Cytarabine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Black Box Warning:
See full prescribing information for complete Boxed Warning.
ConditionName:
Only physicians experienced in cancer chemotherapy should use Cytarabine Injection. For induction therapy patients should be treated in a facility with laboratory and supportive resources sufficient to monitor drug tolerance and protect and maintain a patient compromised by drug toxicity. The main toxic effect of cytarabine is bone marrow suppression with leukopenia, thrombocytopenia and anemia. Less serious toxicity includes nausea, vomiting, diarrhea and abdominal pain, oral ulceration and hepatic dysfunction. The physician must judge possible benefit to the patient against known toxic effects of this drug in considering the advisability of therapy with cytarabine. Before making this judgment or beginning treatment, the physician should be familiar with the following text. |
Overview
Cytarabine is an antineoplastic agent that is FDA approved for the treatment of acute non-lymphocytic leukemia of adults and children, acute lymphocytic leukemia and the blast phase of chronic myelocytic leukemia. Intrathecal administration of cytarabine injection is indicated for the prophylaxis and treatment of meningeal leukemia. There is a Black Box Warning for this drug as shown here. Common adverse reactions include thrombophlebitis, rash, hyperuricemia, anal inflammation, diarrhea, loss of apetite, nauseas, stomatitis, mouth ulceration, vomiting, decreased reticulocyte count, megaloblastic anemia, decreased liver function and fever.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Acute Non-Lymphocytic Leukemia
- Induction: 100 mg/m2/day by continuous IV infusion (Days 1-7) or 100 mg/m2 IV every 12 hours (Days 1-7).
Meningeal Leukemia
- Dosage: doses ranging from 5 mg/m2 to 75 mg/m2 of body surface area. The frequency of administration varied from once a day for 4 days to once every 4 days. The most frequently used dose was 30 mg/m2 every 4 days until cerebrospinal fluid findings were normal, followed by one additional treatment.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cytarabine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Cytarabine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Cytarabine FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cytarabine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Cytarabine in pediatric patients.
Contraindications
- Cytarabine is contraindicated in those patients who are hypersensitive to the drug.
Warnings
|
Black Box Warning:
See full prescribing information for complete Boxed Warning.
ConditionName:
Only physicians experienced in cancer chemotherapy should use Cytarabine Injection. For induction therapy patients should be treated in a facility with laboratory and supportive resources sufficient to monitor drug tolerance and protect and maintain a patient compromised by drug toxicity. The main toxic effect of cytarabine is bone marrow suppression with leukopenia, thrombocytopenia and anemia. Less serious toxicity includes nausea, vomiting, diarrhea and abdominal pain, oral ulceration and hepatic dysfunction. The physician must judge possible benefit to the patient against known toxic effects of this drug in considering the advisability of therapy with cytarabine. Before making this judgment or beginning treatment, the physician should be familiar with the following text. |
- Cytarabine is a potent bone marrow suppressant. Therapy should be started cautiously in patients with pre-existing drug-induced bone marrow suppression. Patients receiving this drug must be under close medical supervision and, during induction therapy, should have leukocyte and platelet counts performed daily. Bone marrow examinations should be performed frequently after blasts have disappeared from the peripheral blood.
- Facilities should be available for management of complications, possibly fatal, of bone marrow suppression (infection resulting from granulocytopenia and other impaired body defenses and hemorrhage secondary to thrombocytopenia). One case of anaphylaxis that resulted in acute cardiopulmonary arrest and required resuscitation has been reported. This occurred immediately after the intravenous administration of cytarabine.
- Severe and at times fatal CNS, GI and pulmonary toxicity (different from that seen with conventional therapy regimens of cytarabine) has been reported following some of the experimental cytarabine dose schedules. These reactions include reversible corneal toxicity, and hemorrhagic conjunctivitis, which may be prevented or diminished by prophylaxis with a local corticosteroid eye drop; cerebral and cerebellar dysfunction including personality changes, somnolence and coma, usually reversible; severe gastrointestinal ulceration, including pneumatosis cystoides intestinalis leading to peritonitis; sepsis and liver abscess; pulmonary edema, liver damage with increased hyperbilirubinemia, bowel necrosis; and necrotizing colitis. Rarely, severe skin rash, leading to desquamation has been reported. Complete alopecia is more commonly seen with experimental high dose therapy than with standard cytarabine treatment programs. If experimental high dose therapy is used, do not use a cytarabine injection containing benzyl alcohol.
