Mitotane
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warnings:
See full prescribing information for complete Boxed Warning.
Warnings:
|
Overview
Mitotane is a antineoplastic Agent that is FDA approved for the treatment of inoperable adrenal cortical carcinoma of both functional and nonfunctional types. There is a Black Box Warning for this drug as shown here. Common adverse reactions include anorexia, nausea or vomiting, somnolence, Skin toxicity.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- LYSODREN is indicated in the treatment of inoperable adrenal cortical carcinoma of both functional and nonfunctional types.
Dosage
- The recommended treatment schedule is to start the patient at 2 g to 6 g of LYSODREN per day in divided doses, either 3 or 4 times a day. Doses are usually increased incrementally to 9 g to 10 g per day. If severe side effects appear, the dose should be reduced until the maximum tolerated dose is achieved. If the patient can tolerate higher doses and improved clinical response appears possible, the dose should be increased until adverse reactions interfere. Experience has shown that the maximum tolerated dose (MTD) will vary from 2 g to 16 g per day, but has usually been 9 g to 10 g per day. The highest doses used in the studies to date were 18 g to 19 g per day.
- Treatment should be instituted in the hospital until a stable dosage regimen is achieved.
- Treatment should be continued as long as clinical benefits are observed. Maintenance of clinical status or slowing of growth of metastatic lesions can be considered clinical benefits if they can clearly be shown to have occurred.
- If no clinical benefits are observed after 3 months at the maximum tolerated dose, the case would generally be considered a clinical failure. However, 10% of the patients who showed a measurable response required more than 3 months at the MTD. Early diagnosis and prompt institution of treatment improve the probability of a positive clinical response. Clinical effectiveness can be shown by reduction in tumor mass; reduction in pain, weakness or anorexia; and reduction of symptoms and signs due to excessive steroid production.
- A number of patients have been treated intermittently with treatment being restarted when severe symptoms have reappeared. Patients often do not respond after the third or fourth such course. Experience accumulated to date suggests that continuous treatment with the maximum possible dosage of LYSODREN is the best approach.
- Procedures for proper handling and disposal of anticancer drugs should be considered. Several guidelines on this subject have been published.1
- To minimize the risk of dermal exposure, always wear impervious gloves when handling bottles containing LYSODREN tablets. LYSODREN tablets should not be crushed. Personnel should avoid exposure to crushed and/or broken tablets. If contact with broken tablets occurs, wash immediately and thoroughly. More information is available in the references listed below.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Mitotane in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Mitotane in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Mitotane in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Mitotane in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Mitotane in pediatric patients.
Contraindications
- LYSODREN (mitotane tablets, USP) should not be given to individuals who have demonstrated a previous hypersensitivity to it.
Warnings
|
Warnings:
See full prescribing information for complete Boxed Warning.
Warnings:
|
- LYSODREN should be temporarily discontinued immediately following shock or severe trauma, since adrenal suppression is its prime action. Exogenous steroids should be administered in such circumstances, since the depressed adrenal may not immediately start to secrete steroids.
- LYSODREN should be administered with care to patients with liver disease other than metastatic lesions from the adrenal cortex, since the metabolism of LYSODREN may be interfered with and the drug may accumulate.
- All possible tumor tissues should be surgically removed from large metastatic masses before LYSODREN administration is instituted. This is necessary to minimize the possibility of infarction and hemorrhage in the tumor due to a rapid cytotoxic effect of the drug.
- Long-term continuous administration of high doses of LYSODREN may lead to brain damage and impairment of function. Behavioral and neurological assessments should be made at regular intervals, since toxicity may be reversible after discontinuation of LYSODREN. Literature reports suggest that mitotane plasma concentrations exceeding 20 mcg/mL are associated with a greater incidence of high grade central nervous system toxicity.
- A substantial percentage of the patients treated show signs of adrenal insufficiency. It therefore appears necessary to watch for and institute steroid replacement in those patients. However, some investigators have recommended that steroid replacement therapy be administered concomitantly with LYSODREN. It has been shown that the metabolism of exogenous steroids is modified and consequently somewhat higher doses than normal replacement therapy may be required. Since LYSODREN increases hormone binding proteins, measurement of free cortisol and corticotropin (ACTH) levels may be useful in achieving optimal steroid replacement.
