Irinotecan: Difference between revisions
m (Robot: Automated text replacement (-{{SIB}} + & -{{EH}} + & -{{EJ}} + & -{{Editor Help}} + & -{{Editor Join}} +)) |
No edit summary |
||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | |||
|authorTag={{AP}} | |||
|genericName=Irinotecan hydrochloride | |||
|aOrAn=an | |||
|drugClass=[[antineoplastic agent]] and [[topoisomerase I inhibitor]] | |||
|indicationType=treatment | |||
|indication=metastatic [[colon carcinoma]] and [[rectum carcinoma]] | |||
|hasBlackBoxWarning=Yes | |||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">WARNING: DIARRHEA AND MYELOSUPPRESSION</span></b> | |||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> | |||
*Early and late forms of diarrhea can occur. Early diarrhea may be accompanied by cholinergic symptoms which may be prevented or ameliorated by atropine. Late diarrhea can be life threatening and should be treated promptly with loperamide. Monitor patients with diarrhea and give fluid and electrolytes as needed. Institute antibiotic therapy if patients develop ileus, fever, or severe neutropenia. Interrupt CAMPTOSAR and reduce subsequent doses if severe diarrhea occurs. | |||
*Severe myelosuppression may occur. | |||
|fdaLIADAdult====Colorectal Cancer Combination Regimens 1 and 2=== | |||
*Administer CAMPTOSAR as a 90-minute intravenous infusion followed by LV and [[5-FU]]. The currently recommended regimens are shown in Table 1. | |||
*A reduction in the starting dose by one dose level of CAMPTOSAR may be considered for patients with any of the following conditions: prior pelvic/abdominal [[radiotherapy]], performance status of 2, or increased [[bilirubin]] levels. Dosing for patients with bilirubin >2 mg/dL cannot be recommended because there is insufficient information to recommend a dose in these patients. | |||
[[file:Irinotecan Dosage1.png|none|300px]] | |||
Dosing for patients with bilirubin >2 mg/dL cannot be recommended because there is insufficient information to recommend a dose in these patients. | |||
====Dose Modification==== | |||
*Based on recommended dose levels described in Table 1, Combination Regimens of CAMPTOSAR and Dose Modifications, subsequent doses should be adjusted as suggested in Table 2, Recommended Dose Modifications for Combination Regimens. All dose modifications should be based on the worst preceding toxicity. | |||
[[file:Irinotecan DOsage2.png|none|300px]] | |||
===Colorectal Single Agent Regimens 1 and 2=== | |||
*Administer CAMPTOSAR as a 90-minute intravenous infusion. The currently recommended regimens are shown in Table 3. | |||
*A reduction in the starting dose by one dose level of CAMPTOSAR may be considered for patients with any of the following conditions: prior pelvic/abdominal radiotherapy, performance status of 2, or increased bilirubin levels. Dosing for patients with bilirubin >2 mg/dL cannot be recommended because there is insufficient information to recommend a dose in these patients. | |||
[[file:Irinotecan Dosage3.png|none|300px]] | |||
====Dose Modifications==== | |||
*Based on recommended dose-levels described in Table 3, Single-Agent Regimens of CAMPTOSAR and Dose Modifications, subsequent doses should be adjusted as suggested in Table 4, Recommended Dose Modifications for Single-Agent Schedules. All dose modifications should be based on the worst preceding toxicity. | |||
[[file:Irinotecan Dosage4.png|none|300px]] | |||
===Dosage in Patients with Reduced UGT1A1 Activity=== | |||
*When administered in combination with other agents, or as a single-agent, a reduction in the starting dose by at least one level of CAMPTOSAR should be considered for patients known to be homozygous for the UGT1A1*28 allele. However, the precise dose reduction in this patient population is not known, and subsequent dose modifications should be considered based on individual patient tolerance to treatment (see Tables 1–4). | |||
===Premedication=== | |||