- Cases of cardiomyopathy with subsequent death has been reported following experimental high dose therapy with cytarabine in combination with cyclophosphamide when used for bone marrow transplant preparation.
- A syndrome of sudden respiratory distress, rapidly progressing to pulmonary edema and radiographically pronounced cardiomegaly has been reported following experimental high dose therapy with cytarabine used for the treatment of relapsed leukemia from one institution in 16/72 patients. The outcome of this syndrome can be fatal.
- Benzyl alcohol is contained in this product. Benzyl alcohol has been reported to be associated with a fatal "Gasping Syndrome" in premature infants.
- Two patients with childhood acute myelogenous leukemia who received intrathecal and intravenous cytarabine at conventional doses (in addition to a number of other concomitantly administered drugs) developed delayed progressive ascending paralysis resulting in death in one of the two patients.
Adverse Reactions
Clinical Trials Experience
Hematological Effects
Infections
Viral, bacterial, fungal, parasitic or saprophytic infections, in any location in the body may be associated with the use of cytarabine alone or in combination with other immunosuppressive agents following immunosuppressant doses that affect cellular or humoral immunity. These infections may be mild, but can be severe and at times fatal.
Cytabarine Syndrome
- Fever
- Myalgia
- Bone pain
- Occasionally chest pain
- Maculopapular rash
- Conjunctivitis and malaise
Cardiovascular effects
- Cardiomyopathy
- Pericarditis: < 0.1% of patients develop this adverse effect, between 2 - 3 days after initiating the therapy, symptoms lasting for 1-6 weeks [1] [2] [3]. Symptoms include:
- Chest pain
- Dyspnea
- Pericardial effusion
- Pericardial friction rub
- Pulsus paradoxus
- Right ventricular diastolic collapse
- ST-Segment elevation on EKG
- Vasculitis: high-dose cytarabine causes small vessel necrotizing vasculitis[4].
Dermatological effects
- Alopecia
- Dermatologic toxicity[5]
- Severe Henoch-Schönlein purpura[6]
- Rash
- Toxic epidermal necrolysis[7]
Endocrine/metabolic Effects
- Disorder of fluid and/or electrolyte: hypokalemia and hypocalcemia, specially when diarrhea is present[8].
- Hyperuricemia: is a result of tumor lysis syndrome[9]
- Increased body temperature[10]
Gastrointestinal Effects
- Gastrointestinal tract finding: nausea and vomiting are common agh high-dosis[9]. GI bleeding has also been reported[8].
- Pancreatitis
- Parotiditis:
- Case report: 2/30 patients developed parotiditis with cytarabine 200 milligrams/square meter/day in continuous infusion for 7 days [13].
- Pseudomembranous enterocolitis
- Case report: 2 patients who developed Pseudomembranous Enterocolitis and as a consequence, needed subtotal colectomy[14].
Hepatic Effects
- Hepatotoxicity[15]: It lead to jaundice and hyperbilirubinemia
Immunological Effects
- Anaphylaxis
- Case report of a 5 year-old patient receiving cytarabine [16].
- Immunosuppression
Musculoeskeletal Effects
Neurologic Effects
- Arachnoiditis[18]
- Aseptic meningitis
- Case report of a 8-year old patient in treatment for Acute Lymphoblastic Leukemia[19].
- Cranial nerve disorder
- Neurological finding: Ataxia[20], dysphagia, nystagmus[21] and decreased level of consciousness[22]
- Neuropathy [23]
- Neurotoxicity[22]: causas cerebellar toxicity[24][25], characterized by:
- Paraplegia: Presents after intrathecal administration[26].
- Paresthesia
- Parkinsonism
- Case report of a 64 year-old patient in treatment for acute myelogenous leukemia with high dose cytarabine[27].
- Pseudotumor cerebri[28]
Ophthalmic Effects
- Conjunctivitis
- Disorder of cornea[29]: After one week with cytarabine treatment
- Vision disorder
- Case report of a 31 year old patient after 2 weeks after receiving consolidation therapy with cytarabine 3 grams per square meter (g/m(2)) every 12 hours for 3 alternate days, developed vision loss[30].
Urinary Tract Effects
- Cystitis[31]
- Renal failure
- Case report: 45 year-old patient treated for hypoplastic myelodysplastic syndrome with low-dose cytarabine[32].
- Urinary retention: its the least frequent of the urinary tract adverse effects
Respiratory Effects
Other
Postmarketing Experience
There is limited information regarding Cytarabine Postmarketing Experience in the drug label.
