Diltiazem (injection)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Diltiazem (injection) is a calcium channel blocker that is FDA approved for the treatment of atrial fibrillation, atrial flutter, and paroxysmal supraventricular tachycardias (PSVT). Common adverse reactions include bradyarrhythmia, peripheral edema, dizziness, headache, cough, and fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Diltiazem hydrochloride injection is indicated for the following:
- Atrial Fibrillation or Atrial Flutter. Temporary control of rapid ventricular rate in atrial fibrillation or atrial flutter. It should not be used in patients with atrial fibrillation or atrial flutter associated with an accessory bypass tract such as in Wolff-Parkinson-White (WPW) syndrome or short PR syndrome.
- Paroxysmal Supraventricular Tachycardia. Rapid conversion of paroxysmal supraventricular tachycardias (PSVT) to sinus rhythm. This includes AV nodal reentrant tachycardias and reciprocating tachycardias associated with an extranodal accessory pathway such as the WPW syndrome or short PR syndrome. Unless otherwise contraindicated, appropriate vagal maneuvers should be attempted prior to administration of diltiazem hydrochloride injection.
- The use of diltiazem hydrochloride injection for control of ventricular response in patients with atrial fibrillation or atrial flutter or conversion to sinus rhythm in patients with PSVT should be undertaken with caution when the patient is compromised hemodynamically or is taking other drugs that decrease any or all of the following: peripheral resistance, myocardial filling, myocardial contractility, or electrical impulse propagation in the myocardium.
- For either indication and particularly when employing continuous intravenous infusion, the setting should include continuous monitoring of the ECG and frequent measurement of blood pressure. A defibrillator and emergency equipment should be readily available.
- In domestic controlled trials in patients with atrial fibrillation or atrial flutter, bolus administration of diltiazem hydrochloride injection was effective in reducing heart rate by at least 20% in 95% of patients. Diltiazem hydrochloride injection rarely converts atrial fibrillation or atrial flutter to normal sinus rhythm. Following administration of one or two intravenous bolus doses of diltiazem hydrochloride injection, response usually occurs within 3 minutes and maximal heart rate reduction generally occurs in 2 to 7 minutes. Heart rate reduction may last from 1 to 3 hours. If hypotension occurs, it is generally short-lived, but may last from 1 to 3 hours.
- A 24-hour continuous infusion of diltiazem hydrochloride injection in the treatment of atrial fibrillation or atrial flutter maintained at least a 20% heart rate reduction during the infusion in 83% of patients. Upon discontinuation of infusion, heart rate reduction may last from 0.5 hours to more than 10 hours (median duration 7 hours). Hypotension, if it occurs, may be similarly persistent.
- In the controlled clinical trials, 3.2% of patients required some form of intervention (typically, use of intravenous fluids or the Trendelenburg position) for blood pressure support following diltiazem hydrochloride injection.
- In domestic controlled trials, bolus administration of diltiazem hydrochloride injection was effective in converting PSVT to normal sinus rhythm in 88% of patients within 3 minutes of the first or second bolus dose.
- Symptoms associated with the arrhythmia were improved in conjunction with decreased heart rate or conversion to normal sinus rhythm following administration of diltiazem hydrochloride injection.
Dosage
- Direct Intravenous Single Injections (Bolus)
- The initial dose of diltiazem hydrochloride injection should be 0.25 mg/kg actual body weight as a bolus administered over 2 minutes (20 mg is a reasonable dose for the average patient). If response is inadequate, a second dose may be administered after 15 minutes. The second bolus dose of diltiazem hydrochloride injection should be 0.35 mg/kg actual body weight administered over 2 minutes (25 mg is a reasonable dose for the average patient). Subsequent intravenous bolus doses should be individualized for each patient. Patients with low body weights should be dosed on a mg/kg basis. Some patients may respond to an initial dose of 0.15 mg/kg, although duration of action may be shorter. Experience with this dose is limited.
