Infra-Hisian Block
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor-In-Chief: Sara Mohsin, M.D.[2]
Overview
Infra-Hisian blocks are defined as impaired conduction in the electrical system of the heart that occur below the AV node.
Historical perspective
- In 1899, Dr. Wenckebach described progressive delay between atrial and ventricular contraction and the eventual failure of a P wave to reach the ventricles.
- Dr. Mobitz then divided the second degree AV block into two subtypes.
- In 1905, Dr. John Hay discovered the second degree of AV block.[1]
- Dr. Hay was examining a patient who complains of slow pulse and dyspnea on exertion for more than 2 years. Dr. Hay noticed the heart rate dropping from 80 beats to 40 beats per minute.
- Dr. Hay noted the a waves and the arterial pulse to remain stable in the beginning. However, recording pulsation for several times resulted in "a" waves that were not followed by c wave. The a-c jugular wave interval was used as a measurement of AV conduction.
- Dr. Hay figured out that the pause following a wave was due to failure of ventricular muscles to respond to a stimulus.
Classification
Infrahisian block describes block of the distal conduction system. Types of infrahisian block include:
Of these types of infrahisian block, Mobitz II heart block is considered most important because of the possible progression to complete heart block.
Pathophysiology
Mobitz type II second degree AV block Mobitz type II second degree AV block, in which the PR interval remains unchanged prior to a P wave that fails to conduct to the ventricles. It almost always results from conduction system disease below the level of the AV node, occurring in the bundle of His in approximately 20 percent of cases and in the bundle branches in the remainder. Patients with bundle branch involvement also have axis shifts and QRS widening depending upon the location of the block. In addition, at least two-thirds of patients with this disorder also have bifascicular or even trifascicular disease. Mobitz type I and Mobitz type II second degree AV block cannot be differentiated from the ECG when 2:1 AV block is present. In this situation, every other P wave is non-conducted and there is no opportunity to observe for the constant PR interval that is characteristic of Mobitz type II second degree AV block.
Type 2 Mobitz II
Conduction delay in Mobitz type II second degree block is almost always infra-nodal (His bundle [20%], bundle branches or fascicles). Usually the morphology of the QRS complex is wide, except when the site of block is the His bundle. In this variant of second degree heart block the PR interval is constant with occasional dropped beats as compared to the gradually prolonging PR interval in Mobitz type I. Bifascicular or trifascicular disease is seen in two thirds of the patients with Mobitz type II.[2][3]
- Type 2 second degree AV block, also known as Mobitz II is almost always a disease of the distal conduction system (His-Purkinje System).
- Although the terms infranodal block or infrahisian block are often applied to this disorder, they are not synonymous with it.
- Infranodal block and infra-Hisian block are terms which refer to the anatomic location of the block, whereas
- Mobitz II refers to an electrocardiographic pattern associated with block at these levels[4].
- Mobitz II heart block is characterized on a surface ECG by intermittently non-conducted P waves not preceded by PR prolongation and not followed by PR shortening. The medical significance of this type of AV block is that it may progress rapidly to complete heart block, in which no escape rhythm may emerge. In this case, the person may experience a Stokes-Adams attack, cardiac arrest, or sudden cardiac death. The definitive treatment for this form of AV Block is an implanted pacemaker[5][6].
Causes
The potential etiologies of Mobitz type II second degree AV block include reversible (both pathologic and iatrogenic) and idiopathic causes that are similar to other degrees of AV block (table 1). Common potentially reversible causes include:
●Pathologic – Myocardial ischemia (acute or chronic) involving the conduction system, cardiomyopathy (eg, amyloidosis, sarcoidosis), myocarditis (eg, Lyme disease), endocarditis with abscess formation, hyperkalemia, and hypervagotonia.
●Iatrogenic – Medication-related (AV nodal blocking medications), post-cardiac surgery, post-catheter ablation, post-transcatheter aortic valve implantation.
