Sexcord/ stromal ovarian tumors pathophysiology: Difference between revisions
(Created page with "___NOTOC___ {{Sexcord/ stromal ovarian tumors}} {{CMG}}; {{AE}} ==Overview== The exact pathogenesis of [disease name] is not fully understood. OR It is thought that [disea...") |
No edit summary |
||
| (124 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | |||
{{Sexcord/ stromal ovarian tumors}} | {{Sexcord/ stromal ovarian tumors}} | ||
{{CMG}}; {{AE}} | {{CMG}}; {{AE}} ;{{M.N}} | ||
==Overview== | ==Overview== | ||
The exact pathogenesis of | The exact [[pathogenesis]] of sexcord/ stromal ovarian tumors is not fully understood. [[Mutations]] mainly involving [[FOXL2]], [[DICER1]], [[STK11]] are involved. They are associated with [[ollier disease]] and [[Maffucci's syndrome|maffucci syndrome]].The [[microscopic]] [[pathology]] varies with the individual subtype of sexcord stromal ovarian tumors. | ||
==Pathophysiology== | |||
===Pathogenesis=== | |||
*The exact [[pathogenesis]] of sexcord/ stromal ovarian tumors is not completely understood.<ref name="pmid27885265">{{cite journal |vauthors=Karnezis AN, Cho KR, Gilks CB, Pearce CL, Huntsman DG |title=The disparate origins of ovarian cancers: pathogenesis and prevention strategies |journal=Nat. Rev. Cancer |volume=17 |issue=1 |pages=65–74 |date=January 2017 |pmid=27885265 |doi=10.1038/nrc.2016.113 |url=}}</ref> | |||
*However [[Genetics|genetic]] factors attribute to the [[pathogenesis]] of some subtypes of the [[tumors]] | |||
*The [[pathophysiology]] of sexcord/ stromal ovarian tumors depends on the [[histological]] subtype. | |||
It is | ==Genetics== | ||
*The recent advancing analyses have made us understand the [[pathophysiology]] of some of these [[tumor]] subtypes | |||
*[[Mutations]] mainly involving [[DICER1]], [[STK11]], and [[FOXL2]] influence the [[development]] of some of these [[neoplasms]] | |||
'''[[FOXL2]]''':<ref name="LimOliva2018">{{cite journal|last1=Lim|first1=Diana|last2=Oliva|first2=Esther|title=Ovarian sex cord-stromal tumours: an update in recent molecular advances|journal=Pathology|volume=50|issue=2|year=2018|pages=178–189|issn=00313025|doi=10.1016/j.pathol.2017.10.008}}</ref><ref name="pmid27813081">{{cite journal |vauthors=Fuller PJ, Leung D, Chu S |title=Genetics and genomics of ovarian sex cord-stromal tumors |journal=Clin. Genet. |volume=91 |issue=2 |pages=285–291 |date=February 2017 |pmid=27813081 |doi=10.1111/cge.12917 |url=}}</ref><ref name="LiBao2018">{{cite journal|last1=Li|first1=Jiaheng|last2=Bao|first2=Riqiang|last3=Peng|first3=Shiwei|last4=Zhang|first4=Chunping|title=The molecular mechanism of ovarian granulosa cell tumors|journal=Journal of Ovarian Research|volume=11|issue=1|year=2018|issn=1757-2215|doi=10.1186/s13048-018-0384-1}}</ref><ref name="pmid29409506">{{cite journal |vauthors=Li J, Bao R, Peng S, Zhang C |title=The molecular mechanism of ovarian granulosa cell tumors |journal=J Ovarian Res |volume=11 |issue=1 |pages=13 |date=February 2018 |pmid=29409506 |pmc=5802052 |doi=10.1186/s13048-018-0384-1 |url=}}</ref><ref name="pmid27858560">{{cite journal |vauthors=Schultz KA, Harris AK, Schneider DT, Young RH, Brown J, Gershenson DM, Dehner LP, Hill DA, Messinger YH, Frazier AL |title=Ovarian Sex Cord-Stromal Tumors |journal=J Oncol Pract |volume=12 |issue=10 |pages=940–946 |date=October 2016 |pmid=27858560 |pmc=5063189 |doi=10.1200/JOP.2016.016261 |url=}}</ref><ref name="BoussiosMoschetta2017">{{cite journal|last1=Boussios|first1=Stergios|last2=Moschetta|first2=Michele|last3=Zarkavelis|first3=George|last4=Papadaki|first4=Alexandra|last5=Kefas|first5=Aristides|last6=Tatsi|first6=Konstantina|title=Ovarian sex-cord stromal tumours and small cell tumours: Pathological, genetic and management aspects|journal=Critical Reviews in Oncology/Hematology|volume=120|year=2017|pages=43–51|issn=10408428|doi=10.1016/j.critrevonc.2017.10.007}}</ref><ref name="LeungFuller2016">{{cite journal|last1=Leung|first1=Dilys T.H.|last2=Fuller|first2=Peter J.|last3=Chu|first3=Simon|title=Impact of FOXL2 mutations on signaling in ovarian granulosa cell tumors|journal=The International Journal of Biochemistry & Cell Biology|volume=72|year=2016|pages=51–54|issn=13572725|doi=10.1016/j.biocel.2016.01.003}}</ref> | |||
*[[FOXL2]] is a [[tumor suppressor gene]] | |||
*It is a member of the [[Forkhead box L2|forkhead box]] (FOX) family of [[Evolution@Home|evolutionarily]] conserved [[transcription factors]] | |||
*It plays a [[Fundamental unit|fundamental]] and crucial role in [[Ovarian|ovarian development]] | |||
*It regulates the [[ovarian]] [[granulosa cell]] [[proliferation]], [[Follicle|follicle development]] and [[Ovarian hormones|ovarian hormones synthesis]] | |||