Drug Interactions
- Reversible decreases in steady-state plasma digoxin concentrations and renal glycoside excretion were observed in patients receiving beta-acetyldigoxin and chemotherapy regimens containing cyclophosphamide, vincristine and prednisone with or without cytarabine or procarbazine. Steady-state plasma digitoxin concentrations did not appear to change. Therefore, monitoring of plasma digoxin levels may be indicated in patients receiving similar combination chemotherapy regimens. The utilization of digitoxin for such patients may be considered as an alternative.
- An in vitro interaction study between gentamicin and cytarabine showed a cytarabine related antagonism for the susceptibility of K. pneumoniae strains. This study suggests that in patients on cytarabine being treated with gentamicin for a K. pneumoniae infection, the lack of a prompt therapeutic response may indicate the need for reevaluation of antibacterial therapy.
- Clinical evidence in one patient showed possible inhibition of fluorocytosine efficacy during therapy with cytarabine. This may be due to potential competitive inhibition of its uptake.
Use in Specific Populations
Pregnancy
- A review of the literature has shown 32 reported cases where cytarabine was given during pregnancy, either alone or in combination with other cytotoxic agents.
- Eighteen normal infants were delivered. Four of these had first trimester exposure. Five infants were premature or of low birth weight. Twelve of the 18 normal infants were followed up at ages ranging from six weeks to seven years, and showed no abnormalities. One apparently normal infant died at 90 days of gastroenteritis.
- Two cases of congenital abnormalities have been reported, one with upper and lower distal limb defects, and the other with extremity and ear deformities. Both of these cases had first trimester exposure.
- There were seven infants with various problems in the neonatal period, including pancytopenia; transient depression of the WBC, hematocrit or platelets; electrolyte abnormalities; transient eosinophilia; and one case of increased IgM levels and hyperpyrexia possibly due to sepsis. Six of the seven infants were also premature. The child with pancytopenia died at 21 days of sepsis.
- Therapeutic abortions were done in five cases. Four fetuses were grossly normal, but one had an enlarged spleen and another showed Trisomy C chromosome abnormality in the chorionic tissue.
- Because of the potential for abnormalities with cytotoxic therapy, particularly during the first trimester, a patient who is or who may become pregnant while on Cytarabine Injection should be apprised of the potential risk to the fetus and the advisability of pregnancy continuation. There is a definite, but considerably reduced risk if therapy is initiated during the second or third trimester. Although normal infants have been delivered to patients treated in all three trimesters of pregnancy, follow-up of such infants would be advisable.
Pregnancy Category (AUS): D
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Cytarabine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Cytarabine during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk: Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from cytarabine, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
There is no FDA guidance on the use of Cytarabine in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Cytarabine in geriatric settings.
Gender
There is no FDA guidance on the use of Cytarabine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Cytarabine with respect to specific racial populations.
Renal Impairment
- In particular, patients with renal or hepatic function impairment may have a higher likelihood of CNS toxicity after high-dose cytarabine treatment. Use the drug with caution and possibly at reduced dose in patients whose liver or kidney function is poor.
Hepatic Impairment
There is no FDA guidance on the use of Cytarabine in patients with hepatic impairment.
Females of Reproductive Potential and Males
- Extensive chromosomal damage, including chromatid breaks have been produced by cytarabine and malignant transformation of rodent cells in culture has been reported.
Immunocompromised Patients
There is no FDA guidance one the use of Cytarabine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Cytarabine Injection (non-preserved) can be administered by intravenous injection or infusion, subcutaneously, or intrathecally. However, the intent of this Pharmacy Bulk Package is for the preparation of solutions for IV infusion only. Intrathecal use of cytarabine requires the use of single-dose, unpreserved solutions only.
- When cytarabine is administered both intrathecally and intravenously within a few days, there is an increased risk of spinal cord toxicity, however, in serious life-threatening disease, concurrent use of intravenous and intrathecal cytarabine is left to the discretion of the treating physician.
Monitoring
- Patients receiving Cytarabine Injection must be monitored closely. Frequent platelet and leukocyte counts and bone marrow examinations are mandatory. Consider suspending or modifying therapy when drug-induced marrow depression has resulted in a platelet count under 50,000 or a polymorphonuclear granulocyte count under 1000/mm3. Counts of formed elements in the peripheral blood may continue to fall after the drug is stopped and reach lowest values after drug-free intervals of 12 to 24 days. When indicated, restart therapy when definite signs of marrow recovery appear (on successive bone marrow studies). Patients whose drug is withheld until "normal" peripheral blood values are attained may escape from control.
