Stomach cancer pathophysiology: Difference between revisions
No edit summary |
No edit summary |
||
| Line 293: | Line 293: | ||
Tumor-like lesion | Tumor-like lesion | ||
Hyperplastic polyp | [[Hyperplastic polyp]] | ||
Fundic gland polyp | [[Fundic gland polyposis|Fundic gland polyp]] | ||
Heterotopic submucosal gland | Heterotopic submucosal gland | ||
Revision as of 22:29, 20 November 2017
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]Associate Editor(s)-in-Chief: Parminder Dhingra, M.D. [2]
|
Stomach cancer Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Stomach cancer pathophysiology On the Web |
|
American Roentgen Ray Society Images of Stomach cancer pathophysiology |
|
Risk calculators and risk factors for Stomach cancer pathophysiology |
Overview
The pathophysiology of stomach cancer depends on histologic subtypes.
Physiology of stomach
- The stomach consists of two functional areas; oxyntic and pyloric glands. The oxyntic area contains parietal cells that produce gastric acid.
- The antrum contains pyloric glands that secrete gastrin and somatostatin.
Pathophysiology
Molecular effect of H.pylori:
- There is a strong correlation between H. pylori and gastric cancer incidence.[1]
- Regression of premalignant lesions has been demonstrated with eradication of H. pylori.[2]
- This is related to nitric oxides accumulation produced by inflammatory cells responding to H. pylori infection.[3]
- Nitric oxides may induce abnormalities in the DNA of epithelial cells.
- The exact pathway for oncogenesis is not known but many trials supported the adenoma-carcinoma sequence.
Oncogenes
- K-ras mutations is found in invasive cancers and intestinal metaplasia.[4]
- Hepatocyte growth factor receptor c-met oncogene, encoding the h is supported by the finding that gene expression is found in intestinal-type gastric cancers. Effector protein CagA made by H.pylori modulates c-met receptor signal transduction pathways.[5]
Tumor suppressor genes
- Almost 50% of gastric cancers have alterations in genes TP53, TP73, APC, TFF, DCC, LOH, and FHIT.[6]
- Inactivation of p53 in gastric epithelial cells reduce their ability to undergo apoptosis.[7]
- Abnormalities are found in intestinal-type , intestinal metaplasia and dysplasia, and H. pylori-associated chronic gastritis.[8]
- Mutations in the APC gene are found in intestinal-type gastric cancers. APC mutations alternate the Wnt/catenin signaling pathway.[9]
- The trefoil factor family (TFF) is normally expressed in the gastroduodenal mucosa. Loss of TFF1 expression has been observed in gastric carcinomas.[10]
Cell cycle regulatory molecules
- Cyclin E overexpression is found in gastric carcinomas.[11]
- Cyclin E and Cyclin-dependent kinase inhibitor 1B are cell cycle regulators.[12]
Epigenetic events
- DNA methylation of gene promoters can silence the expression of CDH1.[13]
- Hypermethylation of the Reprimo gene has been found in the plasma of patients with gastric cancer and can be used as biomarkers in the detection of early gastric cancer.[14]
{{#ev:youtube|_aAhcNjmvhc}}
Beta-catenin/Wnt signaling
- Beta-catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer.[15]
- Beta-catenin is a part of Wnt signaling pathway which regulates coordination of events such as intercellular adhesion junctions, migration, proliferation, and differentiation.
- Beta-catenin is normally bound to protein complexes in the cell membrane that are involved in normal intercellular adhesions.
- APC gene protein prevents the accumulation of beta-catenin. APC mutations lead to loss of regulation of beta-catenin which leads to proliferation, angiogenesis, tumor invasion, and metastasis of cells.[16]
{{#ev:youtube|oweNT288BXo}}
Diffuse-type gastric cancer
- Diffuse gastric carcinomas do not have a precancerous lesion.
- They are highly metastatic with a poorer prognosis than intestinal cancers. When the entire stomach wall is infiltrated, it results in a rigid thickened stomach wall called linitis plastic.[17]
- Intracellular mucin is accumulating pushing the nucleus giving the histological figure of signet ring carcinoma.