*It is recommended that patients receive premedication with antiemetic agents. In clinical studies of the weekly dosage schedule, the majority of patients received 10 mg of dexamethasone given in conjunction with another type of antiemetic agent, such as a 5-HT3 blocker (e.g., ondansetron or granisetron). Antiemetic agents should be given on the day of treatment, starting at least 30 minutes before administration of CAMPTOSAR. Physicians should also consider providing patients with an antiemetic regimen (e.g., prochlorperazine) for subsequent use as needed. A similar antiemetic regimen should be used with Camptosar in combination therapy. | |||
*Prophylactic or therapeutic administration of atropine should be considered in patients experiencing cholinergic symptoms. | |||
===Preparation of Infusion Solution=== | |||
*Inspect vial contents for particulate matter and discoloration and repeat inspection when drug product is withdrawn from vial into syringe. | |||
*CAMPTOSAR Injection 20 mg/mL is intended for single use only and any unused portion should be discarded. | |||
*CAMPTOSAR Injection must be diluted prior to infusion. CAMPTOSAR should be diluted in 5% Dextrose Injection, USP, (preferred) or 0.9% Sodium Chloride Injection, USP, to a final concentration range of 0.12 mg/mL to 2.8 mg/mL. Other drugs should not be added to the infusion solution. | |||
*The solution is physically and chemically stable for up to 24 hours at room temperature and in ambient fluorescent lighting. Solutions diluted in 5% Dextrose Injection, USP, and stored at refrigerated temperatures (approximately 2° to 8°C, 36° to 46°F), and protected from light are physically and chemically stable for 48 hours. Refrigeration of admixtures using 0.9% Sodium Chloride Injection, USP, is not recommended due to a low and sporadic incidence of visible particulates. Freezing CAMPTOSAR and admixtures of CAMPTOSAR may result in precipitation of the drug and should be avoided. | |||
*The CAMPTOSAR Injection solution should be used immediately after reconstitution as it contains no antibacterial preservative. Because of possible microbial contamination during dilution, it is advisable to use the admixture prepared with 5% Dextrose Injection, USP, within 24 hours if refrigerated (2° to 8°C, 36° to 46°F). In the case of admixtures prepared with 5% Dextrose Injection, USP, or Sodium Chloride Injection, USP, the solutions should be used within 4 hours if kept at room temperature. If reconstitution and dilution are performed under strict aseptic conditions (e.g., on Laminar Air Flow bench), CAMPTOSAR Injection solution should be used (infusion completed) within 12 hours at room temperature or 24 hours if refrigerated (2° to 8°C, 36° to 46°F). | |||
===Safe Handling=== | |||
*Care should be exercised in the handling and preparation of infusion solutions prepared from CAMPTOSAR Injection. The use of gloves is recommended. If a solution of CAMPTOSAR contacts the skin, wash the skin immediately and thoroughly with soap and water. If CAMPTOSAR contacts the mucous membranes, flush thoroughly with water. Several published guidelines for handling and disposal of anticancer agents are available. | |||
===Extravasation=== | |||
*Care should be taken to avoid extravasation, and the infusion site should be monitored for signs of inflammation. Should extravasation occur, flushing the site with sterile water and applications of ice are recommended. | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Irinotecan in adult patients. | |||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Irinotecan in adult patients. | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Irinotecan in pediatric patients. | |||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Irinotecan in pediatric patients. | |||
|contraindications=*CAMPTOSAR Injection is contraindicated in patients with a known hypersensitivity to the drug or its excipients. | |||
|alcohol=Alcohol-Irinotecan interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
}} | |||
{{Drugbox| | {{Drugbox| | ||
|IUPAC_name = (''S'')-4,11-diethyl-3,4,12,14-tetrahydro-4-hydroxy-<br>3,14-dioxo1''H''-pyrano[3’,4’:6,7]-indolizino[1,2-b]quinolin-<br>9-yl-[1,4’bipiperidine]-1’-carboxylate | |IUPAC_name = (''S'')-4,11-diethyl-3,4,12,14-tetrahydro-4-hydroxy-<br>3,14-dioxo1''H''-pyrano[3’,4’:6,7]-indolizino[1,2-b]quinolin-<br>9-yl-[1,4’bipiperidine]-1’-carboxylate | ||
Revision as of 19:18, 11 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: DIARRHEA AND MYELOSUPPRESSION
See full prescribing information for complete Boxed Warning.