- When large intravenous doses are given quickly, patients are frequently nauseated and may vomit for several hours post injection. This problem tends to be less severe when the drug is infused.
- The human liver apparently detoxifies a substantial fraction of an administered dose. In particular, patients with renal or hepatic function impairment may have a higher likelihood of CNS toxicity after high-dose cytarabine treatment. Use the drug with caution and possibly at reduced dose in patients whose liver or kidney function is poor.
- Periodic checks of bone marrow, liver and kidney functions should be performed in patients receiving Cytarabine Injection.
- Like other cytotoxic drugs, Cytarabine Injection may induce hyperuricemia secondary to rapid lysis of neoplastic cells. The clinician should monitor the patient's blood uric acid level and be prepared to use such supportive and pharmacologic measures as might be necessary to control this problem.
- Acute pancreatitis has been reported to occur in a patient receiving cytarabine by continuous infusion and in patients being treated with cytarabine who have had prior treatment with L-asparaginase.
IV Compatibility
If used intrathecally, do not use a solution containing benzyl alcohol. This pharmacy bulk package is not intended to be used for the preparation of intrathecal doses.
Overdosage
- There is no antidote for cytarabine over dosage. Doses of 4.5 g/m2 by intravenous infusion over 1 hour every 12 hours for 12 doses has caused an unacceptable increase in irreversible CNS toxicity and death.
- Single doses as high as 3 g/m2 have been administered by rapid intravenous infusion without apparent toxicity.
Pharmacology
Mechanism of Action
- Cytarabine is cytotoxic to a wide variety of proliferating mammalian cells in culture. It exhibits cell phase specificity, primarily killing cells undergoing DNA synthesis (S-phase) and under certain conditions blocking the progression of cells from the G1 phase to the S-phase. Although the mechanism of action is not completely understood, it appears that cytarabine acts through the inhibition of DNA polymerase. A limited, but significant, incorporation of cytarabine into both DNA and RNA has also been reported. Extensive chromosomal damage, including chromatid breaks have been produced by cytarabine and malignant transformation of rodent cells in culture has been reported. Deoxycytidine prevents or delays (but does not reverse) the cytotoxic activity.
Structure
Cytarabine is chemically 4-amino-l- β-Darabinofuranosyl- 2(lH)-pyrimidinone.
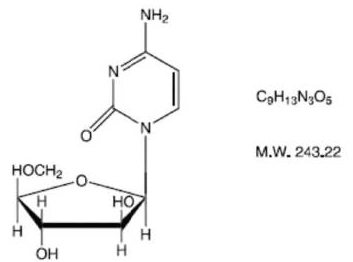
Pharmacodynamics
There is limited information regarding Cytarabine Pharmacodynamics in the drug label.
Pharmacokinetics
- Cytarabine is rapidly metabolized and is not effective orally; less than 20 percent of the orally administered dose is absorbed from the gastrointestinal tract.
- Following rapid intravenous injection of cytarabine, labeled with tritium, the disappearance from plasma is biphasic. There is an initial distributive phase with a half-life of about 10 minutes, followed by a second elimination phase with a half-life of about 1 to 3 hours. After the distributive phase, more than 80 percent of plasma radioactivity can be accounted for by the inactive metabolite 1-β-D-arabinofuranosyluracil (ara-U). Within 24 hours about 80 percent of the administered radioactivity can be recovered in the urine, approximately 90 percent of which is excreted as ara-U.
- Relatively constant plasma levels can be achieved by continuous intravenous infusion.
- After subcutaneous or intramuscular administration of cytarabine labeled with tritium, peak-plasma levels of radioactivity are achieved about 20 to 60 minutes after injection and are considerably lower than those after intravenous administration.
- Cerebrospinal fluid levels of cytarabine are low in comparison to plasma levels after single intravenous injection. However, in one patient in whom cerebrospinal fluid levels are examined after 2 hours of constant intravenous infusion, levels approached 40 percent of the steady state plasma level. With intrathecal administration, levels of cytarabine in the cerebrospinal fluid declined with a first order half-life of about 2 hours. Because cerebrospinal fluid levels of deaminase are low, little conversion to ara-U was observed.