*Almost all like 97% of adult [[granulosa cell tumors]] are characterized by [[Missense mutations|missense]] [[somatic]] [[point mutations]] (402 C→G) in [[FOXL2|FOXL2 gene]] | |||
*Infact this [[mutation]] is a [[Sensitivity|sensitive]] and specific [[biomarker]] for adult [[granulosa cell tumors]] making it a [[pathognomonic]] feature | |||
*The [[Phosphorylation|phosphorylation modification]] of [[FOXL2]] in particular is responsible to the [[growth]] of [[granulosa cell tumors]] | |||
*Importantly this [[mutation]] alter's antiproliferative pathways and also limit the [[apoptosis]], as a result contributing to the [[pathogenesis]] of adult [[granulosa cell tumors]] | |||
*Other factors that play an important role in the [[pathogenesis]] of [[granulosa cell tumors]] are [[PI3K]]/[[AKT]] ([[Phosphatidylinositol|phosphatidylinositol-3-kinase]]; [[serine/threonine kinase]]), [[TGF-β]] ([[Transforming growth factor beta]]) [[signaling pathway]], [[Notch signaling pathway]], [[GATA4]] and [[VEGF]] ([[vascular endothelial growth factor]]) | |||
[[File:Granulosa cell tumor.jpeg|center|thumb|700px|Schematic representation of the cell signaling pathways in GCT development. PI3K, phosphatidylinositol-3-kinase; AKT, serine/threonine kinase; FOXO 1/3, forkhead box O1/3; AMH, [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5802052/ Source:Li J, Bao R, Peng S, Zhang C. The molecular mechanism of ovarian granulosa cell tumors. J Ovarian Res. 2018;11(1):13. Published 2018 Feb 6. doi:10.1186/s13048-018-0384-1]]] | |||
'''[[DICER1]]''':<ref name="pmid27813081">{{cite journal |vauthors=Fuller PJ, Leung D, Chu S |title=Genetics and genomics of ovarian sex cord-stromal tumors |journal=Clin. Genet. |volume=91 |issue=2 |pages=285–291 |date=February 2017 |pmid=27813081 |doi=10.1111/cge.12917 |url=}}</ref><ref name="pmid26033501">{{cite journal |vauthors=Goulvent T, Ray-Coquard I, Borel S, Haddad V, Devouassoux-Shisheboran M, Vacher-Lavenu MC, Pujade-Laurraine E, Savina A, Maillet D, Gillet G, Treilleux I, Rimokh R |title=DICER1 and FOXL2 mutations in ovarian sex cord-stromal tumours: a GINECO Group study |journal=Histopathology |volume=68 |issue=2 |pages=279–85 |date=January 2016 |pmid=26033501 |doi=10.1111/his.12747 |url=}}</ref><ref name="pmid27241106">{{cite journal |vauthors=Stewart CJ, Charles A, Foulkes WD |title=Gynecologic Manifestations of the DICER1 Syndrome |journal=Surg Pathol Clin |volume=9 |issue=2 |pages=227–41 |date=June 2016 |pmid=27241106 |doi=10.1016/j.path.2016.01.002 |url=}}</ref><ref name="pmid29660837">{{cite journal |vauthors=Wang Y, Karnezis AN, Magrill J, Tessier-Cloutier B, Lum A, Senz J, Gilks CB, McCluggage WG, Huntsman DG, Kommoss F |title=DICER1 hot-spot mutations in ovarian gynandroblastoma |journal=Histopathology |volume=73 |issue=2 |pages=306–313 |date=August 2018 |pmid=29660837 |doi=10.1111/his.13630 |url=}}</ref><ref name="LimOliva2018">{{cite journal|last1=Lim|first1=Diana|last2=Oliva|first2=Esther|title=Ovarian sex cord-stromal tumours: an update in recent molecular advances|journal=Pathology|volume=50|issue=2|year=2018|pages=178–189|issn=00313025|doi=10.1016/j.pathol.2017.10.008}}</ref><ref name="pmid30334998">{{cite journal |vauthors=Xu Q, Zou Y, Zhang XF |title=Sertoli-Leydig cell tumors of ovary: A case series |journal=Medicine (Baltimore) |volume=97 |issue=42 |pages=e12865 |date=October 2018 |pmid=30334998 |pmc=6211859 |doi=10.1097/MD.0000000000012865 |url=}}</ref> | |||
*[[DICER1]] [[mutations]] are associated with [[Leydig cell tumor|leydig cell tumors]] and gynandroblastomas | |||
*Although both [[germ line]] and [[somatic]] [[mutations]] play a role, approximately 60% of [[Leydig cell tumor|sexcord leydig cell tumors]] have [[somatic]] [[DICER1]] [[mutations]] | |||
*This particular [[DICER1|gene DICER1]] encodes for a [[RNA]] [[endoribonuclease]] that helps to cleave [[Precursor mRNA|precursor miRNA]] into mature miRNAs | |||
*[[DICER1]] [[mutations]] are associated with a lot of [[tumors]] of which [[pleuropulmonary blastoma]], is the most common [[Lung Disease|lung tumor]] of [[infancy]] and early [[childhood]] | |||
*Others are [[embryonal rhabdomyosarcoma]] of the [[uterine]] [[cervix]], [[renal]] [[tumors]], [[Thyroid Carcinoma|thyroid nodules and carcinoma]], nasal [[Hamartoma|chondromesenchymal hamartoma]], [[ciliary body]] [[medulloepithelioma]], [[pineoblastoma]], and [[pituitary]] [[blastoma]] | |||
*The above mentioned [[tumors]] typically have [[Alleles|biallelic]] [[DICER1]] [[mutations]] that are composed of a loss of function in one [[allele]] and a [[Missense mutations|missense mutation]] in the [[RNase III|RNase IIIb domain]] | |||