- The E-cadherin gene (CDH1) encodes a transmembrane cellular adhesion protein. Its cytoplasmic tail interacts with catenins making the adhesion.
- Somatic mutations in the CDH1 gene by hypermethylation, mutation, and loss of heterozygosity are identified in 40 to 83 percent of sporadic diffuse-type gastric cancers.[18]
- Prostate stem cell antigen gene is also involved in regulating gastric epithelial cell proliferation.[19]
Apoptosis pathway
Neutrophil activation
- H. pylori infection results in the migration of neutrophils to the site of infection and adhesion to the surface epithelium.
- The neutrophils produce nitric oxide synthase which damage DNA.
- CD11a/CD18- and CD11b/CD18-neutrophils interact with intercellular adhesion molecule-1 (ICAM-1).[20]
- Epithelial cells respond by signaling pathways leading to apoptosis, proliferation, differentiation, and autophagy.
Apoptotic pathways
- Apoptosis occurs as a protective mechanism to prevent replication of mutated DNA which leads to atrophy of epithelium so called atrophic gastritis which returns to normal following eradication therapy.[21]
- H. pylori enhances expression of the Fas receptor on gastric epithelial cells and may mediate apoptosis through signaling mechanisms related to the Fas death receptor.
- Another trial supported that the source of tumorigenesis is from bone marrow-derived cells that differentiate into gastric epithelial cells in the presence of H. pylori.[22]
{{#ev:youtube|SyvOPXeg4ig}}
| Helicobacter pylori infection | |||||||||||||||||||||||||||||||||||||
| Inflammatory response secretes IL-8 ,IL-1b | Production of alkaline ammonia | Production of urease bacterial phospholipase A | |||||||||||||||||||||||||||||||||||
| Infux of neutophils and macrophages release of lysosomal enzymes leukotrienes (LT)and reactive oxygen | inhibition of D-cells leads to inappropriate release of somatostatin and hypergastrinemia | Production of urease ,phospholipase A and C release toxic metabolities | |||||||||||||||||||||||||||||||||||
| Mucosal injury | |||||||||||||||||||||||||||||||||||||
Associated disorders
Familial predisposition
- Although most gastric cancers are sporadic, 10 percent of cases are familial.
Hereditary diffuse gastric cancer
- Clinical criteria for HDGC as described by the International Gastric Cancer Linkage Consortium (IGCLC).[23]
- Germline truncating mutations in the CDH1 gene, which encodes the cell adhesion protein E-cadherin, have been identified HDGC is inherited as an autosomal dominant trait with high penetrance.[24]
- The cumulative risk for gastric cancer by age 80 for CDH1 mutation carriers is up to 70 percent in men and up to 56 percent in women.[25]
- Promoter hypermethylation, mutation, and loss of heterozygosity. The end result is loss of expression of the cell adhesion molecule E-cadherin.
- The risk of gastric cancer in asymptomatic carriers of a pathogenetic CDH1 mutation who belong to families with highly penetrant hereditary diffuse gastric cancer is sufficiently high to warrant prophylactic gastrectomy.
- Women in these affected families are also at high risk of developing breast cancer, predominantly lobular. The cumulative risk of breast cancer to age 80 for CDH1 mutation carriers is approximately 42 percent, and like the gastric cancers, the increased relative risk starts early.