Continuous Intravenous Infusion
- For continued reduction of the heart rate (up to 24 hours) in patients with atrial fibrillation or atrial flutter, an intravenous infusion of diltiazem hydrochloride injection may be administered. Immediately following bolus administration of 20 mg (0.25 mg/kg) or 25 mg (0.35 mg/kg) diltiazem hydrochloride injection and reduction of heart rate, begin an intravenous infusion of diltiazem hydrochloride injection. The recommended initial infusion rate of diltiazem hydrochloride injection is 10 mg/h. Some patients may maintain response to an initial rate of 5 mg/h. The infusion rate may be increased in 5 mg/h increments up to 15 mg/h as needed, if further reduction in heart rate is required, the infusion may be maintained for up to 24 hours.
- Diltiazem shows dose-dependent, non-linear pharmacokinetics. Duration of infusion longer than 24 hours and infusion rates greater than 15 mg/h have not been studied. Therefore, infusion duration exceeding 24 hours and infusion rates exceeding 15 mg/h are not recommended.
- Dilution: To prepare diltiazem hydrochloride injection for continuous intravenous infusion aseptically transfer the appropriate quantity (see chart) of diltiazem hydrochloride injection to the desired volume of either Normal Saline, D5W, or D5W/0.45% NaCl. Mix thoroughly. Use within 24 hours. Keep refrigerated until use.
- Diltiazem hydrochloride injection was tested for compatibility with three commonly used intravenous fluids at a maximal concentration of 1 mg diltiazem hydrochloride per milliliter. Diltiazem hydrochloride injection was found to be physically compatible and chemically stable in the following parenteral solutions for at least 24 hours when stored in glass or polyvinylchloride (PVC) bags at controlled room temperature 15° to 30°C (59° to 86°F) or under refrigeration 2° to 8°C (36° to 46°F).
- Dextrose (5%) injection USP
- Sodium chloride (0.9%) injection USP
- Dextrose (5%) and sodium chloride (0.45%) injection USP
- Because of potential physical incompatibilities, it is recommended that diltiazem hydrochloride injection not be mixed with any other drugs in the same container.
- If possible, it is recommended that diltiazem hydrochloride injection not be co-infused in the same intravenous line. Physical incompatibilities (precipitate formation or cloudiness) were observed when diltiazem hydrochloride injection was infused in the same intravenous line with the following drugs: acetazolamide, acyclovir, aminophylline, ampicillin, ampicillin sodium/sulbactam sodium, cefamandole, cefoperazone, diazepam, furosemide, hydrocortisone, sodium succinate, insulin (regular: 100 units/mL), methylprednisolone sodium succinate, mezlocillin, nafcillin, phenytoin, rifampin, and sodium bicarbonate.
- Parental drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Transition to Further Antiarrhythmic Therapy
- Transition to other antiarrhythmic agents following administration of diltiazem hydrochloride injection is generally safe. However, reference should be made to the respective agent manufacturer's package insert for information relative to dosage and administration.
- In controlled clinical trials, therapy with antiarrhythmic agents to maintain reduced heart rate in atrial fibrillation or atrial flutter or for prophylaxis of PSVT was generally started within 3 hours after bolus administration of diltiazem hydrochloride injection. These antiarrhythmic agents were intravenous or oral digoxin, Class I antiarrhythmics (eg, quinidine, procainamide), calcium channel blockers, and oral beta-blockers.
- Experience in the use of antiarrhythmic agents following maintenance infusion of diltiazem hydrochloride injection is limited. Patients should be dosed on an individual basis and reference should be made to respective manufacturer's package insert for information relative to dosage and administration.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Unstable Angina
- Developed by: American College of Cardiology (ACC) and American Heart Association (AHA)
- Class of Recommendation: Class I
- Level of Evidence: Not applicable
- Dosing Information
- 20 mg IV
Non–Guideline-Supported Use
Anal Fissure
- Dosing information
Coronary Artery Bypass Graft
- Dosing information
Diabetic Nephropathy
- Dosing information
- Not applicable
Nephrotoxicity - Post-Transplantation
- Dosing information
Hyperthyroidism
- Dosing information
Prophylaxis of Migraine
- Dosing information
- 60 -90 mg PO qid [9]
Myocardial Infarction
- Dosing information
Painful Spasm of Anus
- 80 mg bid [12]
Pulmonary Hypertension
- Dosing information
Ventricular Arrhythmia
- Dosing information
- 120- 240 mg/day[15]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Diltiazem (injection) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Diltiazem (injection) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Diltiazem (injection) in pediatric patients.