'''[[STK11]]''': | |||
*[[Mutations]] in the [[STK11|STK11 gene]] is associated with sex cord-stromal tumors with annular tubules | |||
==Associated Conditions== | |||
*[[Patients]] with [[ollier disease]] and [[maffucci syndrome]] are associated with an increased risk of [[Granulosa cell tumors|juvenile granulosa cell tumors]]<ref name="pmid27241106">{{cite journal |vauthors=Stewart CJ, Charles A, Foulkes WD |title=Gynecologic Manifestations of the DICER1 Syndrome |journal=Surg Pathol Clin |volume=9 |issue=2 |pages=227–41 |date=June 2016 |pmid=27241106 |doi=10.1016/j.path.2016.01.002 |url=}}</ref><ref name="pmid27858560">{{cite journal |vauthors=Schultz KA, Harris AK, Schneider DT, Young RH, Brown J, Gershenson DM, Dehner LP, Hill DA, Messinger YH, Frazier AL |title=Ovarian Sex Cord-Stromal Tumors |journal=J Oncol Pract |volume=12 |issue=10 |pages=940–946 |date=October 2016 |pmid=27858560 |pmc=5063189 |doi=10.1200/JOP.2016.016261 |url=}}</ref> | |||
*[[Somatic]] [[Mosaic (genetics)|mosaic]] [[mutations]] in [[IDH1]] and [[IDH2]] are observed | |||
*[[Ollier disease]] includes [[Enchondroma|enchondromatosis]], whereas [[maffucci syndrome]] includes [[Enchondroma|enchondromatosis]] and [[hemangiomas]] | |||
[ | ==Gross and Microscopic Pathology== | ||
The [[Gross pathology|gross]] and [[microscopic]] features of the most common [[tumors]] are described below:<ref name="pmid27858560">{{cite journal |vauthors=Schultz KA, Harris AK, Schneider DT, Young RH, Brown J, Gershenson DM, Dehner LP, Hill DA, Messinger YH, Frazier AL |title=Ovarian Sex Cord-Stromal Tumors |journal=J Oncol Pract |volume=12 |issue=10 |pages=940–946 |date=October 2016 |pmid=27858560 |pmc=5063189 |doi=10.1200/JOP.2016.016261 |url=}}</ref><ref name="pmid26782032">{{cite journal |vauthors=Bremmer F, Schweyer S |title=[Leydig cell, Sertoli cell and adult granulosa cell tumors] |language=German |journal=Pathologe |volume=37 |issue=1 |pages=71–7 |date=February 2016 |pmid=26782032 |doi=10.1007/s00292-015-0131-y |url=}}</ref><ref name="pmid24819979">{{cite journal |vauthors=Bremmer F, Behnes CL, Radzun HJ, Bettstetter M, Schweyer S |title=[Sex cord gonadal stromal tumors] |language=German |journal=Pathologe |volume=35 |issue=3 |pages=245–51 |date=May 2014 |pmid=24819979 |doi=10.1007/s00292-014-1901-7 |url=}}</ref><ref name="pmid21317704">{{cite journal |vauthors=Roth LM, Czernobilsky B |title=Perspectives on pure ovarian stromal neoplasms and tumor-like proliferations of the ovarian stroma |journal=Am. J. Surg. Pathol. |volume=35 |issue=3 |pages=e15–33 |date=March 2011 |pmid=21317704 |doi=10.1097/PAS.0b013e31820acb89 |url=}}</ref><ref name="pmid29132723">{{cite journal |vauthors=Young RH |title=Ovarian sex cord-stromal tumours and their mimics |journal=Pathology |volume=50 |issue=1 |pages=5–15 |date=January 2018 |pmid=29132723 |doi=10.1016/j.pathol.2017.09.007 |url=}}</ref><ref name="pmid25400781">{{cite journal |vauthors=Zhang HY, Zhu JE, Huang W, Zhu J |title=Clinicopathologic features of ovarian Sertoli-Leydig cell tumors |journal=Int J Clin Exp Pathol |volume=7 |issue=10 |pages=6956–64 |date=2014 |pmid=25400781 |pmc=4230071 |doi= |url=}}</ref><ref name="ChenRuiz2003">{{cite journal|last1=Chen|first1=Vivien W.|last2=Ruiz|first2=Bernardo|last3=Killeen|first3=Jeffrey L.|last4=Cot�|first4=Timothy R.|last5=Wu|first5=Xiao Cheng|last6=Correa|first6=Catherine N.|last7=Howe|first7=Holly L.|title=Pathology and classification of ovarian tumors|journal=Cancer|volume=97|issue=S10|year=2003|pages=2631–2642|issn=0008-543X|doi=10.1002/cncr.11345}}</ref><ref name="BoussiosMoschetta2017">{{cite journal|last1=Boussios|first1=Stergios|last2=Moschetta|first2=Michele|last3=Zarkavelis|first3=George|last4=Papadaki|first4=Alexandra|last5=Kefas|first5=Aristides|last6=Tatsi|first6=Konstantina|title=Ovarian sex-cord stromal tumours and small cell tumours: Pathological, genetic and management aspects|journal=Critical Reviews in Oncology/Hematology|volume=120|year=2017|pages=43–51|issn=10408428|doi=10.1016/j.critrevonc.2017.10.007}}</ref><ref name="pmid26200099">{{cite journal |vauthors=Irving JA, Lee CH, Yip S, Oliva E, McCluggage WG, Young RH |title=Microcystic Stromal Tumor: A Distinctive Ovarian Sex Cord-Stromal Neoplasm Characterized by FOXL2, SF-1, WT-1, Cyclin D1, and β-catenin Nuclear Expression and CTNNB1 Mutations |journal=Am. J. Surg. Pathol. |volume=39 |issue=10 |pages=1420–6 |date=October 2015 |pmid=26200099 |doi=10.1097/PAS.0000000000000482 |url=}}</ref><ref name="pmid18971779">{{cite journal |vauthors=Irving JA, Young RH |title=Microcystic stromal tumor of the ovary: report of 16 cases of a hitherto uncharacterized distinctive ovarian neoplasm |journal=Am. J. Surg. Pathol. |volume=33 |issue=3 |pages=367–75 |date=March 2009 |pmid=18971779 |doi=10.1097/PAS.0b013e31818479c3 |url=}}</ref><ref name="pmid29676374">{{cite journal |vauthors=Mathur A, Seth A, Pant L |title=Ovarian fibroma with luteinized thecal cells and minor sex cord element: A rare case report |journal=Indian J Pathol Microbiol |volume=61 |issue=2 |pages=264–267 |date=2018 |pmid=29676374 |doi=10.4103/IJPM.IJPM_446_17 |url=}}</ref> | |||
