Reactivation
- Use of TNF blockers, including certolizumab, has been associated with reactivation of hepatitis B virus (HBV) in patients who are chronic carriers of this virus. In some instances, HBV reactivation occurring in conjunction with TNF blocker therapy has been fatal. The majority of reports have occurred in patients concomitantly receiving other medications that suppress the immune system, which may also contribute to HBV reactivation.
- Test patients for HBV infection before initiating treatment with certolizumab. For patients who test positive for HBV infection, consultation with a physician with expertise in the treatment of hepatitis B is recommended. Adequate data are not available on the safety or efficacy of treating patients who are carriers of HBV with anti-viral therapy in conjunction with TNF blocker therapy to prevent HBV reactivation. Patients who are carriers of HBV and require treatment with certolizumab should be closely monitored for clinical and laboratory signs of active HBV infection throughout therapy and for several months following termination of therapy.
- In patients who develop HBV reactivation, discontinue certolizumab and initiate effective anti-viral therapy with appropriate supportive treatment. The safety of resuming TNF blocker therapy after HBV reactivation is controlled is not known. Therefore, exercise caution when considering resumption of certolizumab therapy in this situation and monitor patients closely.
Neurologic Reactions
- Use of TNF blockers, of which certolizumab is a member, has been associated with rare cases of new onset or exacerbation of clinical symptoms and/or radiographic evidence of central nervous system demyelinating disease, including multiple sclerosis, and with peripheral demyelinating disease, including Guillain-Barré syndrome . Exercise caution in considering the use of certolizumab in patients with pre-existing or recent-onset central or peripheral nervous system demyelinating disorders. Rare cases of neurological disorders, including seizure disorder, optic neuritis, and peripheral neuropathy have been reported in patients treated with certolizumab.
Hematological Reactions
- Rare reports of pancytopenia, including aplastic anemia, have been reported with TNF blockers. Adverse reactions of the hematologic system, including medically significant cytopenia (e.g., leukopenia, pancytopenia, thrombocytopenia) have been infrequently reported with certolizumab. The causal relationship of these events to certolizumab remains unclear.
- Although no high risk group has been identified, exercise caution in patients being treated with certolizumab who have ongoing, or a history of, significant hematologic abnormalities. Advise all patients to seek immediate medical attention if they develop signs and symptoms suggestive of blood dyscrasias or infection (e.g., persistent fever, bruising, bleeding, pallor) while on certolizumab. Consider discontinuation of certolizumab therapy in patients with confirmed significant hematologic abnormalities.
Use with Biological Disease-Modifying Antirheumatic Drugs (Biological DMARDs)
- Serious infections were seen in clinical studies with concurrent use of anakinra (an interleukin-1 antagonist) and another TNF blocker, etanercept, with no added benefit compared to etanercept alone. A higher risk of serious infections was also observed in combination use of TNF blockers with abatacept and rituximab. Because of the nature of the adverse events seen with this combination therapy, similar toxicities may also result from the use of certolizumab in this combination. Therefore, the use of certolizumab in combination with other biological DMARDs is not recommended.
Autoimmunity
- Treatment with certolizumab may result in the formation of autoantibodies and rarely, in the development of a lupus-like syndrome. If a patient develops symptoms suggestive of a lupus-like syndrome following treatment with certolizumab, discontinue treatment.
Immunizations
- Patients treated with certolizumab may receive vaccinations, except for live or live attenuated vaccines. No data are available on the response to live vaccinations or the secondary transmission of infection by live vaccines in patients receiving certolizumab.
- In a placebo-controlled clinical trial of patients with rheumatoid arthritis, no difference was detected in antibody response to vaccine between certolizumab and placebo treatment groups when the pneumococcal polysaccharide vaccine and influenza vaccine were administered concurrently with certolizumab. Similar proportions of patients developed protective levels of anti-vaccine antibodies between certolizumab and placebo treatment groups; however patients receiving certolizumab and concomitant methotrexate had a lower humoral response compared with patients receiving certolizumab alone. The clinical significance of this is unknown.
Immunosuppression
- Since TNF mediates inflammation and modulates cellular immune responses, the possibility exists for TNF blockers, including certolizumab, to affect host defenses against infections and malignancies. The impact of treatment with certolizumab on the development and course of malignancies, as well as active and/or chronic infections, is not fully understood. The safety and efficacy of certolizumab in patients with immunosuppression has not been formally evaluated.
Adverse Reactions
Clinical Trials Experience
Clinical Trials Experience
- The most serious adverse reactions were:
- Serious Infections
- Malignancies
- Heart Failure
- Because clinical studies are conducted under widely varying and controlled conditions, adverse reaction rates observed in clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug, and may not predict the rates observed in a broader patient population in clinical practice.
- In premarketing controlled trials of all patient populations combined the most common adverse reactions (≥ 8%) were upper respiratory infections (18%), rash (9%) and urinary tract infections (8%).