Contraindications
- Diltiazem hydrochloride injection is contraindicated in:
- Patients with sick sinus syndrome except in the presence of a functioning ventricular pacemaker.
- Patients with second- or third-degree AV block except in the presence of functioning ventricular pacemaker.
- Patients with severe hypotension or cardiogenic shock.
- Patients who have demonstrated hypersensitivity to the drug.
- Intravenous diltiazem and intravenous beta-blockers should not be administered together or in close proximity (within a few hours).
- Patients with atrial fibrillation or atrial flutter associated with an accessory bypass tract such as in WPW syndrome or short PR syndrome.
- As with other agents which slow AV nodal conduction and do not prolong the refractoriness of the accessory pathway (eg, verapamil, digoxin), in rare instances patients in atrial fibrillation or atrial flutter associated with an accessory bypass tract may experience a potentially life-threatening increase in heart rate accompanied by hypotension when treated with diltiazem hydrochloride injection. As such, the initial use of diltiazem hydrochloride injection should be, if possible, in a setting where monitoring and resuscitation capabilities, including DC cardioversion/defibrillation, are present. Once familiarity of the patient's response is established, use in an office setting may be acceptable.
- Patients with ventricular tachycardia. Administration of other calcium channel blockers to patients with wide complex tachycardia (QRS = 0.12 seconds) has resulted in hemodynamic deterioration and ventricular fibrillation. It is important that an accurate pretreatment diagnosis distinguish wide complex QRS tachycardia of supraventricular origin from that of ventricular origin prior to administration of diltiazem hydrochloride injection.
Warnings
- Cardiac Conduction. Diltiazem prolongs AV nodal conduction and refractoriness that may rarely result in second- or third-degree AV block or sinus rhythm. Concomitant use of diltiazem with agents known to affect cardiac conduction may result in additive effects. If high-degree AV block occurs in sinus rhythm, intravenous diltiazem should be discontinued and appropriate supportive measures instituted.
Congestive Heart Failure. Although diltiazem has a negative inotropic effect in isolated animal tissue preparations, hemodynamic studies in humans with normal ventricular function and in patients with a compromised myocardium, such as severe CHF, acute MI, and hypertrophic cardiomyopathy, have not shown a reduction in cardiac index nor consistent negative effects on contractility (dp/dt). Administration of oral diltiazem in patients with acute myocardial infarction and pulmonary congestion documented by x-ray on admission is contraindicated. Experience with the use of diltiazem hydrochloride injection in patients with impaired ventricular function is limited. Caution should be exercised when using the drug in such patients.
- Hypotension. Decreases in blood pressure associated with diltiazem hydrochloride injection therapy may occasionally result in symptomatic hypotension (3.2%). The use of intravenous diltiazem for control of ventricular response in patients with supraventricular arrhythmias should be undertaken with caution when the patient is compromised hemodynamically. In addition, caution should be used in patients taking other drugs that decrease peripheral resistance, intravascular volume, myocardial contractility or conduction.
- Acute Hepatic Injury. In rare instances, significant elevations in enzymes such as alkaline phosphatase, LDH, SGOT, SGPT, and other phenomena consistent with acute hepatic injury have been noted following oral diltiazem. Therefore, the potential for acute hepatic injury exists following administration of intravenous diltiazem.