{| class="wikitable" | |||
|+ | |||
!Types | |||
!Gross pathology | |||
!Microscopic pathology | |||
!Images | |||
|- | |||
|[[Granulosa cell tumours|Adult granulosa cell tumours]] | |||
| | |||
* They show yellowish [[solid]] or [[solid]]-[[cystic]] appearance on cut surface | |||
| | |||
* It is characterised by [[Dense|densely]] [[Cellular|cellular sheets]] of [[cells]] with scant [[cytoplasm]] imparting a ‘small blue [[cell]] [[tumour]]’ appearance | |||
* Small [[cavities]] called [[Call-Exner bodies|Call–Exner bodies]] that may contain [[eosinophilic]] [[fluid]], [[Degenerative|degenerating]] [[nuclei]], [[Hyaline|hyalinised]] [[Basement membrane|basement membrane material]], or rarely [[basophilic]] [[fluid]] are seen | |||
* The presence of [[nuclear]] grooves and [[Call-Exner bodies|Call–Exner bodies]] are often considered to be [[pathognomonic]] of [[Granulosa cell tumour|AGCTs]] | |||
| | |||
[[File:Call-Exner bodies.png|300px|thumb|Mikael Häggström and Nephron [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)],https://upload.wikimedia.org/wikipedia/commons/9/9b/Call-Exner_bodies.png]] | |||
|- | |||
|[[Granulosa cell tumours|Juvenile granulosa cell tumours]] | |||
| | |||
* They are [[solid]]/[[cystic]] on cut sections | |||
[ | * The [[solid]] areas show a tan-yellow or greyish appearance with [[Haemorrhage|foci of haemorrhage]] and/or [[necrosis]] | ||
* The [[cysts]] have [[serous]] or [[Haemorrhage|haemorrhagic contents]] | |||
| | |||
* Shows a [[nodular]] or [[diffuse]] [[proliferation]] of [[cells]] embedded in an [[Edema|edematous]] or myxoid [[stroma]] | |||
* [[Follicle]]-like spaces of varying sizes and shapes, containing [[eosinophilic]] or [[basophilic]] [[secretions]], is a characteristic feature | |||
* [[Call-Exner bodies|Call–Exner bodies]] are almost never seen | |||
| | |||
[[File:1200px-Juvenile granulosa cell tumour - very high mag.jpg|300px|thumb|Microcystic spaces, cuboidal-to-polygonal cells in sheets or stands or cords, with moderate-to-marked nuclear atypia, and basophilic cytoplasm https://librepathology.org/wiki/File:Juvenile_granulosa_cell_tumour_-_very_high_mag.jpg]] | |||
|- | |||
|Sex cord tumour with annular tubules | |||
| | |||
* They are usually [[solid]] and yellow | |||
| | |||
* They are characterized by simple and complex annular (ring-shaped) [[tubules]] often with [[calcification]] | |||
* They show [[tubules]] with [[sertoli cells]] arranged around one or more [[Hyaline|hyaline bodies]] | |||
* These [[tubules]] may be scattered and admixed with normal [[Ovarian|ovarian tissue]] rather than forming a [[Distinctive feature|distinct]] [[mass]] especially, in [[patients]] with [[Peutz-Jeghers Syndrome|Peutz-Jegher syndrome]] | |||
| | |||
[[File:1200px-Sex cord tumour with annular tubules - 2 - very high mag.jpg|300px|thumb|well-circumscribed nests of cells with nuclei at the periphery, annular tubules (ring-shaped tubules) with dense hyaline material,https://librepathology.org/wiki/Sex_cord_tumour_with_annular_tubules]] | |||
|- | |||
|[[Sertoli-Leydig cell tumour|Sertoli]]–[[Leydig cell tumor|leydig cell tumors]] | |||
| | |||
*They are [[solid]], [[Lobule|lobulated]], yellow on cut surfaces | |||
*Retiform [[tumors]] have a spongy sectioned surface | |||
*Poorly differentiated [[tumors]] may be extensively [[haemorrhagic]] and [[necrotic]] | |||
* | | | ||
*Well differentiated [[tumors]] are characterised by [[sertoli cells]] in a predominantly [[tubular]] pattern, and [[leydig cells]] in the intervening [[stroma]] | |||
*[ | |||
*[[Tumors]] of intermediate differentiation often show a striking so-called [[alveolar]] pattern of [[sertoli cells]] with pale [[cytoplasm]] | |||
[ | *Poorly differentiated [[tumors]] are usually [[Dominant|dominantly]] sarcomatoid | ||
*Retiform [[tumors]] shows elongated [[tubules]] and the [[stroma]] is focally somewhat [[Hyaline|hyalinised]] | |||
*[[Heterologous]] elements include [[mucinous]] [[epithelial]] [[glands]] or rhabdomyosarcomatous and/or chondrosarcomatous elements | |||
| | |||
[[File:1200px-Ovary SertoliLeydigCellTumor 4 PA.jpg|300px|thumb|https://librepathology.org/wiki/File:Ovary_SertoliLeydigCellTumor_4_PA.jpg]] | |||