Nonclinical Toxicology
There is limited information regarding Cytarabine Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Cytarabine Clinical Studies in the drug label.
How Supplied
Cytarabine Injection, PHARMACY BULK PACKAGE. Sterile, Isotonic Solution. Preservative, Free. NDC No. 0069-0154-01.Cytarabine Injection 1000 mg in a 50 mL, (20 mg/mL) flip-top vial (brown cap), packaged individually.
Storage
Store between, 20° - 25°C (68° - 77°F). [See USP Controlled Room Temperature].
Images
Drug Images
{{#ask: Page Name::Cytarabine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Cytarabine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Cytarabine Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Cytarabine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Cytarabine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Woods T, Vidarsson B, Mosher D, Stein JH (1999). "Transient effusive-constrictive pericarditis due to chemotherapy". Clin Cardiol. 22 (4): 316–8. PMID 10198745.
- ↑ Hermans C, Straetmans N, Michaux JL, Ferrant A (1997). "Pericarditis induced by high-dose cytosine arabinoside chemotherapy". Ann Hematol. 75 (1–2): 55–7. PMID 9322684.
- ↑ Vaickus L, Letendre L (1984). "Pericarditis induced by high-dose cytarabine therapy". Arch Intern Med. 144 (9): 1868–9. PMID 6477012.
- ↑ Ahmed I, Chen KR, Nakayama H, Gibson LE (1998). "Cytosine arabinoside-induced vasculitis". Mayo Clin Proc. 73 (3): 239–42. doi:10.1016/S0025-6196(11)64465-0. PMID 9511781.
- ↑ Graves T, Hooks MA (1989). "Drug-induced toxicities associated with high-dose cytosine arabinoside infusions". Pharmacotherapy. 9 (1): 23–8. PMID 2922357.
- ↑ Aktas B, Topcuoglu P, Kurt OK, Ensari A, Demirer T (2009). "Severe Henoch-Schönlein purpura induced by cytarabine". Ann Pharmacother. 43 (4): 792–3. doi:10.1345/aph.1L608. PMID 19336650.
- ↑ Ozkan A, Apak H, Celkan T, Yüksel L, Yildiz I (2001). "Toxic epidermal necrolysis after the use of high-dose cytosine arabinoside". Pediatr Dermatol. 18 (1): 38–40. PMID 11207969.
- ↑ 8.0 8.1 Slavin RE, Dias MA, Saral R (1978). "Cytosine arabinoside induced gastrointestinal toxic alterations in sequential chemotherapeutic protocols: a clinical-pathologic study of 33 patients". Cancer. 42 (4): 1747–59. PMID 709532.
- ↑ 9.0 9.1 Rudnick SA, Cadman EC, Capizzi RL, Skeel RT, Bertino JR, McIntosh S (1979). "High dose cytosine arabinoside (HDARAC) in refractory acute leukemia". Cancer. 44 (4): 1189–93. PMID 498009.
- ↑ Bensinger TA, Fahey JL, Kellon DB, Beutler E (1974). "Letter: Febrile response to cytarabine". JAMA. 229 (12): 1578. PMID 4408256.
- ↑ McBride CE, Yavorski RT, Moses FM, Robson ME, Solimando DA, Byrd JC (1996). "Acute pancreatitis associated with continuous infusion cytarabine therapy: a case report". Cancer. 77 (12): 2588–91. doi:10.1002/(SICI)1097-0142(19960615)77:12<2588::AID-CNCR24>3.0.CO;2-N. PMID 8640710.
- ↑ Siemers RF, Friedenberg WR, Norfleet RG (1985). "High-dose cytosine arabinoside-associated pancreatitis". Cancer. 56 (8): 1940–2. PMID 2411382.
- ↑ Cetkovský P, Koza V (1994). "Salivary glands enlargement in association with cytosine arabinoside application in patients with acute myeloid leukaemia". Eur J Cancer. 30A (11): 1727–8. PMID 7833152.
- ↑ Lea JW, Masys DR, Shackford SR (1980). "Typhlitis: a treatable complication of acute leukemia therapy". Cancer Clin Trials. 3 (4): 355–62. PMID 6933028.
- ↑ Woods WG, Dehner LP, Nesbit ME, Krivit W, Coccia PF, Ramsay NK; et al. (1980). "Fatal veno-occlusive disease of the liver following high dose chemotherapy, irradiation and bone marrow transplantation". Am J Med. 68 (2): 285–90. PMID 6986767.