- Ventricular Premature Beats (VPBs). VPBs may be present on conversion of PSVT to sinus rhythm with diltiazem hydrochloride injection. These VPBs are transient, are typically considered to be benign, and appear to have no clinical significance. Similar ventricular complexes have been noted during cardioversion, other pharmacologic therapy, and during spontaneous conversion of PSVT to sinus rhythm.
Adverse Reactions
Clinical Trials Experience
- The following adverse reaction rates are based on the use of diltiazem hydrochloride injection in over 400 domestic clinical trial patients with atrial fibrillation/flutter or PSVT under double-blind or open-label conditions. Worldwide experience in over 1300 patients was similar.
- Adverse events reported in controlled and uncontrolled clinical trials were generally mild and transient.Hypotension was the most commonly reported adverse event during clinical trials. Asymptomatic hypotension occurred in 4.3% of patients. Symptomatic hypotension occurred in 3.2% of patients. When treatment for hypotension was required, it generally consisted of administration of saline or placing the patient in the Trendelenburg position. Other events reported in at least 1% of the diltiazem-treated patients were injection site reactions (eg, itching, burning) - 3.9%, vasodilation (flushing) -1.7%, and arrhythmia (junctional rhythm or isorhythmic dissociation) - 1.0%.
- In addition, the following events were reported infrequently (less than 1%):
- Cardiovascular: Asystole, atrial flutter, AV block first degree, AV block second degree, bradycardia, chest pain, congestive heart failure, sinus pause, sinus node dysfunction, syncope, ventricular arrhythmia, ventricular fibrillation, ventricular tachycardia.
- Dermatologic: Pruritus, sweating
- Gastrointestinal: Constipation, elevated SGOT or alkaline phosphatase, nausea, vomiting
- Nervous System: Dizziness, paresthesia
- Other: Amblyopia, asthenia, dry mouth, dyspnea, edema, headache, hyperuricemia.
- Although not observed in clinical trials with diltiazem hydrochloride injection, the following events associated with oral diltiazem may occur:
- Cardiovascular: AV block (third degree), bundle branch block, ECG abnormality, palpitations, syncope, tachycardia, ventricular extrasystoles
- Dermatologic: Alopecia, erythema multiforme (including Stevens-Johnson syndrome, toxic epidermal necrolysis) exfoliative dermatitis, leukocytoclastic vasculitis, petechiae, photosensitivity, purpura, rash, urticaria
- Gastrointestinal: Anorexia, diarrhea, dysgeusia, dyspepsia, mild elevations of SGPT and LDH, thirst, weight increase
- Nervous System: Abnormal dreams, amnesia, depression, extrapyramidal symptoms, gait abnormality, hallucinations, insomnia, nervousness, personality change, somnolence, tremor
- Other: Allergic reactions, angioedema (including facial or periorbital edema), CPK elevation, epistaxis, eye irritation, gingival hyperplasia, hemolytic anemia, hyperglycemia, impotence, increased bleeding time, leukopenia, muscle cramps, nasal congestion, nocturia, osteoarticular pain, polyuria, retinopathy, sexual difficulties, thrombocytopenia, tinnitus Events such as myocardial infarction have been observed which are not readily distinguishable from the natural history of the disease for the patient.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Diltiazem (injection) in the drug label.
Drug Interactions
- Due to potential for additive effects, caution is warranted in patients receiving diltiazem hydrochloride injection concomitantly with other agent(s) known to affect cardiac contractility and/or SA node or AV node conduction.
- As with all drugs, care should be exercised when treating patients with multiple medications. Diltiazem hydrochloride undergoes extensive metabolism by the cytochrome P-450 mixed function oxidase system. Although specific pharmacokinetic drug-drug interaction studies have not been conducted with single intravenous injection or constant rate intravenous infusion, coadministration of diltiazem hydrochloride injection with other agents which primarily undergo the same route of biotransformation may result in competitive inhibition of metabolism.
- Digitalis. Intravenous diltiazem has been administered to patients receiving either intravenous or oral digitalis therapy. The combination of the two drugs was well tolerated without serious adverse effects. However, since both drugs affect AV nodal conduction, patients should be monitored for excessive slowing of the heart rate and/or AV block.