|- | |||
|Sclerosing [[stromal]] [[tumor]] | |||
| | |||
* They are mostly [[solid]] [[Mass|masses]] with yellowish foci, [[edema]], and [[cystic]] areas | |||
| | |||
* A pseudolobular pattern, admixed [[Spindle|spindled]] and rounded weakly luteinised [[cells]], | |||
* Prominent typically ectatic branching [[Staghorn coral|staghorn]]-like [[blood vessels]] | |||
| | |||
*[ | [[File:Sclerosing stromal tumor.png|300px|thumb|Pseudolobular pattern showing hypo and hypercellular areas seperated by oedematous fibrous strands://openi.nlm.nih.gov/detailedresult?img=PMC4175603_ogs-57-405-g001&query=sclerosing%20stromal%20tumour%20of%20ovary&it=xg&req=4&npos=5]] | ||
|- | |||
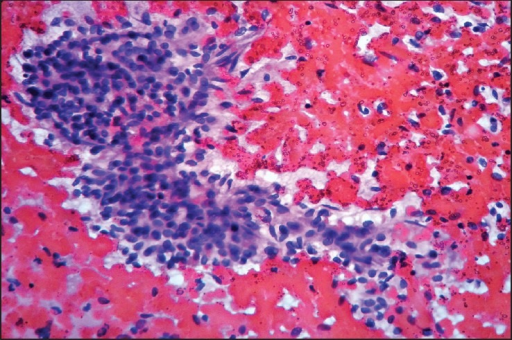
|Luteinised [[Thecoma|thecomas]] with sclerosing peritonitis | |||
| | |||
* They are [[bilateral]] with abnormal in appearance due to a hypercerebriform [[Contour line|contour]] | |||
* often large [[Mass (medicine)|masses]] having a beefy appearance with [[Edema|oedema]] and [[cyst]] formation | |||
| | |||
* [[Microcytic|Microcytic change]] within the [[cellular]] [[neoplasm]] composed of admixed, [[Spindle|spindled]], and weakly luteinised [[cells]] | |||
The | * Normal [[Ovarian|ovarian elements]] are often present between the [[Proliferation|proliferating]] [[spindle cells]] | ||
| | |||
[[File:Luteinised thecomas with sclerosing peritonitis.png|300px|thumb|https://openi.nlm.nih.gov/detailedresult?img=PMC4264285_JMH-5-198-g001&query=Luteinised%20thecomas%20with%20sclerosing%20peritonitis&it=xg&req=4&npos=2]] | |||
|- | |||
|[[Microcytic|Microcystic]] [[stromal]] [[tumor]] | |||
| | |||
* They are [[solid]] and [[cystic]] with [[solid]] [[tissue]] that is tan to white to rarely yellow. | |||
| | |||
*The [[microscopic]] appearance consists of three components: | |||
* [[Microcytic|Microcysts]] (present in 60% of cases) | |||
*[ | * [[Solid]] [[cellular]] areas | ||
* [[Hyaline|Hyalinised]] [[fibrous]] [[stroma]] | |||
*The [[Microcytic|microcystic]] pattern is characterised by small round to oval [[cystic]] spaces, focally coalescing into larger irregular channels; [[Cytoplasmic|intracytoplasmic]] [[vacuoles]] are also common | |||
*[ | *The [[solid]] [[cellular]] areas are usually intersected by [[fibrous]] bands and [[hyaline]][[plaques]] | ||
*[ | | | ||
*[ | |- | ||
|[[Fibroma]] | |||
| | |||
* They are [[solid]] [[ovarian]] [[tumors]] | |||
| | |||
* [[Spindle]]-shaped [[fibroblast]]<nowiki/>s are seen | |||
| | |||
[[File:Ovarian fibroma.png|300px|thumb|Fibroma showing ischemic necrosis,https://openi.nlm.nih.gov/detailedresult?img=PMC4491469_PAMJ-20-322-g007&query=fibroma%20ovarian%20tumor&it=xg&req=4&npos=22]] | |||
|- | |||
|[[Thecoma]] | |||
| | |||
* They appear yellow on [[solid]] sectioned surface | |||
| | |||
* [[Lipid]]-laden [[Stromal cell|stromal cells]] with pale, [[Vacuoles|vaculolated]] [[cytoplasm]] | |||
| | |||
[[File:Thecoma .jpg|300px|thumb|Bland oval or spindled nuclei, abundant cytoplasm that is pale and vaculolated,https://librepathology.org/wiki/Thecoma]] | |||
|- | |||
|[[Fibrosarcoma]] | |||
| | |||
* They are large unilateral [[Mass (medicine)|masses]], often with [[necrosis]] and [[hemorrhage]] | |||
| | |||
* [[Fibroma|Fibromas]] with increased [[Cellular|cellularity]] and [[cell]] [[proliferation]] (''[[mitotic]] activity'') | |||
* They follow a [[malignant]] course | |||
| | |||
|} | |||
== | ==Immunohistochemistry== | ||
[[Granulosa cell tumors|Adult granulosa cell tumors]] stain positive for:<ref name="KasparCrum2015">{{cite journal|last1=Kaspar|first1=Hanna G.|last2=Crum|first2=Christopher P.|title=The Utility of Immunohistochemistry in the Differential Diagnosis of Gynecologic Disorders|journal=Archives of Pathology & Laboratory Medicine|volume=139|issue=1|year=2015|pages=39–54|issn=0003-9985|doi=10.5858/arpa.2014-0057-RA}}</ref><ref name="pmid19033865">{{cite journal |vauthors=Zhao C, Vinh TN, McManus K, Dabbs D, Barner R, Vang R |title=Identification of the most sensitive and robust immunohistochemical markers in different categories of ovarian sex cord-stromal tumors |journal=Am. J. Surg. Pathol. |volume=33 |issue=3 |pages=354–66 |date=March 2009 |pmid=19033865 |doi=10.1097/PAS.0b013e318188373d |url=}}</ref><ref name="pmid17581419">{{cite journal |vauthors=McCluggage WG, McKenna M, McBride HA |title=CD56 is a sensitive and diagnostically useful immunohistochemical marker of ovarian sex cord-stromal tumors |journal=Int. J. Gynecol. Pathol. |volume=26 |issue=3 |pages=322–7 |date=July 2007 |pmid=17581419 |doi=10.1097/01.pgp.0000236947.59463.87 |url=}}</ref><ref name="pmid17721194">{{cite