Mobitz type II second degree AV block is rarely seen in patients without underlying heart disease. When identifiable, the reversible causes most commonly associated with Mobitz type II second degree AV block are myocardial infarction with ischemia of the AV node and medications that alter conduction through the AV node (eg, digoxin, beta blockers, calcium channel blockers). When no specific reversible cause is identified, the block is often felt to be related to idiopathic progressive cardiac conduction disease with myocardial fibrosis and/or sclerosis that affects the conduction system.
| Physiologic and pathophysiologic | |
|---|---|
| Increased vagal tone | |
| Ischemic heart disease, including acute myocardial infarction | |
| Progressive cardiac conduction system disease | With fibrosis and/or sclerosis (Lenegre disease) |
| With calcification (Lev disease) | |
| Infections (eg, viral myocarditis, Lyme carditis) | |
| Cardiomyopathy | Infiltrative processes (eg, sarcoidosis, amyloidosis, hemochromatosis, malignancy, etc) |
| Other non-ischemic cardiomyopathies (eg, idiopathic, infectious, etc) | |
| Congenital AV block | Related to structural congenital heart disease |
| As part of neonatal lupus syndrome | |
| Other | Hyperkalemia |
| severe hypo- or hyperthyroidism | |
| trauma | |
| degenerative neuromuscular diseases | |
| Iatrogenic | |
| Drugs | Beta blockers |
| calcium channel blockers | |
| digoxin | |
| antiarrhythmic drugs | |
| adenosine | |
| Transcatheter aortic valve implantation | |
| Cardiac surgery | Post valvular surgery |
| post surgical correction of congenital heart disease | |
| Catheter ablation of arrhythmias | |
| Alcohol septal ablation for hypertrophic cardiomyopathy | |
| Transcatheter closure of ventricular septal defect | |
Life Threatening Causes
Life-threatening conditions can result in death or permanent disability within 24 hours if left untreated[7].
- Acute myocardial infarction[8][9]
- Acute rheumatic fever
- Bacterial endocarditis
- Myocarditis
- Severe hypothermia
Common Causes
- Acute rheumatic fever
- Bacterial endocarditis[10]
- Calcific aortic stenosis
- Digoxin
- Dilated cardiomyopathy
- Diltiazem
- Enhanced vagal tone
- HCM
- Hypertension
- Iatrogenic after surgical correction of VSD, tetralogy of Fallot, and endocardial cushion defect
- Inferior ST elevation MI
- Massive calcification of the mitral annulus
- Myocarditis
- Normal variants[3]
- Penetrating and non-penetrating trauma of the chest
- Sclerodegenerative disease of the electrical conduction system
- Verapamil
- β blockers
Causes by Organ System
Causes in Alphabetical Order
Epidemiology and Demographics
The incidence/prevalence of [disease name] is approximately [number range] per 100,000 individuals worldwide.
OR
In [year], the incidence/prevalence of [disease name] was estimated to be [number range] cases per 100,000 individuals worldwide.
OR
In [year], the incidence of [disease name] is approximately [number range] per 100,000 individuals with a case-fatality rate of [number range]%.
Patients of all age groups may develop [disease name].
OR
The incidence of [disease name] increases with age; the median age at diagnosis is [#] years.
OR
[Disease name] commonly affects individuals younger than/older than [number of years] years of age.
OR
[Chronic disease name] is usually first diagnosed among [age group].
OR
[Acute disease name] commonly affects [age group].
There is no racial predilection to [disease name].
OR
[Disease name] usually affects individuals of the [race 1] race. [Race 2] individuals are less likely to develop [disease name].
[Disease name] affects men and women equally.
OR
[Gender 1] are more commonly affected by [disease name] than [gender 2]. The [gender 1] to [gender 2] ratio is approximately [number > 1] to 1.
The majority of [disease name] cases are reported in [geographical region].
OR
[Disease name] is a common/rare disease that tends to affect [patient population 1] and [patient population 2].
Risk Factors
There are no established risk factors for [disease name].
OR
The most potent risk factor in the development of [disease name] is [risk factor 1]. Other risk factors include [risk factor 2], [risk factor 3], and [risk factor 4].
OR
Common risk factors in the development of [disease name] include [risk factor 1], [risk factor 2], [risk factor 3], and [risk factor 4].
OR
Common risk factors in the development of [disease name] may be occupational, environmental, genetic, and viral.
Screening
There is insufficient evidence to recommend routine screening for [disease/malignancy].
OR
According to the [guideline name], screening for [disease name] is not recommended.
OR
According to the [guideline name], screening for [disease name] by [test 1] is recommended every [duration] among patients with [condition 1], [condition 2], and [condition 3].
Natural History, Complications, and Prognosis
If left untreated, [#]% of patients with [disease name] may progress to develop [manifestation 1], [manifestation 2], and [manifestation 3].
OR
Common complications of [disease name] include [complication 1], [complication 2], and [complication 3].
OR
Prognosis is generally excellent/good/poor, and the 1/5/10-year mortality/survival rate of patients with [disease name] is approximately [#]%.
