Cefpodoxime: Difference between revisions
Rabin Bista (talk | contribs) No edit summary |
No edit summary |
||
| (14 intermediate revisions by 2 users not shown) | |||
| Line 3: | Line 3: | ||
|genericName=Cefpodoxime proxetil | |genericName=Cefpodoxime proxetil | ||
|aOrAn=a | |aOrAn=a | ||
|drugClass=orally administered, extended spectrum, semi-synthetic antibiotic of the cephalosporin class | |drugClass=orally administered, extended spectrum, semi-synthetic antibiotic of the [[cephalosporin]] class | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=acute otitis media,pharyngitis,tonsillitis,community-acquired pneumonia,acute bacterial exacerbation of chronic bronchitis,acute uncomplicated urethral and cervical gonorrhea,acute maxillary sinusitis,uncomplicated urinary tract infections and uncomplicated skin and skin structure infections | |indication=[[acute otitis media]],[[pharyngitis]],[[tonsillitis]],[[community-acquired pneumonia]],acute bacterial exacerbation of [[chronic bronchitis]],acute uncomplicated urethral and [[cervical gonorrhea]],[[acute maxillary sinusitis]],uncomplicated [[urinary tract infections]] and uncomplicated [[skin and skin structure infections]] | ||
|adverseReactions=[[Diarrhea]],[[Nausea]],[[Vaginal Fungal Infections]],[[Vulvovaginal Infections]],[[Abdominal pain]], | |||
|adverseReactions=Diarrhea,Nausea,Vaginal Fungal Infections,Vulvovaginal Infections,Abdominal pain, | [[Headache]] | ||
Headache | |||
|blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | |blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | ||
|fdaLIADAdult====Indications=== | |fdaLIADAdult====Indications=== | ||
*Acute otitis media caused by Streptococcus pneumoniae (excluding penicillin-resistant strains), Streptococcus pyogenes, Haemophilus influenzae (including beta-lactamase-producing strains), or Moraxella (Branhamella) catarrhalis (including beta-lactamase-producing strains). | *Acute [[otitis media]] caused by [[Streptococcus pneumoniae]] (excluding penicillin-resistant strains), [[Streptococcus pyogenes]], [[Haemophilus influenzae]] (including beta-lactamase-producing strains), or [[Moraxella (Branhamella) catarrhalis]] (including beta-lactamase-producing strains). | ||
*Pharyngitis and/or tonsillitis caused by Streptococcus pyogenes. | *[[Pharyngitis]] and/or [[tonsillitis]] caused by [[Streptococcus pyogenes]]. | ||
*Community-acquired pneumonia caused by S. pneumoniae or H. Influenzae (including beta-lactamase-producing strains). | *[[Community-acquired pneumonia]] caused by [[S. pneumoniae]] or [[H. Influenzae]] (including beta-lactamase-producing strains). | ||
*Acute bacterial exacerbation of chronic bronchitis caused by S. pneumoniae, H. influenzae (non-beta-lactamase-producing strains only), or M. catarrhalis. | *Acute bacterial exacerbation of [[chronic bronchitis]] caused by [[S. pneumoniae]], [[H. influenzae]] (non-beta-lactamase-producing strains only), or [[M. catarrhalis]]. | ||
*Acute, uncomplicated urethral and cervical gonorrhea | *Acute, uncomplicated urethral and [[cervical gonorrhea]] caused by [[Neisseria gonorrhoeae]] (including penicillinase-producing strains). | ||
*Acute, uncomplicated ano-rectal infections in women due to Neisseria gonorrhoeae (including penicillinase-producing strains). | *Acute, uncomplicated [[ano-rectal infections]] in women due to [[Neisseria gonorrhoeae]] (including penicillinase-producing strains). | ||
*Uncomplicated skin and skin structure infections caused by Staphylococcus aureus (including penicillinase-producing strains) or Streptococcus pyogenes. Abscesses should be surgically drained as clinically indicated. | *Uncomplicated [[skin and skin structure infections]] caused by [[Staphylococcus aureus]] (including penicillinase-producing strains) or [[Streptococcus pyogenes]]. [[Abscesses]] should be surgically drained as clinically indicated. | ||
*[[Acute maxillary sinusitis]] caused by [[Haemophilus influenzae]] (including beta-lactamase-producing strains), [[Streptococcus pneumoniae]], and [[Moraxella catarrhalis]]. | |||
*Acute maxillary sinusitis caused by Haemophilus influenzae (including beta-lactamase-producing strains), Streptococcus pneumoniae, and Moraxella catarrhalis. | *Uncomplicated [[urinary tract infections]] (cystitis) caused by [[Escherichia coli]], [[Klebsiella pneumoniae]], [[Proteus mirabilis]], or [[Staphylococcus saprophyticus]]. | ||