Condition Name:
|
Overview
Irinotecan is an antineoplastic agent and topoisomerase I inhibitor that is FDA approved for the treatment of metastatic colon carcinoma and rectum carcinoma. There is a Black Box Warning for this drug as shown here. Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Colorectal Cancer Combination Regimens 1 and 2
- Administer CAMPTOSAR as a 90-minute intravenous infusion followed by LV and 5-FU. The currently recommended regimens are shown in Table 1.
- A reduction in the starting dose by one dose level of CAMPTOSAR may be considered for patients with any of the following conditions: prior pelvic/abdominal radiotherapy, performance status of 2, or increased bilirubin levels. Dosing for patients with bilirubin >2 mg/dL cannot be recommended because there is insufficient information to recommend a dose in these patients.
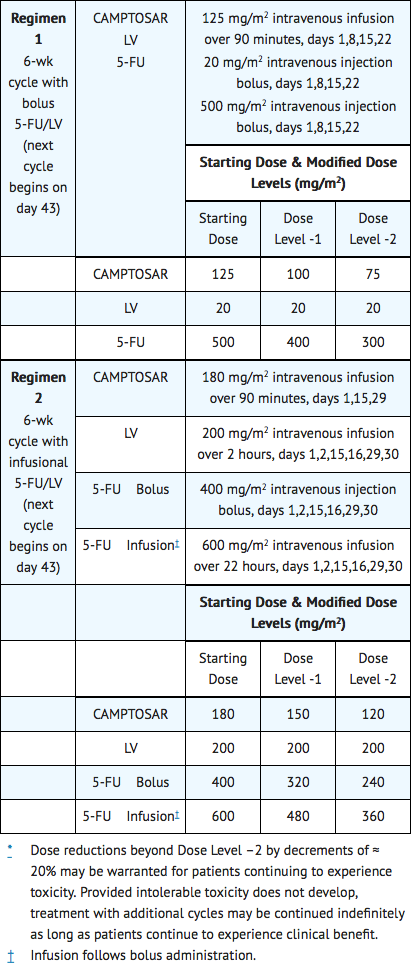
Dosing for patients with bilirubin >2 mg/dL cannot be recommended because there is insufficient information to recommend a dose in these patients.