Gastric Adenocarcinoma and Proximal Polyposis of the Stomach (GAPPS)
- GAPPS was characterized by the autosomal dominant transmission of fundic gland polyposis that is restricted to the proximal stomach, with no evidence of duodenal or colorectal polyposis or other hereditary gastrointestinal (GI) cancer syndrome.[26]
Familial intestinal gastric cancer
- FIGC should be considered a potential diagnosis when histopathological reports denote intestinal-type gastric cancers that segregate within families without gastric polyposis.[27]
Other hereditary cancer syndromes:[27]
- Lynch syndrome (hereditary nonpolyposis colorectal cancer)
- Familial adenomatous polyposis (FAP)
- Li-Fraumeni syndrome
- Peutz Jeghers syndrome
- juvenile polyposis
- Hereditary breast and ovarian cancer syndrome
- Cowden's syndrome
Gross pathology
| Type | Description |
|---|---|
| Type 0 | (superficial) Typical of T1 tumors |
| Type 1 | (mass) Polypoid tumors sharply demarcated from the
surrounding mucosa |
| Type 2 | (ulcerative) Ulcerated tumors with raised margins
surrounded by a thickened gastric wall with clear margins |
| Type 3 | (infiltrative ulcerative)
Ulcerated tumors with raised margins, surrounded by a thickened gastric wall without clear margins |
| Type 4 | (diffuse infiltrative)
Tumors without marked ulceration or raised margins, the gastric wall is thickened and indurated and the margin is unclear |
| Type 5 | (unclassifiable)
Tumors that cannot be classified into any of the above types |
Video shows growth pathology of gastric cancer
{{#ev:youtube|ih-npVIJA6U}}

Histopathology
- Gastric adenocarcinoma is a malignant epithelial tumor, originating from glandular epithelium of the gastric mucosa. It invades the gastric wall, infiltrating the muscularis mucosae, the submucosa and hence the muscular propria. Histologically, there are two major types of gastric cancer (Lauren classification): intestinal type and diffuse type.
- Intestinal type adenocarcinoma: Tumor cells describe irregular tubular structures, harboring pluristratification, multiple lumens, and reduced stroma ("back to back" aspect). Often, it associates intestinal metaplasia in neighboring mucosa. Depending on glandular architecture, cellular pleomorphism and mucosecretion, adenocarcinoma may present 3 degrees of differentiation: well, moderate and poorly differentiated.
- Diffuse type adenocarcinoma (mucinous, colloid): tumor cells are discohesive and secrete mucus which is delivered in the interstitium producing large pools of mucus/colloid (optically "empty" spaces). It is poorly differentiated. If the mucus remains inside the tumor cell, it pushes the nucleus at the periphery - "signet-ring cell".
World Health Organization histological classification of gastric tumors:
| Types | Histological features |
|---|---|
| Epithelial tumors |
|
| Non-epithelial tumors | Leiomyoma
GI stromal tumor Benign Uncertain malignant potential Malignant |
| Malignant lymphomas | Marginal zone B-cell lymphoma of MALT-type |
Japanese histological classification of gastric tumors:
| Types | Histological features |
|---|---|
| Epithelial tumors |
Benign epithelial tumorMalignant epithelial tumorCommon type Papillary adenocarcinoma Tubular adenocarcinoma Well-differentiated Moderately differentiated Poorly differentiated adenocarcinoma Solid type Non-solid type Signet-ring cell carcinoma Mucinous adenocarcinoma |
| Special types | Carcinoid tumor
Endocrine carcinoma Carcinoma with lymphoid stroma Hepatoid adenocarcinoma Adenosquamous carcinoma Squamous cell carcinoma Undifferentiated carcinoma |
| Miscellaneous carcinoma | Non-epithelial tumor
Gastrointestinal stromal tumor (GIST) Smooth muscle tumor Neurogenic tumor Miscellaneous non-epithelial tumors Lymphoma B-cell lymphoma MALT (mucosa-associated lymphoid tissue) lymphoma Follicular lymphoma Mantle cell lymphoma Diffuse large B-cell lymphoma Other B-cell lymphomas T-cell lymphoma Other lymphomas Metastatic tumor Tumor-like lesion Heterotopic submucosal gland Heterotopic pancreas Inflammatory fibroid polyp Gastrointestinal polyposis Familial polyposis coli, Peutz–Jeghers syndrome |

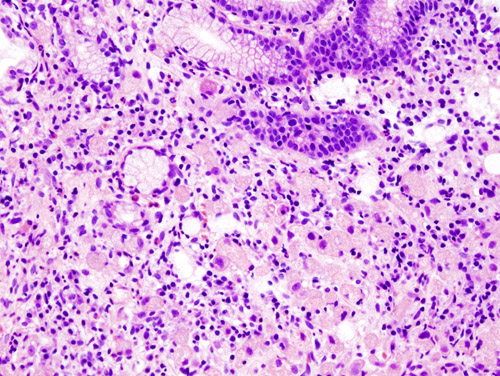
Video shows microscopic pathology of gastric cancer
{{#ev:youtube|lRvq1fEW8sY}}
{{#ev:youtube|lWeECaiEfSs}}
References
- ↑ Eslick GD, Lim LL, Byles JE, Xia HH, Talley NJ (1999). "Association of Helicobacter pylori infection with gastric carcinoma: a meta-analysis". Am J Gastroenterol. 94 (9): 2373–9. doi:10.1111/j.1572-0241.1999.01360.x. PMID 10483994.