Diagnosis
Diagnostic Study of Choice
The diagnosis of [disease name] is made when at least [number] of the following [number] diagnostic criteria are met: [criterion 1], [criterion 2], [criterion 3], and [criterion 4].
OR
The diagnosis of [disease name] is based on the [criteria name] criteria, which include [criterion 1], [criterion 2], and [criterion 3].
OR
The diagnosis of [disease name] is based on the [definition name] definition, which includes [criterion 1], [criterion 2], and [criterion 3].
OR
There are no established criteria for the diagnosis of [disease name].
History and Symptoms
The majority of patients with [disease name] are asymptomatic.
OR
The hallmark of [disease name] is [finding]. A positive history of [finding 1] and [finding 2] is suggestive of [disease name]. The most common symptoms of [disease name] include [symptom 1], [symptom 2], and [symptom 3]. Common symptoms of [disease] include [symptom 1], [symptom 2], and [symptom 3]. Less common symptoms of [disease name] include [symptom 1], [symptom 2], and [symptom 3].
Physical Examination
Patients with [disease name] usually appear [general appearance]. Physical examination of patients with [disease name] is usually remarkable for [finding 1], [finding 2], and [finding 3].
OR
Common physical examination findings of [disease name] include [finding 1], [finding 2], and [finding 3].
OR
The presence of [finding(s)] on physical examination is diagnostic of [disease name].
OR
The presence of [finding(s)] on physical examination is highly suggestive of [disease name].
Laboratory Findings
An elevated/reduced concentration of serum/blood/urinary/CSF/other [lab test] is diagnostic of [disease name].
OR
Laboratory findings consistent with the diagnosis of [disease name] include [abnormal test 1], [abnormal test 2], and [abnormal test 3].
OR
[Test] is usually normal among patients with [disease name].
OR