Precautions
General
- Adrenal insufficiency may develop in patients treated with LYSODREN, and adrenal steroid replacement should be considered for these patients.
- Since sedation, lethargy, vertigo, and other CNS side effects can occur, ambulatory patients should be cautioned about driving, operating machinery, and other hazardous pursuits requiring mental and physical alertness.
- Prolonged bleeding time has been reported in patients treated with LYSODREN. Consider this possibility prior to any surgical intervention.
Adverse Reactions
Clinical Trials Experience
- A very high percentage of patients treated with LYSODREN have shown at least one type of side effect. The main types of adverse reactions consist of the following:
- Gastrointestinal disturbances, which consist of anorexia, nausea or vomiting, and in some cases diarrhea, occur in about 80% of the patients.
Central nervous system side effects occur in 40% of the patients. These consist primarily of depression as manifested by lethargy and somnolence (25%), and dizziness or vertigo (15%).
- Skin toxicity has been observed in about 15% of the cases. These skin changes consist primarily of transient skin rashes which do not seem to be dose related. In some instances, this side effect subsided while the patients were maintained on the drug without a change of dose.
- Infrequently occurring side effects involve the eye (visual blurring, diplopia, lens opacity, toxic retinopathy); the genitourinary system (hematuria, hemorrhagic cystitis, and albuminuria); cardiovascular system (hypertension, orthostatic hypotension, and flushing); and some miscellaneous effects including generalized aching, hyperpyrexia, and lowered protein bound iodine (PBI).
- The following additional adverse reactions have been identified during postapproval use of LYSODREN. Because reports are voluntary from a population of unknown size, an estimate of frequency cannot be made.
- Blood and lymphatic system disorders: neutropenia
- Endocrine disorders: growth retardation, hypothyroidism
- Psychiatric disorders: confusional state
- Nervous system disorders: neuropsychological disturbance, dysarthria, headache, ataxia, mental impairment
- Eye disorders: maculopathy
- Hepatobiliary disorders: hepatitis, elevation of liver enzymes
- Reproductive system and breast disorders: gynecomastia
- General disorders and administration site conditions: asthenia
- Investigations: blood uric acid decreased, blood cholesterol increased, blood triglycerides increased
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Mitotane in the drug label.
Drug Interactions
- LYSODREN is a strong inducer of cytochrome P-450 3A4 (CYP3A4). Monitor patients for a change in dosage requirements for the concomitant drug when administering LYSODREN to patients receiving drugs that are substrates of CYP3A4.
- LYSODREN’s CYP induction effect leads to an increase in dosage requirements for warfarin. Closely monitor patients for a change in anticoagulant dosage requirements when administering LYSODREN to patients receiving coumarin-type anticoagulants.
Use in Specific Populations
Pregnancy
- LYSODREN can cause fetal harm when administered to a pregnant woman. Abnormal pregnancy outcomes such as preterm births and early pregnancy loss have been reported in patients exposed to mitotane during pregnancy. Animal reproduction studies have not been conducted with LYSODREN. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus.
- Advise women of childbearing potential to use effective contraception during treatment and after discontinuation of treatment for as long as mitotane plasma levels are detectable
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Mitotane in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Mitotane during labor and delivery.
Nursing Mothers
- Mitotane has been detected in breast milk. Because of the potential for serious adverse reactions in nursing infants from mitotane, advise women to discontinue nursing during LYSODREN therapy and after treatment discontinuation for as long as mitotane plasma levels are detectable
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- Clinical studies of LYSODREN did not include sufficient numbers of patients aged 65 years and older to determine whether they respond differently than younger patients. Other reported clinical experience has not identified differences in responses between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Mitotane with respect to specific gender populations.
Race
There is no FDA guidance on the use of Mitotane with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Mitotane in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Mitotane in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Mitotane in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Mitotane in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
- Long-term continuous administration of high doses of LYSODREN may lead to brain damage and impairment of function. Behavioral and neurological assessments should be made at regular intervals, since toxicity may be reversible after discontinuation of LYSODREN.