journal |vauthors=Zhao C, Bratthauer GL, Barner R, Vang R |title=Diagnostic utility of WT1 immunostaining in ovarian sertoli cell tumor |journal=Am. J. Surg. Pathol. |volume=31 |issue=9 |pages=1378–86 |date=September 2007 |pmid=17721194 |doi=10.1097/PAS.0b013e3180339961 |url=}}</ref><ref name="pmid18753972">{{cite journal |vauthors=Zhao C, Barner R, Vinh TN, McManus K, Dabbs D, Vang R |title=SF-1 is a diagnostically useful immunohistochemical marker and comparable to other sex cord-stromal tumor markers for the differential diagnosis of ovarian sertoli cell tumor |journal=Int. J. Gynecol. Pathol. |volume=27 |issue=4 |pages=507–14 |date=October 2008 |pmid=18753972 |doi=10.1097/PGP.0b013e31817c1b0a |url=}}</ref><ref name="pmid12808064">{{cite journal |vauthors=Deavers MT, Malpica A, Liu J, Broaddus R, Silva EG |title=Ovarian sex cord-stromal tumors: an immunohistochemical study including a comparison of calretinin and inhibin |journal=Mod. Pathol. |volume=16 |issue=6 |pages=584–90 |date=June 2003 |pmid=12808064 |doi=10.1097/01.MP.0000073133.79591.A1 |url=}}</ref><ref name="pmid17885484">{{cite journal |vauthors=Oliva E, Garcia-Miralles N, Vu Q, Young RH |title=CD10 expression in pure stromal and sex cord-stromal tumors of the ovary: an immunohistochemical analysis of 101 cases |journal=Int. J. Gynecol. Pathol. |volume=26 |issue=4 |pages=359–67 |date=October 2007 |pmid=17885484 |doi=10.1097/PGP.0b013e318064511c |url=}}</ref> | |||
*[[Inhibin]] | |||
*[[Calretinin]] | |||
*[[FOXL2]] and | |||
*[[SF1 (gene)|SF-1]] | |||
*[[WT1]] | |||
*[[CD56]] | |||
They are negative for: | |||
*[[Epithelial]] markers [[CK|CK7]] and EMA | |||
*[[SALL4]] | |||
*[[CD20]] | |||
*[[CD45]] | |||
[[Sertoli-stromal cell tumor|Sertoli–stromal cell tumors]] are positive for: | |||
*[[Calretinin]] | |||
*[[Inhibin]] | |||
*[[SF1 (gene)|SF-1]] | |||
*[[WT1]] | |||
Microcystic [[stromal]] [[tumor]]:<ref name="pmid26200099">{{cite journal |vauthors=Irving JA, Lee CH, Yip S, Oliva E, McCluggage WG, Young RH |title=Microcystic Stromal Tumor: A Distinctive Ovarian Sex Cord-Stromal Neoplasm Characterized by FOXL2, SF-1, WT-1, Cyclin D1, and β-catenin Nuclear Expression and CTNNB1 Mutations |journal=Am. J. Surg. Pathol. |volume=39 |issue=10 |pages=1420–6 |date=October 2015 |pmid=26200099 |doi=10.1097/PAS.0000000000000482 |url=}}</ref><ref name="pmid18971779">{{cite journal |vauthors=Irving JA, Young RH |title=Microcystic stromal tumor of the ovary: report of 16 cases of a hitherto uncharacterized distinctive ovarian neoplasm |journal=Am. J. Surg. Pathol. |volume=33 |issue=3 |pages=367–75 |date=March 2009 |pmid=18971779 |doi=10.1097/PAS.0b013e31818479c3 |url=}}</ref><ref name="pmid25913412">{{cite journal |vauthors=Bi R, Bai QM, Yang F, Wu LJ, Cheng YF, Shen XX, Cai X, Zhou XY, Yang WT |title=Microcystic stromal tumour of the ovary: frequent mutations of β-catenin (CTNNB1) in six cases |journal=Histopathology |volume=67 |issue=6 |pages=872–9 |date=December 2015 |pmid=25913412 |doi=10.1111/his.12722 |url=}}</ref> | |||
*[[CD10]]+ | |||
*[[Vimentin]]+ | |||
*[[Cyclin D1]]+ | |||
*[[Β-catenin|Nuclear β-catenin]]-positive | |||
*[[Inhibin]]- | |||
*[[Calretinin]]- | |||
==References== | ==References== | ||
| Line 81: | Line 204: | ||
{{WH}} | {{WH}} | ||
{{WS}} | {{WS}} | ||
[[Category: | [[Category:Disease]] | ||
[[Category:Types of cancer]] | |||
[[Category:Gynecology]] | |||
[[Category:Up-To-Date]] | |||
[[Category:Oncology]] | |||
[[Category:Medicine]] | |||
[[Category:Gynecology]] | |||
[[Category:Surgery]] | |||
Latest revision as of 02:42, 1 May 2019
|
Sexcord/ stromal ovarian tumors Microchapters |
|
Differentiating Sexcord/ Stromal Ovarian Tumors from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Sexcord/ stromal ovarian tumors pathophysiology On the Web |
|
American Roentgen Ray Society Images of Sexcord/ stromal ovarian tumors pathophysiology |
|
Risk calculators and risk factors for Sexcord/ stromal ovarian tumors pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: ; Maneesha Nandimandalam, M.B.B.S.[2]
Overview
The exact pathogenesis of sexcord/ stromal ovarian tumors is not fully understood. Mutations mainly involving FOXL2, DICER1, STK11 are involved. They are associated with ollier disease and maffucci syndrome.The microscopic pathology varies with the individual subtype of sexcord stromal ovarian tumors.
Pathophysiology
Pathogenesis
- The exact pathogenesis of sexcord/ stromal ovarian tumors is not completely understood.[1]
- However genetic factors attribute to the pathogenesis of some subtypes of the tumors
- The pathophysiology of sexcord/ stromal ovarian tumors depends on the histological subtype.