Some patients with [disease name] may have elevated/reduced concentration of [test], which is usually suggestive of [progression/complication].
OR
There are no diagnostic laboratory findings associated with [disease name].
Electrocardiogram
There are no ECG findings associated with [disease name].
OR
An ECG may be helpful in the diagnosis of [disease name]. Findings on an ECG suggestive of/diagnostic of [disease name] include [finding 1], [finding 2], and [finding 3].
Differentiating Infra-Hisian Block From Other Diseases
| Arrhythmia | Rhythm | Rate | P wave | PR Interval | QRS Complex | Response to Maneuvers | Epidemiology | Co-existing Conditions | |
|---|---|---|---|---|---|---|---|---|---|
| Atrioventricular block[12] | First degree [13][14] |
|
|
|
|
|
|
| |
| Second degree[15][16] |
|
|
|
QRS is normal but dropped as the following:
|
|
| |||
| Third degree[17][18] |
|
|
|
|
|
| |||
| Atrial Fibrillation (AFib)[19][20] |
|
|
|
|
|
|
|
| |
| Atrial Flutter[21] |
|
|
|
|
|
|
|
||
| Atrioventricular nodal reentry tachycardia (AVNRT)[22][23][24][25] |
|
|
|
|
|
|
|
||
| Multifocal Atrial Tachycardia[26][27] |
|
|
|
|
|
|
|
||
| Paroxysmal Supraventricular Tachycardia |
|
|
|
|
|
|
|
||
| Premature Atrial Contractrions (PAC)[28][29] |
|
|
|
|
|
|
|||
| Wolff-Parkinson-White Syndrome[30][31] |
|
|
|
|
|
|
|
| |
| Ventricular Fibrillation (VF)[32][33][34] |
|
|
|
|
|
|
|
| |
| Ventricular Tachycardia[35][36] |
|
|
|
|
|
|
|
| |
The table below provides information on the differential diagnosis of ventricular tachycardia in terms of ECG appearance:
| Disease Name | Causes | ECG Characteristics | ECG view |
|---|---|---|---|
| Ventricular tachycardia [37][38][39][40][41] |
|
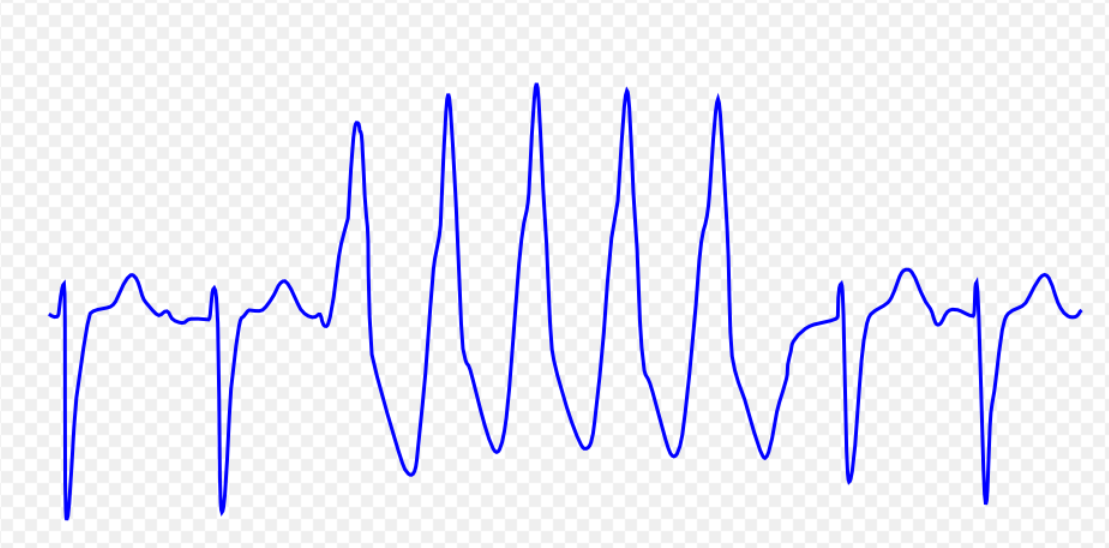 | |
| Ventricular fibrillation [35][43][44][45] |
|
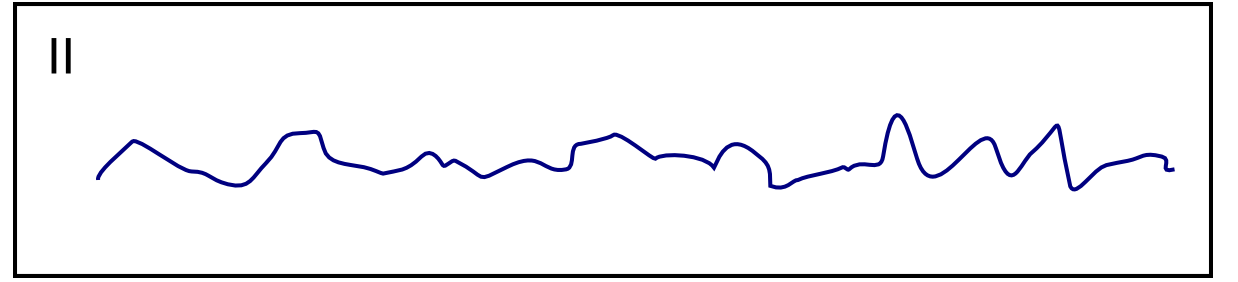 | |
| Ventricular flutter [47][48][49] |
|
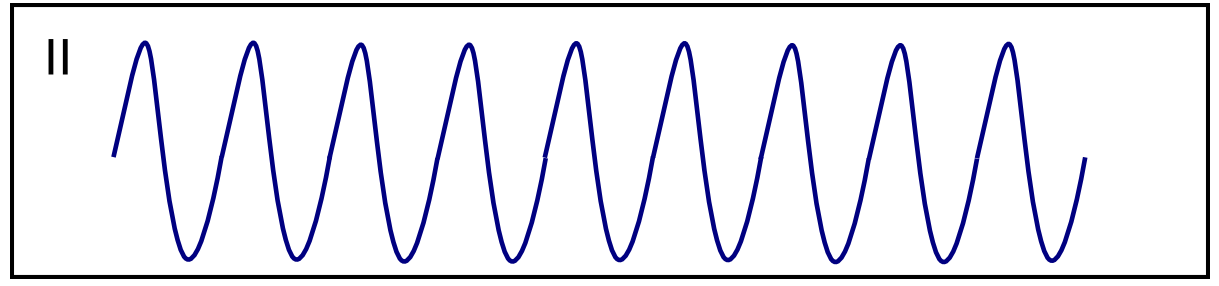 | |
| Asystole [51][52] |
|
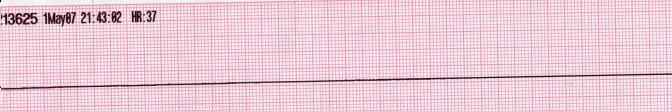 | |