- ↑ Mera R, Fontham ET, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC; et al. (2005). "Long term follow up of patients treated for Helicobacter pylori infection". Gut. 54 (11): 1536–40. doi:10.1136/gut.2005.072009. PMC 1462952. PMID 15985559.
- ↑ Mannick EE, Bravo LE, Zarama G, Realpe JL, Zhang XJ, Ruiz B; et al. (1996). "Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants". Cancer Res. 56 (14): 3238–43. PMID 8764115.
- ↑ Yasui W, Oue N, Kuniyasu H, Ito R, Tahara E, Yokozaki H (2001). "Molecular diagnosis of gastric cancer: present and future". Gastric Cancer. 4 (3): 113–21. doi:10.1007/s101200100001. PMID 11760076.
- ↑ Smith MG, Hold GL, Tahara E, El-Omar EM (2006). "Cellular and molecular aspects of gastric cancer". World J Gastroenterol. 12 (19): 2979–90. PMC 4124370. PMID 16718776.
- ↑ Ushiku T, Chong JM, Uozaki H, Hino R, Chang MS, Sudo M; et al. (2007). "p73 gene promoter methylation in Epstein-Barr virus-associated gastric carcinoma". Int J Cancer. 120 (1): 60–6. doi:10.1002/ijc.22275. PMID 17058198.
- ↑ Ashktorab H, Ahmed A, Littleton G, Wang XW, Allen CR, Tackey R; et al. (2003). "p53 and p14 increase sensitivity of gastric cells to H. pylori-induced apoptosis". Dig Dis Sci. 48 (7): 1284–91. PMID 12870784.
- ↑ Kodama M, Murakami K, Okimoto T, Sato R, Watanabe K, Fujioka T (2007). "Expression of mutant type-p53 products in H pylori-associated chronic gastritis". World J Gastroenterol. 13 (10): 1541–6. PMC 4146896. PMID 17461446.
- ↑ Nakatsuru S, Yanagisawa A, Furukawa Y, Ichii S, Kato Y, Nakamura Y; et al. (1993). "Somatic mutations of the APC gene in precancerous lesion of the stomach". Hum Mol Genet. 2 (9): 1463–5. PMID 8242071.
- ↑ Leung WK, Yu J, Chan FK, To KF, Chan MW, Ebert MP; et al. (2002). "Expression of trefoil peptides (TFF1, TFF2, and TFF3) in gastric carcinomas, intestinal metaplasia, and non-neoplastic gastric tissues". J Pathol. 197 (5): 582–8. doi:10.1002/path.1147. PMID 12210076.
- ↑ Bani-Hani KE, Almasri NM, Khader YS, Sheyab FM, Karam HN (2005). "Combined evaluation of expressions of cyclin E and p53 proteins as prognostic factors for patients with gastric cancer". Clin Cancer Res. 11 (4): 1447–53. doi:10.1158/1078-0432.CCR-04-1730. PMID 15746045.
- ↑ Takano Y, Kato Y, van Diest PJ, Masuda M, Mitomi H, Okayasu I (2000). "Cyclin D2 overexpression and lack of p27 correlate positively and cyclin E inversely with a poor prognosis in gastric cancer cases". Am J Pathol. 156 (2): 585–94. doi:10.1016/S0002-9440(10)64763-3. PMC 1850035. PMID 10666388.
- ↑ Yasui W, Sentani K, Motoshita J, Nakayama H (2006). "Molecular pathobiology of gastric cancer". Scand J Surg. 95 (4): 225–31. doi:10.1177/145749690609500403. PMID 17249269.