- Beta-blockers. Intravenous diltiazem has been administered to patients on chronic oral beta-blocker therapy. The combination of the two drugs was generally well tolerated without serious adverse effects. If intravenous diltiazem is administered to patients receiving chronic oral beta-blocker therapy, the possibility for bradycardia, AV block, and/or depression of contractility should be considered. Oral administration of diltiazem with propranolol in five normal volunteers resulted in increased propranolol levels in all subjects and bioavailability of propranolol was increased approximately 50%. In vitro, propranolol appears to be displaced from its binding sites by diltiazem.
- Anesthetics. The depression of cardiac contractility, conductivity, and automaticity as well as the vascular dilation associated with anesthetics may be potentiated by calcium channel blockers. When used concomitantly, anesthetics and calcium blockers should be titrated carefully.
- Cyclosporine. A pharmacokinetic interaction between diltiazem and cyclosporine has been observed during studies involving renal and cardiac transplant patients. In renal and cardiac transplant recipients, a reduction of cyclosporine dose ranging from 15% to 48% was necessary to maintain cyclosporine trough concentrations similar to those seen prior to the addition of diltiazem. If these agents are to be administered concurrently, cyclosporine concentrations should be monitored, especially when diltiazem therapy is initiated, adjusted or discontinued.
- The effect of cyclosporine on diltiazem plasma concentrations has not been evaluated.
- Carbamazepine. Concomitant administration of oral diltiazem with carbamazepine has been reported to result in elevated plasma levels of carbamazepine (by 40 to 72%), resulting in toxicity in some cases. Patients receiving these drugs concurrently should be monitored for a potential drug interaction
Use in Specific Populations
Pregnancy
- Reproduction studies have been conducted in mice, rats, and rabbits. Administration of oral doses ranging from five to ten times greater (on a mg/kg basis) than the daily recommended oral antianginal therapeutic dose has resulted in embryo and fetal lethality. These doses, in some studies, have been reported to cause skeletal abnormalities. In the perinatal/postnatal studies there was some reduction in early individual pup weights and survival rates. There was an increased incidence of stillbirths at doses of 20 times the human oral antianginal dose or greater.
There are no well-controlled studies in pregnant women; therefore, use diltiazem hydrochloride injection in pregnant women only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Diltiazem (injection) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Diltiazem (injection) during labor and delivery.
Nursing Mothers
- Diltiazem is excreted in human milk. One report with oral diltiazem suggests that concentrations in breast milk may approximate serum levels. If use of diltiazem is deemed essential, an alternative method of infant feeding should be instituted.
Pediatric Use
There is no FDA guidance on the use of Diltiazem (injection) with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Diltiazem (injection) with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Diltiazem (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Diltiazem (injection) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Diltiazem (injection) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Diltiazem (injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Diltiazem (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Diltiazem (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Monitoring of Diltiazem (injection) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Diltiazem (injection) in the drug label.
Overdosage
- Overdosage experience is limited. In the event of overdosage or an exaggerated response, appropriate supportive measures should be employed. The following measures may be considered:
- Bradycardia: Administer atropine (0.6 to 1.0 mg). If there is no response to vagal blockade administer isoproterenol cautiously.
- High-degree AV Block: Treat as for bradycardia above. Fixed high-degree AV block should be treated with cardiac pacing.
- Cardiac Failure: Administer inotropic agents (isoproterenol, dopamine, or dobutamine) and diuretics.
- Hypotension: Vasopressors (eg, dopamine or levarterenol bitartrate).
- Actual treatment and dosage should depend on the severity of the clinical situation and the judgment and experience of the treating physician.
- Diltiazem does not appear to be removed by peritoneal or hemodialysis. Limited data suggest that plasmapheresis or charcoal hemoperfusion may hasten diltiazem elimination following overdose.
- The intravenous LD50's in mice and rats were 60 to 38 mg/kg, respectively. The toxic dose in man is not known.