- Adverse Reactions Most Commonly Leading to Discontinuation of Treatment in Premarketing Controlled Trials
- The proportion of patients with Crohn's disease who discontinued treatment due to adverse reactions in the controlled clinical studies was 8% for certolizumab and 7% for placebo. The most common adverse reactions leading to the discontinuation of certolizumab (for at least 2 patients and with a higher incidence than placebo) were abdominal pain (0.4% certolizumab, 0.2% placebo), diarrhea (0.4% certolizumab, 0% placebo), and intestinal obstruction (0.4% certolizumab, 0% placebo).
- The proportion of patients with rheumatoid arthritis who discontinued treatment due to adverse reactions in the controlled clinical studies was 5% for certolizumab and 2.5% for placebo. The most common adverse reactions leading to discontinuation of certolizumab were tuberculosis infections (0.5%); and pyrexia, urticaria, pneumonia, and rash (0.3%).
Controlled Studies with Crohn's Disease
- The data described below reflect exposure to certolizumab at 400 mg subcutaneous dosing in studies of patients with Crohn's disease. In the safety population in controlled studies, a total of 620 patients with Crohn's disease received certolizumab at a dose of 400 mg, and 614 subjects received placebo (including subjects randomized to placebo in Study CD2 following open label dosing of certolizumab at Weeks 0, 2, 4). In controlled and uncontrolled studies, 1,564 patients received certolizumab at some dose level, of whom 1,350 patients received 400 mg certolizumab. Approximately 55% of subjects were female, 45% were male, and 94% were Caucasian. The majority of patients in the active group were between the ages of 18 and 64.
- During controlled clinical studies, the proportion of patients with serious adverse reactions was 10% for certolizumab and 9% for placebo. The most common adverse reactions (occurring in ≥ 5% of certolizumab-treated patients, and with a higher incidence compared to placebo) in controlled clinical studies with certolizumab were upper respiratory infections (e.g. nasopharyngitis, laryngitis, viral infection) in 20% of certolizumab-treated patients and 13% of placebo-treated patients, urinary tract infections (e.g. bladder infection, bacteriuria, cystitis) in 7% of certolizumab-treated patients and in 6% of placebo-treated patients, and arthralgia (6% certolizumab, 4% placebo).
Other Adverse Reactions
- The most commonly occurring adverse reactions in controlled trials of Crohn's disease were described above. Other serious or significant adverse reactions reported in controlled and uncontrolled studies in Crohn's disease and other diseases, occurring in patients receiving certolizumab at doses of 400 mg or other doses include:
- Blood and lymphatic system disorders: Anemia, leukopenia, lymphadenopathy, pancytopenia, and thrombophilia.
- Cardiac disorders: Angina pectoris, arrhythmias, atrial fibrillation, cardiac failure, hypertensive heart disease, myocardial infarction, myocardial ischemia, pericardial effusion, pericarditis, stroke and transient ischemic attack.
- Eye disorders: Optic neuritis, retinal hemorrhage, and uveitis.
- General disorders and administration site conditions: Bleeding and injection site reactions.
- Hepatobiliary disorders: Elevated liver enzymes and hepatitis.
- Immune system disorders: Alopecia totalis.
- Psychiatric disorders: Anxiety, bipolar disorder, and suicide attempt.
- Renal and urinary disorders: Nephrotic syndrome and renal failure.
- Reproductive system and breast disorders: Menstrual disorder
- Skin and subcutaneous tissue disorders: Dermatitis, erythema nodosum, and urticaria.
- Vascular disorders: Thrombophlebitis, vasculitis.
- Controlled Studies with Rheumatoid Arthritis.
- Certolizumab was studied primarily in placebo-controlled trials and in long-term follow-up studies. The data described below reflect the exposure to certolizumab in 2,367 RA patients, including 2,030 exposed for at least 6 months, 1,663 exposed for at least one year and 282 for at least 2 years; and 1,774 in adequate and well-controlled studies. In placebo-controlled studies, the population had a median age of 53 years at entry; approximately 80% were females, 93% were Caucasian and all patients were suffering from active rheumatoid arthritis, with a median disease duration of 6.2 years. Most patients received the recommended dose of certolizumab or higher.
- Table 1 summarizes the reactions reported at a rate of at least 3% in patients treated with certolizumab 200 mg every other week compared to placebo (saline formulation), given concomitantly with methotrexate.
- Hypertensive adverse reactions were observed more frequently in patients receiving CIMZIA than in controls. These adverse reactions occurred more frequently among patients with a baseline history of hypertension and among patients receiving concomitant corticosteroids and non-steroidal anti-inflammatory drugs.
- Patients receiving certolizumab 400 mg as monotherapy every 4 weeks in rheumatoid arthritis controlled clinical trials had similar adverse reactions to those patients receiving certolizumab 200 mg every other week.