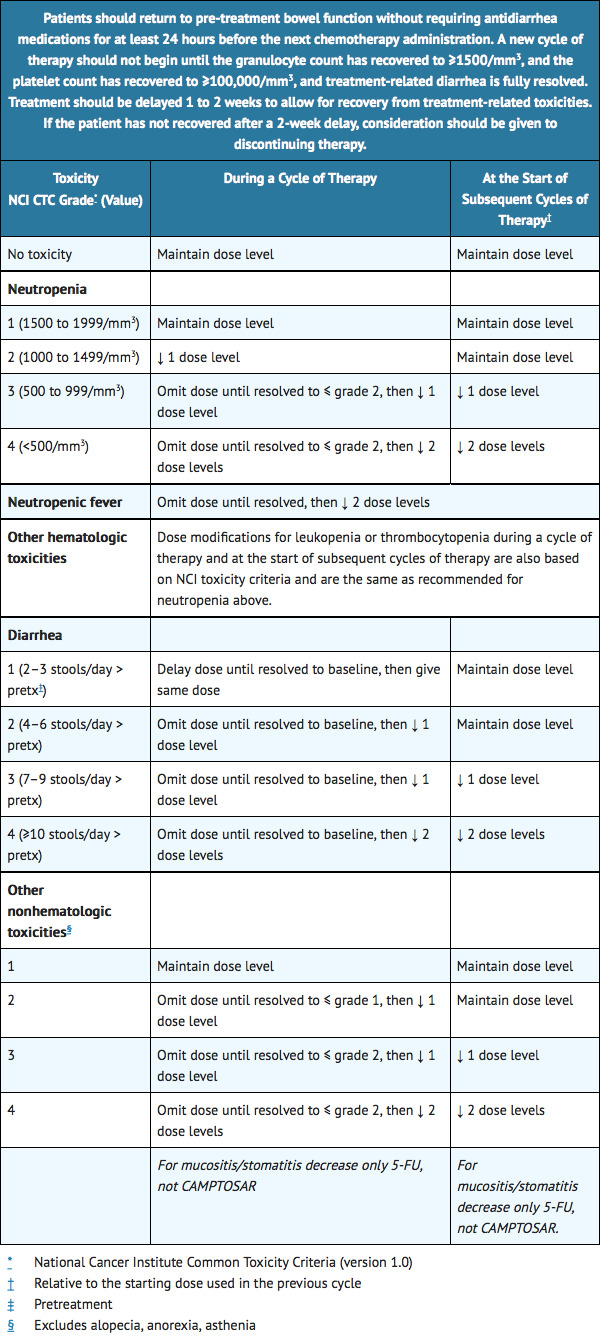
Dose Modification
- Based on recommended dose levels described in Table 1, Combination Regimens of CAMPTOSAR and Dose Modifications, subsequent doses should be adjusted as suggested in Table 2, Recommended Dose Modifications for Combination Regimens. All dose modifications should be based on the worst preceding toxicity.
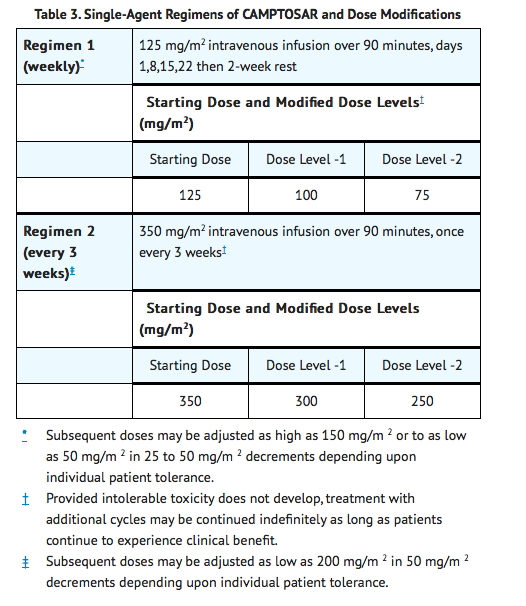
Colorectal Single Agent Regimens 1 and 2
- Administer CAMPTOSAR as a 90-minute intravenous infusion. The currently recommended regimens are shown in Table 3.
- A reduction in the starting dose by one dose level of CAMPTOSAR may be considered for patients with any of the following conditions: prior pelvic/abdominal radiotherapy, performance status of 2, or increased bilirubin levels. Dosing for patients with bilirubin >2 mg/dL cannot be recommended because there is insufficient information to recommend a dose in these patients.