| Pulseless electrical activity [54][55] |
|
|
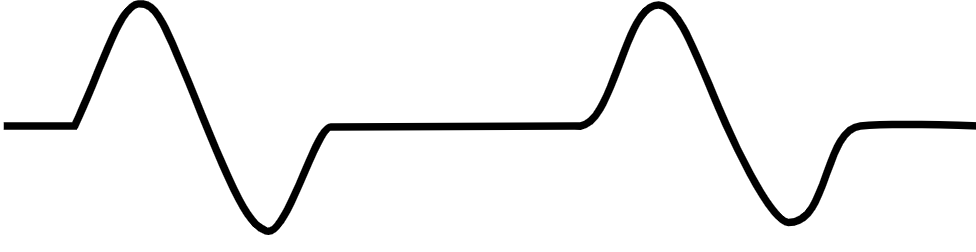 |
| Torsade de Pointes [57][58][59] |
|
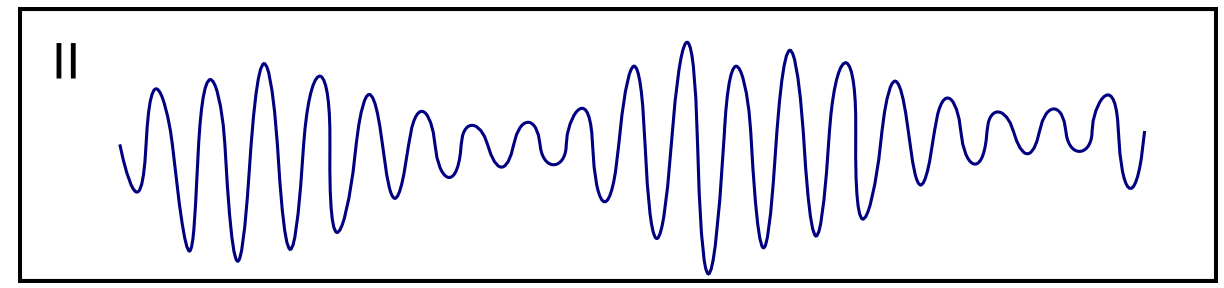 |
References
- ↑ Upshaw CB, Silverman ME (2000). "John Hay: discoverer of type II atrioventricular block". Clin Cardiol. 23 (11): 869–71. doi:10.1002/clc.4960231118. PMC 6655013 Check
|pmc=value (help). PMID 11097138. - ↑ Puech P, Wainwright RJ (1983). "Clinical electrophysiology of atrioventricular block". Cardiol Clin. 1 (2): 209–24. PMID 6544636.
- ↑ 3.0 3.1 Wogan JM, Lowenstein SR, Gordon GS (1993). "Second-degree atrioventricular block: Mobitz type II". J Emerg Med. 11 (1): 47–54. doi:10.1016/0736-4679(93)90009-v. PMID 8445186.
- ↑ Li X, Xue Y, Wu H (2018). "A Case of Atrioventricular Block Potentially Associated with Right Coronary Artery Lesion and Ticagrelor Therapy Mediated by the Increasing Adenosine Plasma Concentration". Case Rep Vasc Med. 2018: 9385017. doi:10.1155/2018/9385017. PMC 5933017. PMID 29850368.
- ↑ Fu Md J, Bhatta L (2018). "Lyme carditis: Early occurrence and prolonged recovery". J Electrocardiol. 51 (3): 516–518. doi:10.1016/j.jelectrocard.2017.12.035. PMID 29275956.
- ↑ Tuohy S, Saliba W, Pai M, Tchou P (January 2018). "Catheter ablation as a treatment of atrioventricular block". Heart Rhythm. 15 (1): 90–96. doi:10.1016/j.hrthm.2017.08.015. PMID 28823599.
- ↑ Mangi MA, Jones WM, Napier L. PMID 29493981. Missing or empty
|title=(help) - ↑ Misumida N, Ogunbayo GO, Kim SM, Abdel-Latif A, Ziada KM, Elayi CS (November 2018). "Frequency and Significance of High-Degree Atrioventricular Block and Sinoatrial Node Dysfunction in Patients With Non-ST-Elevation Myocardial Infarction". Am. J. Cardiol. 122 (10): 1598–1603. doi:10.1016/j.amjcard.2018.08.001. PMID 30227965.
- ↑ Barold SS, Herweg B (December 2012). "Second-degree atrioventricular block revisited". Herzschrittmacherther Elektrophysiol. 23 (4): 296–304. doi:10.1007/s00399-012-0240-8. PMID 23224264.