Other Adverse Reactions
- Other infrequent adverse reactions (occurring in less than 3% of RA patients) were similar to those seen in Crohn's disease patients.
Psoriatic Arthritis Clinical Study
- Certolizumab has been studied in 409 patients with psoriatic arthritis (PsA) in a placebo-controlled trial. The safety profile for patients with PsA treated with certolizumab was similar to the safety profile seen in patients with RA and previous experience with certolizumab.
Ankylosing Spondylitis Clinical Study
- Certolizumab has been studied in 325 patients with axial spondyloarthritis of whom the majority had ankylosing spondylitis (AS) in a placebo-controlled study (AS-1). The safety profile for patients in study AS-1 treated with certolizumab was similar to the safety profile seen in patients with RA.
Infections
- The incidence of infections in controlled studies in Crohn's disease was 38% for certolizumab-treated patients and 30% for placebo-treated patients. The infections consisted primarily of [upper respiratory infections]] (20% for certolizumab, 13% for placebo). The incidence of serious infections during the controlled clinical studies was 3% per patient-year for certolizumab-treated patients and 1% for placebo-treated patients. Serious infections observed included bacterial and viral infections, pneumonia, and pyelonephritis.
- The incidence of new cases of infections in controlled clinical studies in rheumatoid arthritis was 0.91 per patient-year for all certolizumab-treated patients and 0.72 per patient-year for placebo-treated patients. The infections consisted primarily of upper respiratory tract infections, herpes infections, urinary tract infections, and lower respiratory tract infections. In the controlled rheumatoid arthritis studies, there were more new cases of serious infection adverse reactions in the certolizumab treatment groups, compared to the placebo groups (0.06 per patient-year for all certolizumab doses vs. 0.02 per patient-year for placebo). Rates of serious infections in the 200 mg every other week dose group were 0.06 per patient-year and in the 400 mg every 4 weeks dose group were 0.04 per patient-year. Serious infections included tuberculosis, pneumonia, cellulitis, and pyelonephritis. In the placebo group, no serious infection occurred in more than one subject. There is no evidence of increased risk of infections with continued exposure over time.
Tuberculosis and Opportunistic Infections
- In completed and ongoing global clinical studies in all indications including 5,118 certolizumab-treated patients, the overall rate of tuberculosis is approximately 0.61 per 100 patient-years across all indications.
- The majority of cases occurred in countries with high endemic rates of TB. Reports include cases of miliary, lymphatic, peritoneal, as well as pulmonary TB. The median time to onset of TB for all patients exposed to certolizumab across all indications was 345 days. In the studies with certolizumab in RA, there were 36 cases of TB among 2,367 exposed patients, including some fatal cases. Rare cases of opportunistic infections have also been reported in these clinical trials.
Malignancies
- In clinical studies of certolizumab, the overall incidence rate of malignancies was similar for certolizumab-treated and control patients. For some TNF blockers, more cases of malignancies have been observed among patients receiving those TNF blockers compared to control patients.
Heart Failure
- In placebo-controlled and open-label rheumatoid arthritis studies, cases of new or worsening heart failure have been reported for certolizumab-treated patients. The majority of these cases were mild to moderate and occurred during the first year of exposure.
Autoantibodies
- In clinical studies in Crohn's disease, 4% of patients treated with certolizumab and 2% of patients treated with placebo that had negative baseline ANA titers developed positive titers during the studies. One of the 1,564 Crohn's disease patients treated with certolizumab developed symptoms of a lupus-like syndrome.
- In clinical trials of TNF blockers, including certolizumab, in patients with RA, some patients have developed ANA. Four patients out of 2,367 patients treated with certolizumab in RA clinical studies developed clinical signs suggestive of a lupus-like syndrome. The impact of long-term treatment with certolizumab on the development of autoimmune diseases is unknown.
Immunogenicity
- Patients were tested at multiple time points for antibodies to certolizumab pegol during Studies CD1 and CD2. The overall percentage of antibody positive patients was 8% in patients continuously exposed to certolizumab, approximately 6% were neutralizing in vitro. No apparent correlation of antibody development to adverse events or efficacy was observed. Patients treated with concomitant immunosuppressants had a lower rate of antibody development than patients not taking immunosuppressants at baseline (3% and 11%, respectively). The following adverse events were reported in Crohn's disease patients who were antibody-positive (N = 100) at an incidence at least 3% higher compared to antibody-negative patients (N = 1,242): abdominal pain, arthralgia, edema peripheral, erythema nodosum, injection site erythema, injection site pain, pain in extremity, and upper respiratory tract infection.