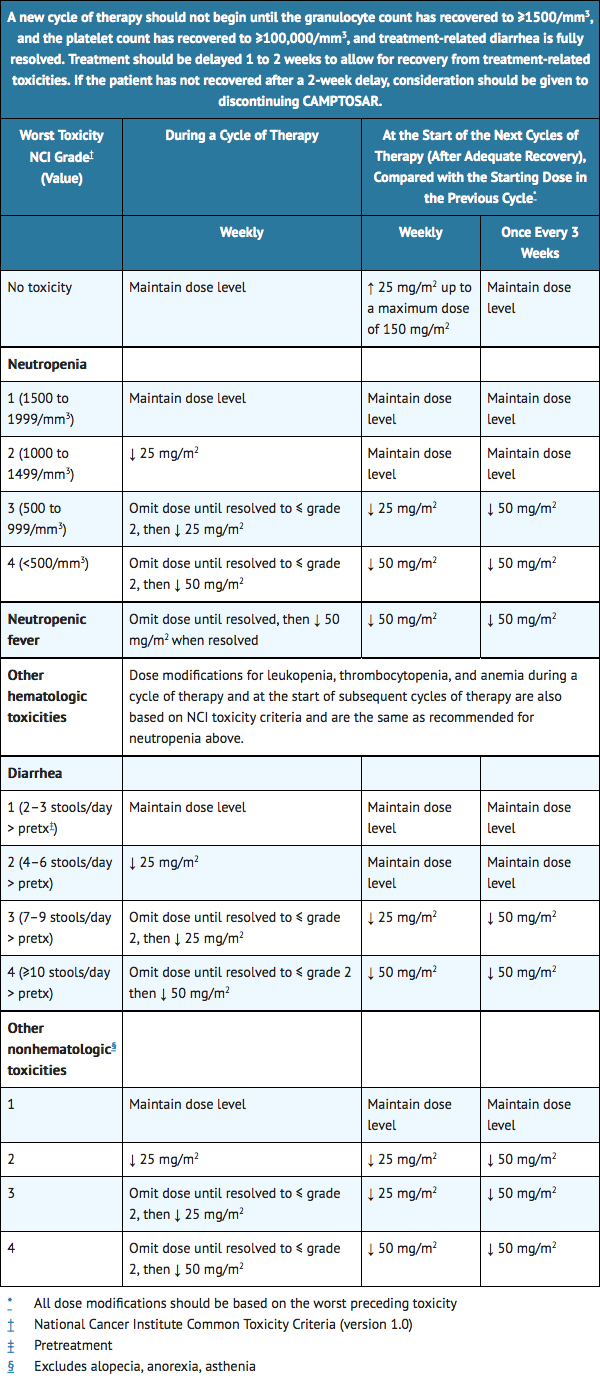
Dose Modifications
- Based on recommended dose-levels described in Table 3, Single-Agent Regimens of CAMPTOSAR and Dose Modifications, subsequent doses should be adjusted as suggested in Table 4, Recommended Dose Modifications for Single-Agent Schedules. All dose modifications should be based on the worst preceding toxicity.
Dosage in Patients with Reduced UGT1A1 Activity
- When administered in combination with other agents, or as a single-agent, a reduction in the starting dose by at least one level of CAMPTOSAR should be considered for patients known to be homozygous for the UGT1A1*28 allele. However, the precise dose reduction in this patient population is not known, and subsequent dose modifications should be considered based on individual patient tolerance to treatment (see Tables 1–4).
Premedication
- It is recommended that patients receive premedication with antiemetic agents. In clinical studies of the weekly dosage schedule, the majority of patients received 10 mg of dexamethasone given in conjunction with another type of antiemetic agent, such as a 5-HT3 blocker (e.g., ondansetron or granisetron). Antiemetic agents should be given on the day of treatment, starting at least 30 minutes before administration of CAMPTOSAR. Physicians should also consider providing patients with an antiemetic regimen (e.g., prochlorperazine) for subsequent use as needed. A similar antiemetic regimen should be used with Camptosar in combination therapy.
- Prophylactic or therapeutic administration of atropine should be considered in patients experiencing cholinergic symptoms.
Preparation of Infusion Solution
- Inspect vial contents for particulate matter and discoloration and repeat inspection when drug product is withdrawn from vial into syringe.
- CAMPTOSAR Injection 20 mg/mL is intended for single use only and any unused portion should be discarded.
- CAMPTOSAR Injection must be diluted prior to infusion. CAMPTOSAR should be diluted in 5% Dextrose Injection, USP, (preferred) or 0.9% Sodium Chloride Injection, USP, to a final concentration range of 0.12 mg/mL to 2.8 mg/mL. Other drugs should not be added to the infusion solution.