*Uncomplicated urinary tract infections (cystitis) caused by Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, or Staphylococcus saprophyticus. | |||
===Dosage=== | ===Dosage=== | ||
====Film Coated Tablets==== | ====Film Coated Tablets==== | ||
| Line 33: | Line 26: | ||
====Patients with Renal Dysfunction==== | ====Patients with Renal Dysfunction==== | ||
*For patients with severe renal impairment (<30 mL/min creatinine clearance), the dosing intervals should be increased to Q 24 hours. In patients maintained on hemodialysis, the dose frequency should be 3 times/week after hemodialysis. | *For patients with severe renal impairment (<30 mL/min creatinine clearance), the dosing intervals should be increased to Q 24 hours. In patients maintained on [[hemodialysis]], the dose frequency should be 3 times/week after [[hemodialysis]]. | ||
*When only the serum creatinine level is available, the following formula (based on sex, weight, and age of the patient) may be used to estimate creatinine clearance (mL/min). For this estimate to be valid, the serum creatinine level should represent a steady state of renal function. | *When only the serum creatinine level is available, the following formula (based on sex, weight, and age of the patient) may be used to estimate creatinine clearance (mL/min). For this estimate to be valid, the serum creatinine level should represent a steady state of renal function. | ||
:*Males: | :*Males: Weight (kg) x (140 - age) | ||
(mL/min) 72 x serum creatinine (mg/100 mL) | |||
:*Females: | :*Females: 0.85 x above value | ||
(mL/min) | |||
====Patients with Cirrhosis==== | ====Patients with [[Cirrhosis]]==== | ||
*Cefpodoxime pharmacokinetics in cirrhotic patients (with or without ascites) are similar to those in healthy subjects. Dose adjustment is not necessary in this population | *Cefpodoxime pharmacokinetics in cirrhotic patients (with or without [[ascites]]) are similar to those in healthy subjects. Dose adjustment is not necessary in this population | ||
|offLabelAdultGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Cefpodoxime proxetil in adult patients | |offLabelAdultGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Cefpodoxime proxetil in adult patients | ||
|offLabelAdultNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Cefpodoxime proxetil in adult patients. | |offLabelAdultNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Cefpodoxime proxetil in adult patients. | ||
| Line 54: | Line 47: | ||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications=Cefpodoxime proxetil is contraindicated in patients with a known allergy to cefpodoxime or to the cephalosporin group of antibiotics. | |contraindications=* Cefpodoxime proxetil is contraindicated in patients with a known allergy to cefpodoxime or to the cephalosporin group of antibiotics. | ||
|warnings= | |warnings=* BEFORE THERAPY WITH CEFPODOXIME PROXETIL IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS [[HYPERSENSITIVITY REACTIONS]] TO CEFPODOXIME, OTHER [[CEPHALOSPORINS]], [[PENICILLINS]], OR OTHER DRUGS. IF CEFPODOXIME IS TO BE ADMINISTERED TO PENICILLIN SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS HYPERSENSITIVITY AMONG [[BETA-LACTAM ANTIBIOTICS]] HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY. IF AN ALLERGIC REACTION TO CEFPODOXIME PROXETIL OCCURS, DISCONTINUE THE DRUG. SERIOUS ACUTE [[HYPERSENSITIVITY REACTIONS]] MAY REQUIRE TREATMENT WITH [[EPINEPHRINE]] AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, INTRAVENOUS FLUIDS, INTRAVENOUS ANTIHISTAMINE, AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED. | ||
* [[Clostridium difficile associated diarrhea]] (CDAD) has been reported with use of nearly all antibacterial agents, including cefpodoxime proxetil, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile. | |||
* In patients with transient or persistent reduction in urinary output due to renal insufficiency, the total daily dose of cefpodoxime proxetil should be reduced because high and prolonged serum antibiotic concentrations can occur in such individuals following usual doses. Cefpodoxime, like other cephalosporins, should be administered with caution to patients receiving concurrent treatment with potent diuretics. | * [[C. difficile]] produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents. | ||