Pharmacology
| |
| |
Diltiazem (injection)
| |
| Systematic (IUPAC) name | |
| cis-(+)-[2-(2-dimethylaminoethyl)-5-(4-methoxyphenyl) -3-oxo-6-thia-2-azabicyclo[5.4.0]undeca-7,9, 11-trien-4-yl]ethanoate | |
| Identifiers | |
| CAS number | |
| ATC code | C08 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 414.519 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 40% |
| Metabolism | Hepatic |
| Half life | 3–4.5 hours |
| Excretion | Renal Biliary Lactic (in lactating females) |
| Therapeutic considerations | |
| Pregnancy cat. |
C: (USA) |
| Legal status | |
| Routes | Oral |
Mechanism of Action
- Diltiazem inhibits the influx of calcium (Ca2+) ions during membrane depolarization of cardiac and vascular smooth muscle. The therapeutic benefits of diltiazem in supraventricular tachycardias are related to its ability to slow AV nodal conduction time and prolong AV nodal refractoriness.
- Diltiazem exhibits frequency (use) dependent effects on AV nodal conduction such that it may selectively reduce the heart rate during tachycardias involving the AV node with little or no effect on normal AV nodal conduction at normal heart rates.
- Diltiazem slows the ventricular rate in patients with a rapid ventricular response during atrial fibrillation or atrial flutter. Diltiazem converts Paroxysmal Supraventricular Tachycardia (PSVT) to normal sinus rhythm by interrupting the reentry circuit in AV nodal reentrant tachycardias and reciprocating tachycardias, e.g., Wolff-Parkinson-White syndrome (WPW).
- Diltiazem prolongs the sinus cycle length. It has no effect on the sinus node recovery time or on the sinoatrial conduction time in patients without SA nodal dysfunction. Diltiazem has no significant electrophysiologic effect on tissues in the heart that are fast sodium channel dependent, e.g., His-Purkinje tissue, atrial and ventricular muscle, and extranodal accessory pathways.
- Like other calcium channel antagonists, because of its effect on vascular smooth muscle, diltiazem decreases total peripheral resistance resulting in a decrease in both systolic and diastolic blood pressure.
Structure
- Diltiazem® (diltiazem hydrochloride) is a calcium ion cellular influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1,5-Benzothiazepin-4(5H)-one, 3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-, monohydrochloride,(+)-cis-. The chemical structure is:
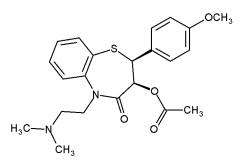
Pharmacodynamics
- The prolongation of PR interval correlated significantly with plasma diltiazem concentration in normal volunteers using the Sigmoidal Emax model. Changes in heart rate, systolic blood pressure, and diastolic blood pressure did not correlate with diltiazem plasma concentration in normal volunteers. Reduction in mean arterial pressure correlated linearly with diltiazem plasma concentration in a group of hypertensive patients.
- In patients with atrial fibrillation and atrial flutter, a significant correlation was observed between the percent reduction in HR and plasma diltiazem concentration using the Signmoidal Emax model. Based on this relationship, the mean plasma diltiazem concentration required to produce a 20% decrease in heart rate was determined to be 80 ng/mL. Mean plasma diltiazem concentrations of 130 ng/mL and 300 ng/mL were determined to produce reductions in heart rate of 30% to 40%.
Pharmacokinetics
- Following a single intravenous injection in healthy male volunteers, diltiazem hydrochloride appears to obey linear pharmacokinetics over a dose range of 10.5 to 21 mg. The plasma elimination half-life is approximately 3.4 hours. The apparent volume of distribution of diltiazem hydrochloride is approximately 305 L. Diltiazem is extensively metabolized in the liver with a systemic clearance of approximately 65 L/h.