- The solution is physically and chemically stable for up to 24 hours at room temperature and in ambient fluorescent lighting. Solutions diluted in 5% Dextrose Injection, USP, and stored at refrigerated temperatures (approximately 2° to 8°C, 36° to 46°F), and protected from light are physically and chemically stable for 48 hours. Refrigeration of admixtures using 0.9% Sodium Chloride Injection, USP, is not recommended due to a low and sporadic incidence of visible particulates. Freezing CAMPTOSAR and admixtures of CAMPTOSAR may result in precipitation of the drug and should be avoided.
- The CAMPTOSAR Injection solution should be used immediately after reconstitution as it contains no antibacterial preservative. Because of possible microbial contamination during dilution, it is advisable to use the admixture prepared with 5% Dextrose Injection, USP, within 24 hours if refrigerated (2° to 8°C, 36° to 46°F). In the case of admixtures prepared with 5% Dextrose Injection, USP, or Sodium Chloride Injection, USP, the solutions should be used within 4 hours if kept at room temperature. If reconstitution and dilution are performed under strict aseptic conditions (e.g., on Laminar Air Flow bench), CAMPTOSAR Injection solution should be used (infusion completed) within 12 hours at room temperature or 24 hours if refrigerated (2° to 8°C, 36° to 46°F).
Safe Handling
- Care should be exercised in the handling and preparation of infusion solutions prepared from CAMPTOSAR Injection. The use of gloves is recommended. If a solution of CAMPTOSAR contacts the skin, wash the skin immediately and thoroughly with soap and water. If CAMPTOSAR contacts the mucous membranes, flush thoroughly with water. Several published guidelines for handling and disposal of anticancer agents are available.
Extravasation
- Care should be taken to avoid extravasation, and the infusion site should be monitored for signs of inflammation. Should extravasation occur, flushing the site with sterile water and applications of ice are recommended.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Irinotecan in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Irinotecan in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Irinotecan FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Irinotecan in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Irinotecan in pediatric patients.
Contraindications
- CAMPTOSAR Injection is contraindicated in patients with a known hypersensitivity to the drug or its excipients.
Warnings
|
WARNING: DIARRHEA AND MYELOSUPPRESSION
See full prescribing information for complete Boxed Warning.
Condition Name:
|
There is limited information regarding Irinotecan Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Irinotecan Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Irinotecan Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Irinotecan Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Irinotecan in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Irinotecan in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Irinotecan during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Irinotecan in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Irinotecan in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Irinotecan in geriatric settings.
Gender
There is no FDA guidance on the use of Irinotecan with respect to specific gender populations.
Race
There is no FDA guidance on the use of Irinotecan with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Irinotecan in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Irinotecan in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Irinotecan in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Irinotecan in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Irinotecan Administration in the drug label.
Monitoring
There is limited information regarding Irinotecan Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Irinotecan and IV administrations.
Overdosage
There is limited information regarding Irinotecan overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Irinotecan Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Irinotecan Mechanism of Action in the drug label.
Structure
There is limited information regarding Irinotecan Structure in the drug label.
Pharmacodynamics
There is limited information regarding Irinotecan Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Irinotecan Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Irinotecan Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Irinotecan Clinical Studies in the drug label.
How Supplied
There is limited information regarding Irinotecan How Supplied in the drug label.
Storage
There is limited information regarding Irinotecan Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Irinotecan |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Irinotecan |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Irinotecan Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Irinotecan interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Irinotecan Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Irinotecan Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
| Error creating thumbnail: File missing | |
| Clinical data | |
|---|---|
| Pregnancy category | |
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | NA |
| Metabolism | Hepatic glucuronidation |
| Elimination half-life | 6 to 12 hours |
| Excretion | Biliary and renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C33H38N4O6 |
| Molar mass | 586.678 g/mol 677.185 g/mol (hydrochloride) |
|
WikiDoc Resources for Irinotecan |
|
Articles |
|---|
|
Most recent articles on Irinotecan |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Irinotecan at Clinical Trials.gov Clinical Trials on Irinotecan at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Irinotecan
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Irinotecan Discussion groups on Irinotecan Patient Handouts on Irinotecan Directions to Hospitals Treating Irinotecan Risk calculators and risk factors for Irinotecan
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Irinotecan |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Overview
Irinotecan is a chemotherapy agent that is a topoisomerase 1 inhibitor. Chemically, it is a semisynthetic analogue of the natural alkaloid camptothecin.