IV Compatibility
There is limited information regarding IV Compatibility of Mitotane in the drug label.
Overdosage
- No proven antidotes have been established for LYSODREN overdosage. The long half-life of mitotane will require prolonged observation for toxicity
Pharmacology
 | |
| Clinical data | |
|---|---|
| Trade names | Lysodren |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608050 |
| [[Regulation of therapeutic goods |Template:Engvar data]] | |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 40% |
| Protein binding | 6% |
| Elimination half-life | 18 to 159 days |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C14H10Cl4 |
| Molar mass | 320.04 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Mechanism of Action
- LYSODREN can best be described as an adrenal cytotoxic agent, although it can cause adrenal inhibition, apparently without cellular destruction. Its biochemical mechanism of action is unknown. Data are available to suggest that the drug modifies the peripheral metabolism of steroids as well as directly suppressing the adrenal cortex. The administration of LYSODREN alters the extra-adrenal metabolism of cortisol in man; leading to a reduction in measurable 17-hydroxy corticosteroids, even though plasma levels of corticosteroids do not fall. The drug apparently causes increased formation of 6-β-hydroxycortisol.
Structure
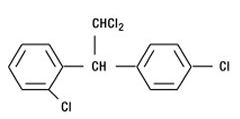
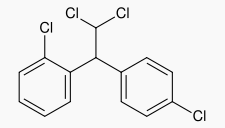
- LYSODREN® (mitotane tablets, USP) is an oral chemotherapeutic agent. It is best known by its trivial name, o,p′-DDD, and is chemically, 1,1-dichloro-2-(o-chlorophenyl)-2-(p-chlorophenyl) ethane. The chemical structure is shown below:
- LYSODREN is a white granular solid composed of clear colorless crystals. It is tasteless and has a slight pleasant aromatic odor. It is soluble in ethanol, isooctane, and carbon tetrachloride. It has a molecular weight of 320.05.
- Inactive ingredients in LYSODREN tablets are: avicel, Polyethylene Glycol 3350, silicon dioxide, and starch.
- LYSODREN is available as 500 mg scored tablets for oral administration.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Mitotane in the drug label.
Pharmacokinetics
- Data in adrenal carcinoma patients indicate that about 40% of oral LYSODREN is absorbed and approximately 10% of the administered dose is recovered in the urine as a water-soluble metabolite. A variable amount of metabolite (1%-17%) is excreted in the bile and the balance is apparently stored in the tissues.
- Following discontinuation of LYSODREN, the plasma terminal half-life has ranged from 18 to 159 days. In most patients blood levels become undetectable after 6 to 9 weeks. Autopsy data have provided evidence that LYSODREN is found in most tissues of the body; however, fat tissues are the primary site of storage. LYSODREN is converted to a water-soluble metabolite.
- No unchanged LYSODREN has been found in urine or bile.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- The carcinogenic and mutagenic potentials of LYSODREN (mitotane tablets, USP) are unknown. However, the mechanism of action of this compound suggests that it probably has less carcinogenic potential than other cytotoxic chemotherapeutic drugs.
Clinical Studies
There is limited information regarding Clinical Studies of Mitotane in the drug label.
How Supplied
- LYSODREN® (mitotane tablets, USP)
NDC 0015-3080-60—500 mg Tablets, bottle of 100
Storage
- Store at 25°C (77°F); excursions permitted to 15°C-30°C (59°F-86°F)
Images
Drug Images
{{#ask: Page Name::Mitotane |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
REPRESENTATIVE PACKAGING
See How Supplied section for a complete list of available packages of LYSODREN.
NDC 0015-3080-60 100 TABLETS LYSODREN® (mitotane tablets, USP) EACH TABLET CONTAINS 500 mg Rx only Bristol-Myers Squibb
Ingredients and Appearance
{{#ask: Label Page::Mitotane |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Mitotane in the drug label.
Precautions with Alcohol
- Alcohol-Mitotane interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Lysodren®[1]
Look-Alike Drug Names
There is limited information regarding Mitotane Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.