- ↑ Bernal C, Aguayo F, Villarroel C, Vargas M, Díaz I, Ossandon FJ; et al. (2008). "Reprimo as a potential biomarker for early detection in gastric cancer". Clin Cancer Res. 14 (19): 6264–9. doi:10.1158/1078-0432.CCR-07-4522. PMID 18829507.
- ↑ Clements WM, Wang J, Sarnaik A, Kim OJ, MacDonald J, Fenoglio-Preiser C; et al. (2002). "beta-Catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer". Cancer Res. 62 (12): 3503–6. PMID 12067995.
- ↑ Lowy AM, Clements WM, Bishop J, Kong L, Bonney T, Sisco K; et al. (2006). "beta-Catenin/Wnt signaling regulates expression of the membrane type 3 matrix metalloproteinase in gastric cancer". Cancer Res. 66 (9): 4734–41. doi:10.1158/0008-5472.CAN-05-4268. PMID 16651426.
- ↑ Graziano F, Humar B, Guilford P (2003). "The role of the E-cadherin gene (CDH1) in diffuse gastric cancer susceptibility: from the laboratory to clinical practice". Ann Oncol. 14 (12): 1705–13. PMID 14630673.
- ↑ Ramos-de la Medina A, More H, Medina-Franco H, Humar B, Gamboa A, Ortiz LJ; et al. (2006). "Single nucleotide polymorphisms (SNPs) at CDH1 promoter region in familial gastric cancer". Rev Esp Enferm Dig. 98 (1): 36–41. PMID 16555931.
- ↑ Study Group of Millennium Genome Project for Cancer. Sakamoto H, Yoshimura K, Saeki N, Katai H, Shimoda T; et al. (2008). "Genetic variation in PSCA is associated with susceptibility to diffuse-type gastric cancer". Nat Genet. 40 (6): 730–40. doi:10.1038/ng.152. PMID 18488030.
- ↑ Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M; et al. (2001). "Helicobacter pylori infection and the development of gastric cancer". N Engl J Med. 345 (11): 784–9. doi:10.1056/NEJMoa001999. PMID 11556297.
- ↑ Xia HH, Talley NJ (2001). "Apoptosis in gastric epithelium induced by Helicobacter pylori infection: implications in gastric carcinogenesis". Am J Gastroenterol. 96 (1): 16–26. doi:10.1111/j.1572-0241.2001.03447.x. PMID 11197247.
- ↑ Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H; et al. (2004). "Gastric cancer originating from bone marrow-derived cells". Science. 306 (5701): 1568–71. doi:10.1126/science.1099513. PMID 15567866.
- ↑ Hansford S, Kaurah P, Li-Chang H, Woo M, Senz J, Pinheiro H; et al. (2015). "Hereditary Diffuse Gastric Cancer Syndrome: CDH1 Mutations and Beyond". JAMA Oncol. 1 (1): 23–32. doi:10.1001/jamaoncol.2014.168. PMID 26182300.
- ↑ van der Post RS, Vogelaar IP, Carneiro F, Guilford P, Huntsman D, Hoogerbrugge N; et al. (2015). "Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers". J Med Genet. 52 (6): 361–74. doi:10.1136/jmedgenet-2015-103094. PMC 4453626. PMID 25979631.
- ↑ van der Post RS, Vogelaar IP, Manders P, van der Kolk LE, Cats A, van Hest LP; et al. (2015). "Accuracy of Hereditary Diffuse Gastric Cancer Testing Criteria and Outcomes in Patients With a Germline Mutation in CDH1". Gastroenterology. 149 (4): 897–906.e19. doi:10.1053/j.gastro.2015.06.003. PMID 26072394.
- ↑ Brosens LA, Giardiello FM, Offerhaus GJ, Montgomery EA (2016). "Syndromic Gastric Polyps: At the Crossroads of Genetic and Environmental Cancer Predisposition". Adv Exp Med Biol. 908: 347–69. doi:10.1007/978-3-319-41388-4_17. PMID 27573780.
- ↑ 27.0 27.1 Choi YJ, Kim N (2016). "Gastric cancer and family history". Korean J Intern Med. 31 (6): 1042–1053. doi:10.3904/kjim.2016.147. PMC 5094936. PMID 27809451.