- The overall percentage of patients with antibodies to certolizumab pegol detectable on at least one occasion was 7% (105 of 1,509) in the rheumatoid arthritis placebo-controlled trials. Approximately one third (3%, 39 of 1,509) of these patients had antibodies with neutralizing activity in vitro. Patients treated with concomitant immunosuppressants (MTX) had a lower rate of antibody development than patients not taking immunosuppressants at baseline. Patients treated with concomitant immunosuppressant therapy (MTX) in RA-I, RA-II, RA-III had a lower rate of neutralizing antibody formation overall than patients treated with certolizumab monotherapy in RA-IV (2% vs. 8%). Both the loading dose of 400 mg every other week at Weeks 0, 2 and 4 and concomitant use of MTX were associated with reduced immunogenicity.
- Antibody formation was associated with lowered drug plasma concentration and reduced efficacy. In patients receiving the recommended certolizumab dosage of 200 mg every other week with concomitant MTX, the ACR20 response was lower among antibody positive patients than among antibody-negative patients (Study RA-I, 48% versus 60%; Study RA-II 35% versus 59%, respectively). In Study RA-III, too few patients developed antibodies to allow for meaningful analysis of ACR20 response by antibody status. In Study RA-IV (monotherapy), the ACR20 response was 33% versus 56%, antibody-positive versus antibody-negative status, respectively. No association was seen between antibody development and the development of adverse events.
- The data reflect the percentage of patients whose test results were considered positive for antibodies to certolizumab pegol in an ELISA, and are highly dependent on the sensitivity and specificity of the assay. The observed incidence of antibody (including neutralizing antibody) positivity in an assay is highly dependent on several factors, including assay sensitivity and specificity, assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to certolizumab pegol with the incidence of antibodies to other products may be misleading.
Hypersensitivity Reactions
- The following symptoms that could be compatible with hypersensitivity reactions have been reported rarely following certolizumab administration to patients: angioedema, dermatitis allergic, dizziness (postural), dyspnea, hot flush, hypotension, injection site reactions, malaise, pyrexia, rash, serum sickness, and (vasovagal) syncope.
Postmarketing Experience
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of CIMZIA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate reliably their frequency or establish a causal relationship to drug exposure.
- Vascular disorder: systemic vasculitis has been identified during post-approval use of TNF blockers.
- Skin: case of severe skin reactions, including Stevens-Johnson syndrome, toxic epidermal necrolysis, erythema multiforme, and new or worsening psoriasis (all sub-types including pustular and palmoplantar) have been identified during post-approval use of TNF blockers.
- Immune System Disorders: Sarcoidosis
Drug Interactions
Use with Anakinra, Abatacept, Rituximab, and Natalizumab
- An increased risk of serious infections has been seen in clinical studies of other TNF-blocking agents used in combination with anakinra or abatacept, with no added benefit. Formal drug interaction studies have not been performed with rituximab or natalizumab. Because of the nature of the adverse events seen with these combinations with TNF blocker therapy, similar toxicities may also result from the use of certolizumab in these combinations. There is not enough information to assess the safety and efficacy of such combination therapy. Therefore, the use of certolizumab in combination with anakinra, abatacept, rituximab, or natalizumab is not recommended.
Live Vaccines
- Avoid use of live (including attenuated) vaccines concurrently with certolizumab.
Laboratory Tests
- Interference with certain coagulation assays has been detected in patients treated with CIMZIA. Certolizumab pegol may cause erroneously elevated activated partial thromboplastin time (aPTT) assay results in patients without coagulation abnormalities. This effect has been observed with the PTT-Lupus Anticoagulant (LA) test and Standard Target Activated Partial Thromboplastin time (STA-PTT) Automate tests from Diagnostica Stago, and the HemosIL APTT-SP liquid and HemosIL lyophilized silica tests from Instrumentation Laboratories. Other aPTT assays may be affected as well. Interference with thrombin time (TT) and prothrombin time (PT) assays has not been observed. There is no evidence that certolizumab therapy has an effect on in vivo coagulation.
Use in Specific Populations
Pregnancy
Risk Summary
- Adequate and well-controlled studies with certolizumab have not been conducted in pregnant women.
- Certolizumab pegol plasma concentrations obtained from 10 women treated with certolizumab during pregnancy and their newborn infants demonstrated low placental transfer of certolizumab pegol. Certolizumab may be eliminated at a slower rate in exposed infants than in adult patients. No fetal harm was observed in animal reproduction studies. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
- There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to certolizumab during pregnancy. To enroll, healthcare providers or patients can call 1-877-311-8972.
Human Data
- In an independent clinical study conducted in 10 pregnant women with Crohn´s disease treated with certolizumab, certolizumab pegol concentrations were measured in maternal blood as well as in cord and infant blood (n=12) at the day of birth. The last dose of certolizumab (400 mg for every mother) was given on average 19 days prior to delivery (range 5-42 days). Plasma certolizumab pegol concentrations were <0.41 –1.66 μg/mL in cord blood, <0.41 – 1.58 μg/mL in infant blood, and 1.87–59.57 μg/mL in maternal blood. Plasma certolizumab pegol concentrations were lower (by at least 75%) in the infants than in mothers suggesting low placental transfer of certolizumab pegol. In one infant, the plasma certolizumab pegol concentration declined from 1.02 to 0.84 μg /mL over 4 weeks suggesting that certolizumab may be eliminated at a slower rate in infants than adults.