Genetics
- The recent advancing analyses have made us understand the pathophysiology of some of these tumor subtypes
- Mutations mainly involving DICER1, STK11, and FOXL2 influence the development of some of these neoplasms
- FOXL2 is a tumor suppressor gene
- It is a member of the forkhead box (FOX) family of evolutionarily conserved transcription factors
- It plays a fundamental and crucial role in ovarian development
- It regulates the ovarian granulosa cell proliferation, follicle development and ovarian hormones synthesis
- Almost all like 97% of adult granulosa cell tumors are characterized by missense somatic point mutations (402 C→G) in FOXL2 gene
- Infact this mutation is a sensitive and specific biomarker for adult granulosa cell tumors making it a pathognomonic feature
- The phosphorylation modification of FOXL2 in particular is responsible to the growth of granulosa cell tumors
- Importantly this mutation alter's antiproliferative pathways and also limit the apoptosis, as a result contributing to the pathogenesis of adult granulosa cell tumors
- Other factors that play an important role in the pathogenesis of granulosa cell tumors are PI3K/AKT (phosphatidylinositol-3-kinase; serine/threonine kinase), TGF-β (Transforming growth factor beta) signaling pathway, Notch signaling pathway, GATA4 and VEGF (vascular endothelial growth factor)

- DICER1 mutations are associated with leydig cell tumors and gynandroblastomas
- Although both germ line and somatic mutations play a role, approximately 60% of sexcord leydig cell tumors have somatic DICER1 mutations
- This particular gene DICER1 encodes for a RNA endoribonuclease that helps to cleave precursor miRNA into mature miRNAs
- DICER1 mutations are associated with a lot of tumors of which pleuropulmonary blastoma, is the most common lung tumor of infancy and early childhood
- Others are embryonal rhabdomyosarcoma of the uterine cervix, renal tumors, thyroid nodules and carcinoma, nasal chondromesenchymal hamartoma, ciliary body medulloepithelioma, pineoblastoma, and pituitary blastoma
- The above mentioned tumors typically have biallelic DICER1 mutations that are composed of a loss of function in one allele and a missense mutation in the RNase IIIb domain
- Mutations in the STK11 gene is associated with sex cord-stromal tumors with annular tubules
Associated Conditions
- Patients with ollier disease and maffucci syndrome are associated with an increased risk of juvenile granulosa cell tumors[10][6]
- Somatic mosaic mutations in IDH1 and IDH2 are observed
- Ollier disease includes enchondromatosis, whereas maffucci syndrome includes enchondromatosis and hemangiomas
Gross and Microscopic Pathology
The gross and microscopic features of the most common tumors are described below:[6][13][14][15][16][17][18][7][19][20][21]
| Types | Gross pathology | Microscopic pathology | Images |
|---|---|---|---|
| Adult granulosa cell tumours |
|
 | |
| Juvenile granulosa cell tumours |
|
|
 |
| Sex cord tumour with annular tubules |
|
|
 |
| Sertoli–leydig cell tumors |
|
|
 |
| Sclerosing stromal tumor |
|
 | |
| Luteinised thecomas with sclerosing peritonitis |
|
 | |
| Microcystic stromal tumor |
|
||
| Fibroma |
|
 | |
| Thecoma |
|
|
 |
| Fibrosarcoma |
|
|
Immunohistochemistry
Adult granulosa cell tumors stain positive for:[22][23][24][25][26][27][28]
They are negative for:
- Epithelial markers CK7 and EMA
- SALL4
- CD20
- CD45
Sertoli–stromal cell tumors are positive for:
Microcystic stromal tumor:[19][20][29]
- CD10+
- Vimentin+
- Cyclin D1+
- Nuclear β-catenin-positive
- Inhibin-
- Calretinin-
References
- ↑ Karnezis AN, Cho KR, Gilks CB, Pearce CL, Huntsman DG (January 2017). "The disparate origins of ovarian cancers: pathogenesis and prevention strategies". Nat. Rev. Cancer. 17 (1): 65–74. doi:10.1038/nrc.2016.113. PMID 27885265.
- ↑ 2.0 2.1 Lim, Diana; Oliva, Esther (2018). "Ovarian sex cord-stromal tumours: an update in recent molecular advances". Pathology. 50 (2): 178–189. doi:10.1016/j.pathol.2017.10.008. ISSN 0031-3025.
- ↑ 3.0 3.1 Fuller PJ, Leung D, Chu S (February 2017). "Genetics and genomics of ovarian sex cord-stromal tumors". Clin. Genet. 91 (2): 285–291. doi:10.1111/cge.12917. PMID 27813081.
- ↑ Li, Jiaheng; Bao, Riqiang; Peng, Shiwei; Zhang, Chunping (2018). "The molecular mechanism of ovarian granulosa cell tumors". Journal of Ovarian Research. 11 (1). doi:10.1186/s13048-018-0384-1. ISSN 1757-2215.
- ↑ Li J, Bao R, Peng S, Zhang C (February 2018). "The molecular mechanism of ovarian granulosa cell tumors". J Ovarian Res. 11 (1): 13. doi:10.1186/s13048-018-0384-1. PMC 5802052. PMID 29409506.
- ↑ 6.0 6.1 6.2 Schultz KA, Harris AK, Schneider DT, Young RH, Brown J, Gershenson DM, Dehner LP, Hill DA, Messinger YH, Frazier AL (October 2016). "Ovarian Sex Cord-Stromal Tumors". J Oncol Pract. 12 (10): 940–946. doi:10.1200/JOP.2016.016261. PMC 5063189. PMID 27858560.
- ↑ 7.0 7.1 Boussios, Stergios; Moschetta, Michele; Zarkavelis, George; Papadaki, Alexandra; Kefas, Aristides; Tatsi, Konstantina (2017). "Ovarian sex-cord stromal tumours and small cell tumours: Pathological, genetic and management aspects". Critical Reviews in Oncology/Hematology. 120: 43–51. doi:10.1016/j.critrevonc.2017.10.007. ISSN 1040-8428.
- ↑ Leung, Dilys T.H.; Fuller, Peter J.; Chu, Simon (2016). "Impact of FOXL2 mutations on signaling in ovarian granulosa cell tumors". The International Journal of Biochemistry & Cell Biology. 72: 51–54. doi:10.1016/j.biocel.2016.01.003. ISSN 1357-2725.
- ↑ Goulvent T, Ray-Coquard I, Borel S, Haddad V, Devouassoux-Shisheboran M, Vacher-Lavenu MC, Pujade-Laurraine E, Savina A, Maillet D, Gillet G, Treilleux I, Rimokh R (January 2016). "DICER1 and FOXL2 mutations in ovarian sex cord-stromal tumours: a GINECO Group study". Histopathology. 68 (2): 279–85. doi:10.1111/his.12747. PMID 26033501.
- ↑ 10.0 10.1 Stewart CJ, Charles A, Foulkes WD (June 2016). "Gynecologic Manifestations of the DICER1 Syndrome". Surg Pathol Clin. 9 (2): 227–41. doi:10.1016/j.path.2016.01.002. PMID 27241106.
- ↑ Wang Y, Karnezis AN, Magrill J, Tessier-Cloutier B, Lum A, Senz J, Gilks CB, McCluggage WG, Huntsman DG, Kommoss F (August 2018). "DICER1 hot-spot mutations in ovarian gynandroblastoma". Histopathology. 73 (2): 306–313. doi:10.1111/his.13630. PMID 29660837.