- ↑ Kamatani T, Akizuki A, Kondo S, Shirota T (Fall 2016). "Second-Degree Atrioventricular Block Occurring After Tooth Extraction". Anesth Prog. 63 (3): 156–9. doi:10.2344/15-00042.1. PMC 5011958. PMID 27585419.
- ↑ Menicagli F, Lanza A, Sbrocca F, Baldi A, Spugnini EP (2016). "A case of advanced second-degree atrioventricular block in a ferret secondary to lymphoma". Open Vet J. 6 (1): 68–70. doi:10.4314/ovj.v6i1.10. PMC 4833871. PMID 27200273.
- ↑ Kerola T, Eranti A, Aro AL, Haukilahti MA, Holkeri A, Junttila MJ; et al. (2019). "Risk Factors Associated With Atrioventricular Block". JAMA Netw Open. 2 (5): e194176. doi:10.1001/jamanetworkopen.2019.4176. PMC 6632153 Check
|pmc=value (help). PMID 31125096. - ↑ Barold SS (1996). "Indications for permanent cardiac pacing in first-degree AV block: class I, II, or III?". Pacing Clin Electrophysiol. 19 (5): 747–51. doi:10.1111/j.1540-8159.1996.tb03355.x. PMID 8734740.
- ↑ Upshaw CB (2004). "Comparison of the prevalence of first-degree atrioventricular block in African-American and in Caucasian patients: an electrocardiographic study III". J Natl Med Assoc. 96 (6): 756–60. PMC 2568382. PMID 15233485.
- ↑ Zehender M, Meinertz T, Keul J, Just H (1990). "ECG variants and cardiac arrhythmias in athletes: clinical relevance and prognostic importance". Am Heart J. 119 (6): 1378–91. doi:10.1016/s0002-8703(05)80189-9. PMID 2191578.
- ↑ Friedman HS, Gomes JA, Haft JI (1975). "An analysis of Wenckebach periodicity". J Electrocardiol. 8 (4): 307–15. doi:10.1016/s0022-0736(75)80003-3. PMID 1176840.
- ↑ OSTRANDER LD, BRANDT RL, KJELSBERG MO, EPSTEIN FH (June 1965). "ELECTROCARDIOGRAPHIC FINDINGS AMONG THE ADULT POPULATION OF A TOTAL NATURAL COMMUNITY, TECUMSEH, MICHIGAN". Circulation. 31: 888–98. doi:10.1161/01.cir.31.6.888. PMID 14297523.
- ↑ Movahed MR, Hashemzadeh M, Jamal MM (October 2005). "Increased prevalence of third-degree atrioventricular block in patients with type II diabetes mellitus". Chest. 128 (4): 2611–4. doi:10.1378/chest.128.4.2611. PMID 16236932.
- ↑ Lankveld TA, Zeemering S, Crijns HJ, Schotten U (July 2014). "The ECG as a tool to determine atrial fibrillation complexity". Heart. 100 (14): 1077–84. doi:10.1136/heartjnl-2013-305149. PMID 24837984.
- ↑ Harris K, Edwards D, Mant J (2012). "How can we best detect atrial fibrillation?". J R Coll Physicians Edinb. 42 Suppl 18: 5–22. doi:10.4997/JRCPE.2012.S02. PMID 22518390.
- ↑ Cosío FG (June 2017). "Atrial Flutter, Typical and Atypical: A Review". Arrhythm Electrophysiol Rev. 6 (2): 55–62. doi:10.15420/aer.2017.5.2. PMC 5522718. PMID 28835836.
- ↑ Katritsis DG, Josephson ME (August 2016). "Classification, Electrophysiological Features and Therapy of Atrioventricular Nodal Reentrant Tachycardia". Arrhythm Electrophysiol Rev. 5 (2): 130–5. doi:10.15420/AER.2016.18.2. PMC 5013176. PMID 27617092.
- ↑ Letsas KP, Weber R, Siklody CH, Mihas CC, Stockinger J, Blum T, Kalusche D, Arentz T (April 2010). "Electrocardiographic differentiation of common type atrioventricular nodal reentrant tachycardia from atrioventricular reciprocating tachycardia via a concealed accessory pathway". Acta Cardiol. 65 (2): 171–6. doi:10.2143/AC.65.2.2047050. PMID 20458824.
- ↑ "Atrioventricular Nodal Reentry Tachycardia (AVNRT) - StatPearls - NCBI Bookshelf".