- After constant rate intravenous infusion to healthy male volunteers, diltiazem exhibits nonlinear pharmacokinetics over an infusion range of 4.8 to 13.2 mg/h for 24 hours. Over this infusion range, as the dose is increased, systemic clearance decreases from 64 to 48 L/h while the plasma elimination half-life increases from 4.1 to 4.9 hours. The apparent volume of distribution remains unchanged (360 to 391 L). In patients with atrial fibrillation or atrial flutter, diltiazem systemic clearance has been found to be decreased compared to healthy volunteers. In patients administered bolus doses ranging from 2.5 mg to 38.5 mg, systemic clearance averaged 36 L/h. In patients administered continuous infusions at 10 mg/h or 15 mg/h for 24 hours, diltiazem systemic clearance averaged 42 L/h and 31 L/h, respectively.
- Based on the results of pharmacokinetic studies in healthy volunteers administered different oral diltiazem hydrochloride formulations, constant rate intravenous infusions of diltiazem hydrochloride at 3, 5, 7, and 11 mg/h are predicted to produce steady-state plasma diltiazem concentrations equivalent to 120-, 180-, 240-, and 360-mg total daily oral doses of diltiazem hydrochloride tablets or capsules.
- After oral administration, diltiazem hydrochloride undergoes extensive metabolism in man by deacetylation, N-demethylation, and O-demethylation via cytochrome P-450 (oxidative metabolism) in addition to conjugation. Metabolites N-monodesmethyldiltiazem, desacetyldiltiazem, desacetyl-N-monodesmethyldiltiazem, desacetyl-O-desmethyldiltiazem, and desacetyl-N, O-desmethyldiltiazem have been identified in human urine. Following oral administration, 2% to 4% of the unchanged diltiazem appears in the urine. Drugs which induce or inhibit hepatic microsomal enzymes may alter diltiazem disposition.
- Following single intravenous injection of diltiazem hydrochloride, however, plasma concentrations of N-monodesmethyldiltiazem and desacetyldiltiazem, two principal metabolites found in plasma after oral administration, are typically not detected. These metabolites are observed, however, following 24 hour constant rate intravenous infusion. Total radioactivity measurement following short IV administration in healthy volunteers suggests the presence of other unidentified metabolites which attain higher concentrations than those of diltiazem and are more slowly eliminated; half-life of total radioactivity is about 20 hours compared to 2 to 5 hours for diltiazem.
- Diltiazem hydrochloride is 70% to 80% bound to plasma proteins. In vitro studies suggest alpha1-acid glycoprotein binds approximately 40% of the drug at clinically significant concentrations. Albumin appears to bind approximately 30% of the drug, while other constituents bind the remaining bound fraction. Competitive in vitro ligand binding studies have shown that diltiazem binding is not altered by therapeutic concentrations of digoxin, phenytoin, hydrochlorothiazide, indomethacin, phenylbutazone, propranolol, salicylic acid, tolbutamide, or warfarin.
- Renal insufficiency, or even end-stage renal disease, does not appear to influence diltiazem disposition following oral administration. Liver cirrhosis was shown to reduce diltiazem's apparent oral clearance and prolong its half-life.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Diltiazem (injection) in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Diltiazem (injection) in the drug label.
How Supplied
- Diltiazem Hydrochloride Injection, 0.5% (5 mg/mL) is supplied:
- 5 mL vials NDC 17478-937-05
- 10 mL vials NDC 17478-937-10
- 25 mL vials NDC 17478-937-25
- SINGLE-USE CONTAINERS. DISCARD UNUSED PORTION.
Storage
- Store under refrigeration 2° to 8°C (36° to 46°F). DO NOT FREEZE. May be stored at room temperature for up to 1 month. Destroy after 1 month at room temperature.
Images
Drug Images
{{#ask: Page Name::Diltiazem (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Diltiazem (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Diltiazem (injection) in the drug label.
Precautions with Alcohol
- Alcohol-Diltiazem (injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- DILTIAZEM HYDROCHLORIDE®[16]
Look-Alike Drug Names
There is limited information regarding Diltiazem (injection) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Carapeti EA, Kamm MA, Phillips RK (2000). "Topical diltiazem and bethanechol decrease anal sphincter pressure and heal anal fissures without side effects". Dis Colon Rectum. 43 (10): 1359–62. PMID 11052511.