- ↑ Xu Q, Zou Y, Zhang XF (October 2018). "Sertoli-Leydig cell tumors of ovary: A case series". Medicine (Baltimore). 97 (42): e12865. doi:10.1097/MD.0000000000012865. PMC 6211859. PMID 30334998.
- ↑ Bremmer F, Schweyer S (February 2016). "[Leydig cell, Sertoli cell and adult granulosa cell tumors]". Pathologe (in German). 37 (1): 71–7. doi:10.1007/s00292-015-0131-y. PMID 26782032.
- ↑ Bremmer F, Behnes CL, Radzun HJ, Bettstetter M, Schweyer S (May 2014). "[Sex cord gonadal stromal tumors]". Pathologe (in German). 35 (3): 245–51. doi:10.1007/s00292-014-1901-7. PMID 24819979.
- ↑ Roth LM, Czernobilsky B (March 2011). "Perspectives on pure ovarian stromal neoplasms and tumor-like proliferations of the ovarian stroma". Am. J. Surg. Pathol. 35 (3): e15–33. doi:10.1097/PAS.0b013e31820acb89. PMID 21317704.
- ↑ Young RH (January 2018). "Ovarian sex cord-stromal tumours and their mimics". Pathology. 50 (1): 5–15. doi:10.1016/j.pathol.2017.09.007. PMID 29132723.
- ↑ Zhang HY, Zhu JE, Huang W, Zhu J (2014). "Clinicopathologic features of ovarian Sertoli-Leydig cell tumors". Int J Clin Exp Pathol. 7 (10): 6956–64. PMC 4230071. PMID 25400781.
- ↑ Chen, Vivien W.; Ruiz, Bernardo; Killeen, Jeffrey L.; Cot�, Timothy R.; Wu, Xiao Cheng; Correa, Catherine N.; Howe, Holly L. (2003). "Pathology and classification of ovarian tumors". Cancer. 97 (S10): 2631–2642. doi:10.1002/cncr.11345. ISSN 0008-543X. replacement character in
|last4=at position 4 (help) - ↑ 19.0 19.1 Irving JA, Lee CH, Yip S, Oliva E, McCluggage WG, Young RH (October 2015). "Microcystic Stromal Tumor: A Distinctive Ovarian Sex Cord-Stromal Neoplasm Characterized by FOXL2, SF-1, WT-1, Cyclin D1, and β-catenin Nuclear Expression and CTNNB1 Mutations". Am. J. Surg. Pathol. 39 (10): 1420–6. doi:10.1097/PAS.0000000000000482. PMID 26200099.
- ↑ 20.0 20.1 Irving JA, Young RH (March 2009). "Microcystic stromal tumor of the ovary: report of 16 cases of a hitherto uncharacterized distinctive ovarian neoplasm". Am. J. Surg. Pathol. 33 (3): 367–75. doi:10.1097/PAS.0b013e31818479c3. PMID 18971779.
- ↑ Mathur A, Seth A, Pant L (2018). "Ovarian fibroma with luteinized thecal cells and minor sex cord element: A rare case report". Indian J Pathol Microbiol. 61 (2): 264–267. doi:10.4103/IJPM.IJPM_446_17. PMID 29676374.
- ↑ Kaspar, Hanna G.; Crum, Christopher P. (2015). "The Utility of Immunohistochemistry in the Differential Diagnosis of Gynecologic Disorders". Archives of Pathology & Laboratory Medicine. 139 (1): 39–54. doi:10.5858/arpa.2014-0057-RA. ISSN 0003-9985.
- ↑ Zhao C, Vinh TN, McManus K, Dabbs D, Barner R, Vang R (March 2009). "Identification of the most sensitive and robust immunohistochemical markers in different categories of ovarian sex cord-stromal tumors". Am. J. Surg. Pathol. 33 (3): 354–66. doi:10.1097/PAS.0b013e318188373d. PMID 19033865.
- ↑ McCluggage WG, McKenna M, McBride HA (July 2007). "CD56 is a sensitive and diagnostically useful immunohistochemical marker of ovarian sex cord-stromal tumors". Int. J. Gynecol. Pathol. 26 (3): 322–7. doi:10.1097/01.pgp.0000236947.59463.87. PMID 17581419.
- ↑ Zhao C, Bratthauer GL, Barner R, Vang R (September 2007). "Diagnostic utility of WT1 immunostaining in ovarian sertoli cell tumor". Am. J. Surg. Pathol. 31 (9): 1378–86. doi:10.1097/PAS.0b013e3180339961. PMID 17721194.
- ↑ Zhao C, Barner R, Vinh TN, McManus K, Dabbs D, Vang R (October 2008). "SF-1 is a diagnostically useful immunohistochemical marker and comparable to other sex cord-stromal tumor markers for the differential diagnosis of ovarian sertoli cell tumor". Int. J. Gynecol. Pathol. 27 (4): 507–14. doi:10.1097/PGP.0b013e31817c1b0a. PMID 18753972.
- ↑ Deavers MT, Malpica A, Liu J, Broaddus R, Silva EG (June 2003). "Ovarian sex cord-stromal tumors: an immunohistochemical study including a comparison of calretinin and inhibin". Mod. Pathol. 16 (6): 584–90. doi:10.1097/01.MP.0000073133.79591.A1. PMID 12808064.
- ↑ Oliva E, Garcia-Miralles N, Vu Q, Young RH (October 2007). "CD10 expression in pure stromal and sex cord-stromal tumors of the ovary: an immunohistochemical analysis of 101 cases". Int. J. Gynecol. Pathol. 26 (4): 359–67. doi:10.1097/PGP.0b013e318064511c. PMID 17885484.
- ↑ Bi R, Bai QM, Yang F, Wu LJ, Cheng YF, Shen XX, Cai X, Zhou XY, Yang WT (December 2015). "Microcystic stromal tumour of the ovary: frequent mutations of β-catenin (CTNNB1) in six cases". Histopathology. 67 (6): 872–9. doi:10.1111/his.12722. PMID 25913412.