- ↑ Schernthaner C, Danmayr F, Strohmer B (2014). "Coexistence of atrioventricular nodal reentrant tachycardia with other forms of arrhythmias". Med Princ Pract. 23 (6): 543–50. doi:10.1159/000365418. PMC 5586929. PMID 25196716.
- ↑ Scher DL, Arsura EL (September 1989). "Multifocal atrial tachycardia: mechanisms, clinical correlates, and treatment". Am. Heart J. 118 (3): 574–80. doi:10.1016/0002-8703(89)90275-5. PMID 2570520.
- ↑ Goodacre S, Irons R (March 2002). "ABC of clinical electrocardiography: Atrial arrhythmias". BMJ. 324 (7337): 594–7. doi:10.1136/bmj.324.7337.594. PMC 1122515. PMID 11884328.
- ↑ Lin CY, Lin YJ, Chen YY, Chang SL, Lo LW, Chao TF, Chung FP, Hu YF, Chong E, Cheng HM, Tuan TC, Liao JN, Chiou CW, Huang JL, Chen SA (August 2015). "Prognostic Significance of Premature Atrial Complexes Burden in Prediction of Long-Term Outcome". J Am Heart Assoc. 4 (9): e002192. doi:10.1161/JAHA.115.002192. PMC 4599506. PMID 26316525.
- ↑ Strasburger JF, Cheulkar B, Wichman HJ (December 2007). "Perinatal arrhythmias: diagnosis and management". Clin Perinatol. 34 (4): 627–52, vii–viii. doi:10.1016/j.clp.2007.10.002. PMC 3310372. PMID 18063110.
- ↑ Rao AL, Salerno JC, Asif IM, Drezner JA (July 2014). "Evaluation and management of wolff-Parkinson-white in athletes". Sports Health. 6 (4): 326–32. doi:10.1177/1941738113509059. PMC 4065555. PMID 24982705.
- ↑ Rosner MH, Brady WJ, Kefer MP, Martin ML (November 1999). "Electrocardiography in the patient with the Wolff-Parkinson-White syndrome: diagnostic and initial therapeutic issues". Am J Emerg Med. 17 (7): 705–14. doi:10.1016/s0735-6757(99)90167-5. PMID 10597097.
- ↑ Glinge C, Sattler S, Jabbari R, Tfelt-Hansen J (September 2016). "Epidemiology and genetics of ventricular fibrillation during acute myocardial infarction". J Geriatr Cardiol. 13 (9): 789–797. doi:10.11909/j.issn.1671-5411.2016.09.006. PMC 5122505. PMID 27899944.
- ↑ Samie FH, Jalife J (May 2001). "Mechanisms underlying ventricular tachycardia and its transition to ventricular fibrillation in the structurally normal heart". Cardiovasc. Res. 50 (2): 242–50. doi:10.1016/s0008-6363(00)00289-3. PMID 11334828.
- ↑ Adabag AS, Luepker RV, Roger VL, Gersh BJ (April 2010). "Sudden cardiac death: epidemiology and risk factors". Nat Rev Cardiol. 7 (4): 216–25. doi:10.1038/nrcardio.2010.3. PMC 5014372. PMID 20142817.
- ↑ 35.0 35.1 Koplan BA, Stevenson WG (March 2009). "Ventricular tachycardia and sudden cardiac death". Mayo Clin. Proc. 84 (3): 289–97. doi:10.1016/S0025-6196(11)61149-X. PMC 2664600. PMID 19252119.
- ↑ Levis JT (2011). "ECG Diagnosis: Monomorphic Ventricular Tachycardia". Perm J. 15 (1): 65. doi:10.7812/tpp/10-130. PMC 3048638. PMID 21505622.
- ↑ Ajijola, Olujimi A.; Tung, Roderick; Shivkumar, Kalyanam (2014). "Ventricular tachycardia in ischemic heart disease substrates". Indian Heart Journal. 66: S24–S34. doi:10.1016/j.ihj.2013.12.039. ISSN 0019-4832.
- ↑ Meja Lopez, Eliany; Malhotra, Rohit (2019). "Ventricular Tachycardia in Structural Heart Disease". Journal of Innovations in Cardiac Rhythm Management. 10 (8): 3762–3773. doi:10.19102/icrm.2019.100801. ISSN 2156-3977.
- ↑ Coughtrie, Abigail L; Behr, Elijah R; Layton, Deborah; Marshall, Vanessa; Camm, A John; Shakir, Saad A W (2017). "Drugs and life-threatening ventricular arrhythmia risk: results from the DARE study cohort". BMJ Open. 7 (10): e016627. doi:10.1136/bmjopen-2017-016627. ISSN 2044-6055.