- ↑ Jonas M, Neal KR, Abercrombie JF, Scholefield JH (2001). "A randomized trial of oral vs. topical diltiazem for chronic anal fissures". Dis Colon Rectum. 44 (8): 1074–8. PMID 11535842.
- ↑ Christakis GT, Fremes SE, Weisel RD, Tittley JG, Mickle DA, Ivanov J; et al. (1986). "Diltiazem cardioplegia. A balance of risk and benefit". J Thorac Cardiovasc Surg. 91 (5): 647–61. PMID 3517506.
- ↑ Costa P, Donegani E, DePaulis R, Ottino GM, Villani M, Matani A; et al. (1986). "Diltiazem cold cardioplegia in coronary artery surgery: effects on myocardial function and ischemia". Tex Heart Inst J. 13 (1): 53–60. PMC 324598. PMID 15226832.
- ↑ Dönmez A, Karaaslan D, Sekerci S, Akpek E, Karakayali H, Arslan G (1999). "The effects of diltiazem and dopamine on early graft function in renal transplant recipients". Transplant Proc. 31 (8): 3305–6. PMID 10616487.
- ↑ Choy BY, Walker RG, Becker GJ (1994). "Vasculopathy in cyclosporine-treated renal allografts: possible protection by diltiazem". Clin Transplant. 8 (3 Pt 1): 271–3. PMID 8061366.
- ↑ Roti E, Montermini M, Roti S, Gardini E, Robuschi G, Minelli R; et al. (1988). "The effect of diltiazem, a calcium channel-blocking drug, on cardiac rate and rhythm in hyperthyroid patients". Arch Intern Med. 148 (9): 1919–21. PMID 2458079.
- ↑ Milner MR, Gelman KM, Phillips RA, Fuster V, Davies TF, Goldman ME (1990). "Double-blind crossover trial of diltiazem versus propranolol in the management of thyrotoxic symptoms". Pharmacotherapy. 10 (2): 100–6. PMID 2349134.
- ↑ Smith R, Schwartz A (1984). "Diltiazem prophylaxis in refractory migraine". N Engl J Med. 310 (20): 1327–8. doi:10.1056/NEJM198405173102015. PMID 6144044.
- ↑ "Correction: Outcomes in Patients with Acute Non-Q-Wave Myocardial Infarction Randomly Assigned to an Invasive as Compared with a Conservative Management Strategy". N Engl J Med. 339 (15): 1091. 1998. doi:10.1056/NEJM199810083391520. PMID 9761812.
- ↑ Ogawa H, Yasue H, Nakamura N, Obata K, Sonoda R (1987). "Hemodynamic effects of intravenous diltiazem in patients with acute myocardial infarction". Clin Cardiol. 10 (6): 323–8. PMID 3594955.
- ↑ Boquet J, Moore N, Lhuintre JP, Boismare F (1986). "Diltiazem for proctalgia fugax". Lancet. 1 (8496): 1493. PMID 2873295.
- ↑ Kambara H, Fujimoto K, Wakabayashi A, Kawai C (1981). "Primary pulmonary hypertension: beneficial therapy with diltiazem". Am Heart J. 101 (2): 230–1. PMID 7468425.
- ↑ Shinohara S, Murata I, Yamada H, Sato T, Kikutani T, Inoue T; et al. (1994). "Combined effects of diltiazem and oxygen in pulmonary hypertension of mixed connective tissue disease". J Rheumatol. 21 (9): 1763–5. PMID 7799364.
- ↑ Papademetriou V, Narayan P, Kokkinos P (1994). "Effects of diltiazem, metoprolol, enalapril and hydrochlorothiazide on frequency of ventricular premature complexes". Am J Cardiol. 73 (4): 242–6. PMID 7507638.
- ↑ "DILTIAZEM HYDROCHLORIDE- diltiazem hydrochloride injection".