Animal Data
- Because certolizumab pegol does not cross-react with mouse or rat TNFα, reproduction studies were performed in rats using a rodent anti-murine TNFα pegylated Fab' fragment (cTN3 PF) similar to certolizumab pegol. Reproduction studies have been performed in rats at doses up to 100 mg/kg and have revealed no evidence of impaired fertility or harm to the fetus due to cTN3 PF.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Certolizumab pegol in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Certolizumab pegol during labor and delivery.
Nursing Mothers
- It is not known whether certolizumab pegol is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from certolizumab, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established. Due to its inhibition of TNFα, certolizumab administered during pregnancy could affect immune responses in the in utero-exposed newborn and infant. Although certolizumab pegol levels were low in 12 infants exposed to certolizumab in utero, the clinical significance of these low levels is unknown. Additional data available from one exposed infant suggests that certolizumab may be eliminated at a slower rate in infants than in adults. The safety of administering live or live-attenuated vaccines in exposed infants is unknown. Risks and benefits should be considered prior to vaccinating (live or live-attenuated) exposed infants.
Geriatic Use
- Clinical studies of certolizumab did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. Population pharmacokinetic analyses of patients enrolled in certolizumab clinical studies concluded that there was no apparent difference in drug concentration regardless of age. Because there is a higher incidence of infections in the elderly population in general, use caution when treating the elderly with certolizumab.
Gender
There is no FDA guidance on the use of Certolizumab pegol with respect to specific gender populations.
Race
There is no FDA guidance on the use of Certolizumab pegol with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Certolizumab pegol in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Certolizumab pegol in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Certolizumab pegol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Certolizumab pegol in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Monitoring of Certolizumab pegol in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Certolizumab pegol in the drug label.
Overdosage
- The maximum tolerated dose of certolizumab pegol has not been established. Doses of up to 800 mg subcutaneous and 20 mg/kg intravenous have been administered without evidence of dose-limiting toxicities. In cases of overdosage, it is recommended that patients be monitored closely for any adverse reactions or effects, and appropriate symptomatic treatment instituted immediately.
Pharmacology
Certolizumab pegol?
| |
| Therapeutic monoclonal antibody | |
| Source | zu/o |
| Target | TNF alpha |
| Identifiers | |
| CAS number | |
| ATC code | L04 |
| PubChem | ? |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 47.75 kDa |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
B(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | Subcutaneous |
Mechanism of Action
- Certolizumab (certolizumab pegol) is a TNF blocker. Certolizumab is a recombinant, humanized antibody Fab' fragment, with specificity for human tumor necrosis factor alpha (TNFα), conjugated to an approximately 40kDa polyethylene glycol (PEG2MAL40K). The Fab' fragment is manufactured in E. coli and is subsequently subjected to purification and conjugation to PEG2MAL40K, to generate certolizumab pegol. The Fab' fragment is composed of a light chain with 214 amino acids and a heavy chain with 229 amino acids. The molecular weight of certolizumab pegol is approximately 91 kiloDaltons.
- Certolizumab is supplied as either a sterile, white, lyophilized powder for solution or as a sterile, solution in a single-use prefilled 1 mL glass syringe for subcutaneous injection. After reconstitution of the lyophilized powder with 1 mL sterile Water for Injection, USP, the resulting pH is approximately 5.2. Each single-use vial provides approximately 200 mg certolizumab pegol, 0.9 mg lactic acid, 0.1 mg polysorbate, and 100 mg sucrose.
- Each single-use prefilled syringe of certolizumab delivers 200 mg in 1 mL of solution with a pH of approximately 4.7 for subcutaneous use. Each 1 mL syringe of certolizumab contains certolizumab pegol (200 mg), sodium acetate (1.36 mg), sodium chloride (7.31 mg), and Water for Injection, USP.
- Certolizumab is a clear to opalescent solution that is colorless to pale yellow and essentially free from particulates. No preservatives are present.
- Certolizumab pegol binds to human TNFα with a KD of 90pM. TNFα is a key pro-inflammatory cytokine with a central role in inflammatory processes. Certolizumab pegol selectively neutralizes TNFα (IC90 of 4 ng/mL for inhibition of human TNFα in the in vitro L929 murine fibrosarcoma cytotoxicity assay) but does not neutralize lymphotoxin α (TNFβ). Certolizumab pegol cross-reacts poorly with TNF from rodents and rabbits, therefore in vivo efficacy was evaluated using animal models in which human TNFα was the physiologically active molecule.