Its main use is in colon cancer, particularly in combination with other chemotherapy agents. This includes the regimen FOLFIRI which consists of infusional 5-fluorouracil, leucovorin, and irinotecan.
Irinotecan was first introduced in Japan by the Pharmaceutical arm of Yakult Honsha as Campto®. In 1994, it received accelerated FDA approval in the United States, where it is now marketed by Pfizer as Camptosar®. It is also known as CPT-11.
Mechanism
Irinotecan is activated by hydrolysis to SN-38, an inhibitor of topoisomerase I. This is then inactivated by glucuronidation by uridine diphosphate glucoronosyltransferase 1A1 (UGT1A1). The inhibition of topoisomerase I by the active metabolite SN-38 eventually leads to inhibition of both DNA replication and transcription.
Side effects
The most significant adverse effects of irinotecan are severe diarrhea and extreme suppression of the immune systems.
Diarrhea
Irinotecan-associated diarrhea is severe and clinically significant, sometimes leading severe dehydration requiring hospitalization or intensive care unit admission. This side effect is managed with the aggressive use of antidiarrheals such as loperamide or Lomotil with the first loose bowel movement.
Immunosuppression
The immune system is adversely impacted by irinotecan. This is reflected in dramatically lowered white blood cell counts in the blood, in particular the neutrophils. While the bone marrow, where neutrophils are made, cranks up production to compensate, the patient may experience a period of neutropenia, that is, a clinical lack of neutrophils in the blood.
Pharmacogenomics
Irinotecan is converted by an enzyme into its active metabolite SN-38, which is in turn inactivated by the enzyme UGT1A1. People with variants of the UGT1A1 called TA7, also known as the *28 variant, express fewer UGT1A1 enzymes in their liver. During chemotherapy, these patients effectively receive a larger than expected dose because their bodies are not able to clear irinotecan as fast as others. In studies this corresponds to higher incidences of severe diarrhea and neutropenia [1].
In 2004, a clinical study was performed that both validated prospectively the association of the *28 variant with greater toxicity and the ability of genetic testing in predicting that toxicity before chemotherapy administration [2].
In 2005, the FDA made changes to the labelling of irinotecan to add pharmacogenomics recommendations that patients with polymorphisms in UGT1A1 gene, specifically the *28 variant, should perhaps receive reduced drug doses. Irinotecan is one of the first widely-used chemotherapy agents that is dosed for each patient according to his genotype[3].
See also
- Camptothecin
- Topotecan (Hycamtin®)
- Pharmacogenomics
References
- ↑ Journal of Clinical Oncology, Vol 22, No 8 April 15, 2004: pp. 1382-1388
- ↑ Innocenti F; et al. (2004). "Genetic Variants in the UDP-glucuronosyltransferase 1A1 Gene Predict the Risk of Severe Neutropenia of Irinotecan". Journal of Clinical Oncology. 22 (8): 1382–88. PMID 15007088. Unknown parameter
|month=ignored (help) - ↑ O'Dwyer PJ, Catalano RP (2006). "Uridine Diphosphate Glucuronosyltransferase (UGT) 1A1 and Irinotecan: Practical Pharmacogenomics Arrives in Cancer Therapy". Journal of Clinical Oncology. 24 (28): 4534–38. PMID 17008691. Unknown parameter
|month=ignored (help)
External links
- Pages with script errors
- Pages with citations using unsupported parameters
- CS1 maint: Explicit use of et al.
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Cancer treatments
- Chemotherapeutic agents
- Prodrugs