- ↑ Berkowitz FE, Wehde S, Ngwenya ET, Greeff M, Wadee AA, Rabson AR (1987). "Anaphylactic shock due to cytarabine in a leukemic child". Am J Dis Child. 141 (9): 1000–1. PMID 3475975.
- ↑ Margolis D, Ross E, Miller KB (1987). "Rhabdomyolysis associated with high-dose cytarabine". Cancer Treat Rep. 71 (12): 1325–6. PMID 3690552.
- ↑ Glantz MJ, LaFollette S, Jaeckle KA, Shapiro W, Swinnen L, Rozental JR; et al. (1999). "Randomized trial of a slow-release versus a standard formulation of cytarabine for the intrathecal treatment of lymphomatous meningitis". J Clin Oncol. 17 (10): 3110–6. PMID 10506606.
- ↑ Pease CL, Horton TM, McClain KL, Kaplan SL (2001). "Aseptic meningitis in a child after systemic treatment with high dose cytarabine". Pediatr Infect Dis J. 20 (1): 87–9. PMID 11176579.
- ↑ Baker WJ, Royer GL, Weiss RB (1991). "Cytarabine and neurologic toxicity". J Clin Oncol. 9 (4): 679–93. PMID 1648599.
- ↑ Grossman L, Baker MA, Sutton DM, Deck JH (1983). "Central nervous system toxicity of high-dose cytosine arabinoside". Med Pediatr Oncol. 11 (4): 246–50. PMID 6888324.
- ↑ 22.0 22.1 Herzig RH, Hines JD, Herzig GP, Wolff SN, Cassileth PA, Lazarus HM; et al. (1987). "Cerebellar toxicity with high-dose cytosine arabinoside". J Clin Oncol. 5 (6): 927–32. PMID 3585447.
- ↑ Powell BL, Zekan PJ, Muss HB, Richards F, Lyerly ES, Capizzi RL (1986). "Ara-C syndrome during low-dose continuous infusion therapy". Med Pediatr Oncol. 14 (6): 310–2. PMID 3784982.
- ↑ Hasle H (1990). "Cerebellar toxicity during cytarabine therapy associated with renal insufficiency". Cancer Chemother Pharmacol. 27 (1): 76–8. PMID 2245495.
- ↑ Smith GA, Damon LE, Rugo HS, Ries CA, Linker CA (1997). "High-dose cytarabine dose modification reduces the incidence of neurotoxicity in patients with renal insufficiency". J Clin Oncol. 15 (2): 833–9. PMID 9053511.
- ↑ Wolff L, Zighelboim J, Gale RP (1979). "Paraplegia following intrathecal cytosine arabinoside". Cancer. 43 (1): 83–5. PMID 282937.
- ↑ Luque FA, Selhorst JB, Petruska P (1987). "Parkinsonism induced by high-dose cytosine arabinoside". Mov Disord. 2 (3): 219–22. doi:10.1002/mds.870020309. PMID 3504550.
- ↑ Fort JA, Smith LD (1999). "Pseudotumor cerebri secondary to intermediate-dose cytarabine HCl". Ann Pharmacother. 33 (5): 576–8. PMID 10369621.
- ↑ Hopen G, Mondino BJ, Johnson BL, Chervenick PA (1981). "Corneal toxicity with systemic cytarabine". Am J Ophthalmol. 91 (4): 500–4. PMID 6939330.
- ↑ Schwartz J, Alster Y, Ben-Tal O, Lowenstein A (2000). "Visual loss following high-dose cytosine arabinoside (ARA-C)". Eur J Haematol. 64 (3): 208–9. PMID 10997890.
- ↑ Renert WA, Berdon WE, Baker DH (1973). "Hemorrhagic cystitis and vesicoureteral reflux secondary to cytotoxic therapy for childhood malignancies". Am J Roentgenol Radium Ther Nucl Med. 117 (3): 664–9. PMID 4511884.
- ↑ Tanaka M, Kanamori H, Yamaji S, Mishima A, Fujita H, Fujisawa S; et al. (1999). "Low-dose cytarabine-induced hepatic and renal dysfunction in a patient with myelodysplastic syndrome". Anticancer Drugs. 10 (3): 289–91. PMID 10327034.
{{#subobject:
|Label Page=Cytarabine |Label Name=Cyt.png
}}
{{#subobject:
|Label Page=Cytarabine |Label Name=Captura de pantalla 2014-12-19 a la(s) 13.17.52.png
}}