- Certolizumab pegol was shown to neutralize membrane-associated and soluble human TNFα in a dose-dependent manner. Incubation of monocytes with certolizumab pegol resulted in a dose-dependent inhibition of LPS-induced TNFα and IL-1β production in human monocytes.
- Certolizumab pegol does not contain a fragment crystallizable (Fc) region, which is normally present in a complete antibody, and therefore does not fix complement or cause antibody-dependent cell-mediated cytotoxicity in vitro. It does not induce apoptosis in vitro in human peripheral blood-derived monocytes or lymphocytes, nor does certolizumab pegol induce neutrophil degranulation.
- A tissue reactivity study was carried out ex vivo to evaluate potential cross-reactivity of certolizumab pegol with cryosections of normal human tissues. Certolizumab pegol showed no reactivity with a designated standard panel of normal human tissues.
Structure
- Certolizumab (certolizumab pegol) is a TNF blocker. certolizumab is a recombinant, humanized antibody Fab' fragment, with specificity for human tumor necrosis factor alpha (TNFα), conjugated to an approximately 40kDa polyethylene glycol (PEG2MAL40K). The Fab' fragment is manufactured in E. coli and is subsequently subjected to purification and conjugation to PEG2MAL40K, to generate certolizumab pegol. The Fab' fragment is composed of a light chain with 214 amino acids and a heavy chain with 229 amino acids. The molecular weight of certolizumab pegol is approximately 91 kiloDaltons.
- Certolizumab is supplied as either a sterile, white, lyophilized powder for solution or as a sterile, solution in a single-use prefilled 1 mL glass syringe for subcutaneous injection. After reconstitution of the lyophilized powder with 1 mL sterile Water for Injection, USP, the resulting pH is approximately 5.2. Each single-use vial provides approximately 200 mg certolizumab pegol, 0.9 mg lactic acid, 0.1 mg polysorbate, and 100 mg sucrose.
- Each single-use prefilled syringe of certolizumab delivers 200 mg in 1 mL of solution with a pH of approximately 4.7 for subcutaneous use. Each 1 mL syringe of certolizumab contains certolizumab pegol (200 mg), sodium acetate (1.36 mg), sodium chloride (7.31 mg), and Water for Injection, USP.
- certolizumab is a clear to opalescent solution that is colorless to pale yellow and essentially free from particulates. No preservatives are present.
Pharmacodynamics
- Certolizumab pegol binds to human TNFα with a KD of 90pM. TNFα is a key pro-inflammatory cytokine with a central role in inflammatory processes. Certolizumab pegol selectively neutralizes TNFα (IC90 of 4 ng/mL for inhibition of human TNFα in the in vitro L929 murine fibrosarcoma cytotoxicity assay) but does not neutralize lymphotoxin α (TNFβ). Certolizumab pegol cross-reacts poorly with TNF from rodents and rabbits, therefore in vivo efficacy was evaluated using animal models in which human TNFα was the physiologically active molecule.
- Certolizumab pegol was shown to neutralize membrane-associated and soluble human TNFα in a dose-dependent manner. Incubation of monocytes with certolizumab pegol resulted in a dose-dependent inhibition of LPS-induced TNFα and IL-1β production in human monocytes.
- Certolizumab pegol does not contain a fragment crystallizable (Fc) region, which is normally present in a complete antibody, and therefore does not fix complement or cause antibody-dependent cell-mediated cytotoxicity in vitro. It does not induce apoptosis in vitro in human peripheral blood-derived monocytes or lymphocytes, nor does certolizumab pegol induce neutrophil degranulation.
- A tissue reactivity study was carried out ex vivo to evaluate potential cross-reactivity of certolizumab pegol with cryosections of normal human tissues. Certolizumab pegol showed no reactivity with a designated standard panel of normal human tissues.
Pharmacokinetics
Absorption
- A total of 126 healthy subjects received doses of up to 800 mg certolizumab pegol subcutaneously (sc) and up to 10 mg/kg intravenously (IV) in four pharmacokinetic studies. Data from these studies demonstrate that single intravenous and subcutaneous doses of certolizumab pegol have predictable dose-related plasma concentrations with a linear relationship between the dose administered and the maximum plasma concentration (Cmax), and the Area Under the certolizumab pegol plasma concentration versus time Curve (AUC). A mean Cmax of approximately 43 to 49 mcg/mL occurred at Week 5 during the initial loading dose period using the recommended dose regimen for the treatment of patients with rheumatoid arthritis (400 mg sc at Weeks 0, 2 and 4 followed by 200 mg every other week).
- Certolizumab pegol plasma concentrations were broadly dose-proportional and pharmacokinetics observed in patients with rheumatoid arthritis and Crohn's disease were consistent with those seen in healthy subjects.
