Triplatin tetranitrate
 | |
| Identifiers | |
|---|---|
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
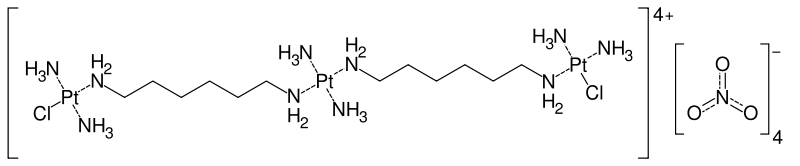
Triplatin tetranitrate (rINN; also known as BBR3464) is a new platinum-based cytotoxic drug that is currently undergoing clinical trials throughout the world for the treatment of human cancer. It is a trinuclear platinum coordination complex, with chloride and amine ligands. The drug acts by forming coordinate covalent adducts with cellular DNA, preventing DNA transcription and replication, and through this inducing apoptosis. It is structurally similar to, but in a different family from, the anticancer drugs cisplatin, carboplatin and oxaliplatin.
History
It was invented by Professor Nicholas Farrell at Virginia Commonwealth University in the United States. The development of this drug came from his earlier work looking at dinuclear platinum derivatives of cisplatin, research he began in the late 1980s. Similar work was also being undertaken by Dr John Broomhead at the Australian National University in Australia. BBR3464 was patented in the mid-1990s and was originally licensed to the pharmaceutical company Roche. In preclinical trials, it demonstrated cytotoxic activity in cancer cell lines which have either intrinsic or acquired resistance to cisplatin. On this basis it entered Phase I (Toxicity) clinical trials under the auspices of Novuspharma before the rights were transferred to Cell Therapeutics.
This drug is currently undergoing Phase II (Efficacy) trials with mixed results. So far trials of the drug with patients suffering from ovarian cancer, small cell lung cancer and gastric or gastro-oesophageal adenocarcinomas have been reported in the literature.
Mode of action
The main target of triplatin is cellular DNA, similar to cisplatin. Outside of the cell, the concentration of chloride (approx. 100 millimolar) prevents the drugs from hydrolysing, but once inside the cell, where the concentration of chloride drops to between 4 and 20 millimolar, the chloride ligands of BBR3464 come off and the drug is capable of forming coordinate covalent bonds with purine bases on DNA. The novel adducts BBR3464 forms with DNA are though to be the mechanism by which this drug acts; the adducts are able to prevent DNA transcription and replication, thus inducing cell apoptosis.
Side-effects
All platinum based drugs, and particularly BBR3464, cause large dose limiting side-effects. For BBR3464 these are largely diarrhea, cramps and vomiting, but are so severe that the maximum tolerated dose (MTD) in humans is between 0.9 to 1.1 milligrams per square metre. This is considerably lower than the MTD for all the platinum based drugs currently used in the clinic, like cisplatin (60–120 mg) and carboplatin (approx. 800 mg).
References
- Wheate, Nial J. and Collins, J. Grant (2003). Multi-nuclear platinum complexes as anti-cancer drugs. Coord Chem Rev, 241, 133–145. doi:10.1016/S0010-8545(03)00050-X.
- Wheate, Nial J. and Collins, J. Grant (2005). Multi-nuclear platinum drugs: A new paradigm in chemotherapy. Curr Med Chem Anticancer Agents, 5, 267–279. PMID 15992354.
{{jb1}
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Platinum compounds
- Chemotherapeutic agents
- IARC Group 2A carcinogens
- Coordination compounds