- ↑ El-Sherif, Nabil (2001). "Mechanism of Ventricular Arrhythmias in the Long QT Syndrome: On Hermeneutics". Journal of Cardiovascular Electrophysiology. 12 (8): 973–976. doi:10.1046/j.1540-8167.2001.00973.x. ISSN 1045-3873.
- ↑ de Riva, Marta; Watanabe, Masaya; Zeppenfeld, Katja (2015). "Twelve-Lead ECG of Ventricular Tachycardia in Structural Heart Disease". Circulation: Arrhythmia and Electrophysiology. 8 (4): 951–962. doi:10.1161/CIRCEP.115.002847. ISSN 1941-3149.
- ↑ ECG found in of https://en.ecgpedia.org/index.php?title=Main_Page
- ↑ Maury P, Sacher F, Rollin A, Mondoly P, Duparc A, Zeppenfeld K, Hascoet S (May 2017). "Ventricular arrhythmias and sudden death in tetralogy of Fallot". Arch Cardiovasc Dis. 110 (5): 354–362. doi:10.1016/j.acvd.2016.12.006. PMID 28222965.
- ↑ Saumarez RC, Camm AJ, Panagos A, Gill JS, Stewart JT, de Belder MA, Simpson IA, McKenna WJ (August 1992). "Ventricular fibrillation in hypertrophic cardiomyopathy is associated with increased fractionation of paced right ventricular electrograms". Circulation. 86 (2): 467–74. doi:10.1161/01.cir.86.2.467. PMID 1638716.
- ↑ Bektas, Firat; Soyuncu, Secgin (2012). "Hypokalemia-induced Ventricular Fibrillation". The Journal of Emergency Medicine. 42 (2): 184–185. doi:10.1016/j.jemermed.2010.05.079. ISSN 0736-4679.
- ↑ ECG found in https://en.ecgpedia.org/index.php?title=Main_Page
- ↑ Thies, Karl-Christian; Boos, Karin; Müller-Deile, Kai; Ohrdorf, Wolfgang; Beushausen, Thomas; Townsend, Peter (2000). "Ventricular flutter in a neonate—severe electrolyte imbalance caused by urinary tract infection in the presence of urinary tract malformation". The Journal of Emergency Medicine. 18 (1): 47–50. doi:10.1016/S0736-4679(99)00161-4. ISSN 0736-4679.
- ↑ Koster, Rudolph W.; Wellens, Hein J.J. (1976). "Quinidine-induced ventricular flutter and fibrillation without digitalis therapy". The American Journal of Cardiology. 38 (4): 519–523. doi:10.1016/0002-9149(76)90471-9. ISSN 0002-9149.
- ↑ Dhurandhar RW, Nademanee K, Goldman AM (1978). "Ventricular tachycardia-flutter associated with disopyramide therapy: a report of three cases". Heart Lung. 7 (5): 783–7. PMID 250503.
- ↑ ECG found in https://en.ecgpedia.org/index.php?title=Main_Page
- ↑ ACLS: Principles and Practice. p. 71-87. Dallas: American Heart Association, 2003. ISBN 0-87493-341-2.
- ↑ ACLS for Experienced Providers. p. 3-5. Dallas: American Heart Association, 2003. ISBN 0-87493-424-9.
- ↑ ECG found in https://en.ecgpedia.org/index.php?title=Main_Page
- ↑ "2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care - Part 7.2: Management of Cardiac Arrest." Circulation 2005; 112: IV-58 - IV-66.
- ↑ Foster B, Twelve Lead Electrocardiography, 2nd edition, 2007
- ↑ ECG found in wikimedia Commons
- ↑ Li M, Ramos LG (July 2017). "Drug-Induced QT Prolongation And Torsades de Pointes". P T. 42 (7): 473–477. PMC 5481298. PMID 28674475.
- ↑ Sharain, Korosh; May, Adam M.; Gersh, Bernard J. (2015). "Chronic Alcoholism and the Danger of Profound Hypomagnesemia". The American Journal of Medicine. 128 (12): e17–e18. doi:10.1016/j.amjmed.2015.06.051. ISSN 0002-9343.
- ↑ Khan IA (2001). "Twelve-lead electrocardiogram of torsades de pointes". Tex Heart Inst J. 28 (1): 69. PMC 101137. PMID 11330748.
- ↑ ECG found in https://en.ecgpedia.org/index.php?title=Main_Page