- Following subcutaneous administration, peak plasma concentrations of certolizumab pegol were attained between 54 and 171 hours post-injection. Certolizumab pegol has bioavailability (F) of approximately 80% (ranging from 76% to 88%) following subcutaneous administration compared to intravenous administration.
Distribution
- The steady state volume of distribution (Vss) was estimated as 6 to 8 L in the population pharmacokinetic analysis for patients with Crohn's disease and patients with rheumatoid arthritis.
Metabolism
- The metabolism of certolizumab pegol has not been studied in human subjects. Data from animals indicate that once cleaved from the Fab' fragment the PEG moiety is mainly excreted in urine without further metabolism.
Elimination
- PEGylation, the covalent attachment of PEG polymers to peptides, delays the metabolism and elimination of these entities from the circulation by a variety of mechanisms, including decreased renal clearance, proteolysis, and immunogenicity.
- Accordingly, certolizumab pegol is an antibody Fab' fragment conjugated with PEG in order to extend the terminal plasma elimination half-life (t1/2) of the Fab'. The terminal elimination phase half-life (t1/2) was approximately 14 days for all doses tested. The clearance following IV administration to healthy subjects ranged from 9.21 mL/h to 14.38 mL/h. The clearance following sc dosing was estimated 17 mL/h in the Crohn's disease population PK analysis with an inter-subject variability of 38% (CV) and an inter-occasion variability of 16%. Similarly, the clearance following sc dosing was estimated as 21.0 mL/h in the RA population PK analysis, with an inter-subject variability of 30.8% (%CV) and inter-occasion variability 22.0%. The route of elimination of certolizumab pegol has not been studied in human subjects. Studies in animals indicate that the major route of elimination of the PEG component is via urinary excretion.
Special Populations
- Population pharmacokinetic analysis was conducted on data from patients with rheumatoid arthritis and patients with Crohn's disease, to evaluate the effect of age, race, gender, methotrexate use, concomitant medication, creatinine clearance and presence of anti-certolizumab antibodies on pharmacokinetics of certolizumab pegol.
- Only bodyweight and presence of anti-certolizumab antibodies significantly affected certolizumab pegol pharmacokinetics. Pharmacokinetic exposure was inversely related to body weight but pharmacodynamic exposure-response analysis showed that no additional therapeutic benefit would be expected from a weight-adjusted dose regimen. The presence of anti-certolizumab antibodies was associated with a 3.6-fold increase in clearance.
- Age: Pharmacokinetics of certolizumab pegol was not different in elderly compared to young adults.
- Gender: Pharmacokinetics of certolizumab pegol was similar in male and female subjects.
- Renal Impairment: Specific clinical studies have not been performed to assess the effect of renal impairment on the pharmacokinetics of certolizumab. The pharmacokinetics of the PEG (polyethylene glycol) fraction of certolizumab pegol is expected to be dependent on renal function but has not been assessed in renal impairment. There are insufficient data to provide a dosing recommendation in moderate and severe renal impairment.
- Race: A specific clinical study showed no difference in pharmacokinetics between Caucasian and Japanese subjects.
Drug Interaction Studies
- Methotrexate pharmacokinetics is not altered by concomitant administration with certolizumab in patients with rheumatoid arthritis. The effect of methotrexate on certolizumab pharmacokinetics was not studied. However, methotrexate-treated patients have lower incidence of antibodies to certolizumab. Thus, therapeutic plasma levels are more likely to be sustained when certolizumab is administered with methotrexate in patients with rheumatoid arthritis.
- Formal drug-drug interaction studies have not been conducted with certolizumab upon concomitant administration with corticosteroids, nonsteroidal anti-inflammatory drugs, analgesics or immunosuppressants.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, and Impairment of Fertility
- Long-term animal studies of certolizumab have not been conducted to assess its carcinogenic potential. Certolizumab pegol was not genotoxic in the Ames test, the human peripheral blood lymphocytes chromosomal aberration assay, or the mouse bone marrow micronucleus assay.
- Since certolizumab pegol does not cross-react with mouse or rat TNFα, reproduction studies were performed in rats using a rodent anti-murine TNFα pegylated Fab fragment (cTN3 PF), similar to certolizumab pegol. The cTN3 PF had no effects on the fertility and general reproductive performance of male and female rats at intravenous doses up 100 mg/kg, administered twice weekly.
Clinical Studies
Crohn's Disease
- The efficacy and safety of certolizumab were assessed in two double-blind, randomized, placebo-controlled studies in patients aged 18 years and older with moderately to severely active Crohn's disease, as defined by a Crohn's Disease Activity Index (CDAI1) of 220 to 450 points, inclusive. certolizumab was administered subcutaneously at a dose of 400 mg in both studies. Stable concomitant medications for Crohn's disease were permitted.
Study CD1
- Study CD1 was a randomized placebo-controlled study in 662 patients with active Crohn's disease. Certolizumab or placebo was administered at Weeks 0, 2, and 4 and then every four weeks to Week 24. Assessments were done at Weeks 6 and 26. Clinical response was defined as at least a 100-point reduction in CDAI score compared to baseline, and clinical remission was defined as an absolute CDAI score of 150 points or lower.
- The results for Study CD1 are provided in Table 2. At Week 6, the proportion of clinical responders was statistically significantly greater for certolizumab-treated patients compared to controls. The difference in clinical remission rates was not statistically significant at Week 6. The difference in the proportion of patients who were in clinical response at both Weeks 6 and 26 was also statistically significant, demonstrating maintenance of clinical response.
How Supplied
- Refrigerate intact carton between 2 to 8 °C (36 to 46 °F)
Storage
- Do not freeze. Do not separate contents of carton prior to use. Do not use beyond expiration date, which is located on the drug label and carton. Protect solution from light.
Images
Drug Images
{{#ask: Page Name::Certolizumab pegol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

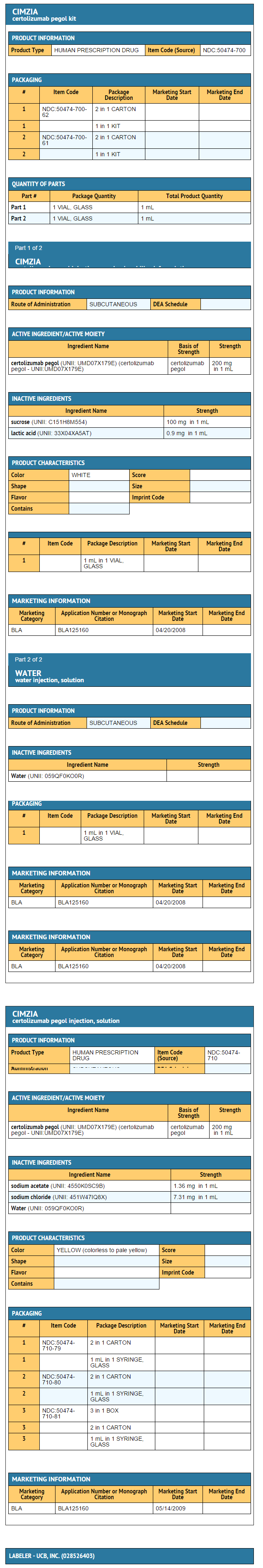
{{#ask: Label Page::Certolizumab pegol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Patient Counseling
- Advise patients of the potential risks and benefits of certolizumab therapy. Be sure that patients receive the Medication Guide and allow them time to read it prior to starting certolizumab therapy and to review it periodically. Any questions resulting from the patient's reading of the Medication Guide should be discussed. Because caution should be exercised in prescribing certolizumab to patients with clinically important active infections, advise patients of the importance of informing their health care providers about all aspects of their health.
Immunosuppression
- Inform patients that certolizumab may lower the ability of the immune system to fight infections. Instruct patients of the importance of contacting their doctor if they develop any symptoms of infection, including tuberculosis and reactivation of hepatitis B virus infections.
- Counsel patients about the possible risk of lymphoma and other malignancies while receiving certolizumab.
Allergic Reactions
- Advise patients to seek immediate medical attention if they experience any symptoms of severe allergic reactions. The prefilled syringe components do not contain any latex or dry natural rubber.
Other Medical Conditions
- Advise patients to report any signs of new or worsening medical conditions such as heart disease, neurological disease, or autoimmune disorders. Advise patients to report promptly any symptoms suggestive of a cytopenia such as bruising, bleeding, or persistent fever.
Instruction on Prefilled Syringe Self-Injection Technique
- After proper training by a qualified healthcare professional in subcutaneous injection technique, a patient may self inject with certolizumab using the Prefilled Syringe if a healthcare provider determines that it is appropriate. A patient's ability to administer certolizumab subcutaneous injections should be checked to ensure correct administration. Suitable sites for injection include the thigh or abdomen. Certolizumab should be injected when the liquid is at room temperature.
- Full injection instructions are provided in the Instructions for Use booklet for the Prefilled Syringe, packaged in each certolizumab Prefilled Syringe kit.
- To avoid needle-stick injury, patients and healthcare providers should not attempt to place the needle cover back on the syringe or otherwise recap the needle. Be sure to properly dispose of needles and syringes in a puncture-proof container, and instruct patients and caregivers in proper syringe and needle disposal technique. Actively discourage any reuse of the injection materials.
Precautions with Alcohol
- Alcohol-Certolizumab pegol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- CIMZIA®[1]
Look-Alike Drug Names
There is limited information regarding Certolizumab pegol Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Certolizumab pegol
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Certolizumab pegol |Label Name=Certolizumab pegol11.png
}}
{{#subobject:
|Label Page=Certolizumab pegol |Label Name=Certolizumab pegol11.png
}}