* If CDAD is suspected or confirmed, ongoing antibiotic use not directed against [[C. difficile]] may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated. | |||
* A concerted effort to monitor for [[C. difficile]] in cefpodoxime-treated patients with diarrhea was undertaken because of an increased incidence of diarrhea associated with C. difficile in early trials in normal subjects. C. difficile organisms or toxin was reported in 10% of the cefpodoxime-treated adult patients with diarrhea; however, no specific diagnosis of pseudomembranous colitis was made in these patients. | |||
* In post-marketing experience outside the United States, reports of [[pseudomembranous colitis]] associated with the use of cefpodoxime proxetil have been received. | |||
====Precautions==== | |||
* In patients with transient or persistent reduction in urinary output due to [[renal insufficiency]], the total daily dose of cefpodoxime proxetil should be reduced because high and prolonged serum antibiotic concentrations can occur in such individuals following usual doses. Cefpodoxime, like other cephalosporins, should be administered with caution to patients receiving concurrent treatment with potent diuretics. | |||
*As with other antibiotics, prolonged use of cefpodoxime proxetil may result in overgrowth of non-susceptible organisms. Repeated evaluation of the patient’s condition is essential. If superinfection occurs during therapy, appropriate measures should be taken. | *As with other antibiotics, prolonged use of cefpodoxime proxetil may result in overgrowth of non-susceptible organisms. Repeated evaluation of the patient’s condition is essential. If superinfection occurs during therapy, appropriate measures should be taken. | ||
*Prescribing cefpodoxime proxetil in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria. | *Prescribing cefpodoxime proxetil in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria. | ||
| Line 65: | Line 70: | ||
<!--Clinical Trials Experience--> | <!--Clinical Trials Experience--> | ||
|clinicalTrials======Body as a Whole===== | |clinicalTrials======Body as a Whole===== | ||
*Diarrhea | *[[Diarrhea]] | ||
*Nausea | *[[Nausea]] | ||
*Vaginal Fungal Infections | *[[Vaginal Fungal Infections]] | ||
*Vulvovaginal Infections | *[[Vulvovaginal Infections]] | ||
*Abdominal Pain | *[[Abdominal Pain]] | ||
*Headache | *[[Headache]] | ||
=====Cardiovascular===== | =====Cardiovascular===== | ||
*congestive heart failure | *[[congestive heart failure]] | ||
* migraine | *[[migraine]] | ||
* palpitations | *[[palpitations]] | ||
* vasodilation | *[[vasodilation]] | ||
* hematoma | *[[hematoma]] | ||
* hypertension | *[[hypertension]] | ||
* hypotension | *[[hypotension]] | ||
=====Digestive===== | =====Digestive===== | ||
*vomiting | *[[vomiting]] | ||
* dyspepsia | *[[dyspepsia]] | ||
* dry mouth | *[[dry mouth]] | ||
* flatulence | *[[flatulence]] | ||
*decreased appetite | *[[decreased appetite]] | ||
* constipation | *[[constipation]] | ||
* oral moniliasis | *[[oral moniliasis]] | ||
*anorexia | *[[anorexia]] | ||
* eructation | *[[eructation]] | ||
*gastritis | *[[gastritis]] | ||
* mouth ulcer | *[[mouth ulcer]] | ||
* gastrointestinal disorders | *[[gastrointestinal disorders]] | ||
*rectal disorders | *[[rectal disorders]] | ||
* tongue disorders | *[[tongue disorders]] | ||
*tooth disorders | *[[tooth disorders]] | ||
*increased thirst | *increased thirst | ||
* oral lesions | *[[oral lesions]] | ||
* tenesmus | *[[tenesmus]] | ||
* dry throat | *[[dry throat]] | ||
* toothache | *[[toothache]] | ||
=====Hematologic and Lymphatic===== | =====Hematologic and Lymphatic===== | ||
*anemia | *[[anemia]] | ||
*Thrombocythemia | *[[Thrombocythemia]] | ||
* positive direct Coombs’ test | *positive [[direct Coombs’ test]] | ||
* eosinophilia | *[[eosinophilia]] | ||
* leukocytosis | *[[leukocytosis]] | ||
* leukopenia | *[[leukopenia]] | ||
* prolonged partial thromboplastin time | * prolonged [[partial thromboplastin time]] | ||
* thrombocytopenic purpura | *[[thrombocytopenic purpura]] | ||
=====Metabolic and Nutritional===== | =====Metabolic and Nutritional===== | ||
*dehydration | *[[dehydration]] | ||
* gout | *[[gout]] | ||
* peripheral edema | *[[peripheral edema]] | ||
* weight increase | *[[weight increase]] | ||
* Increased SGPT | * Increased [[SGPT]] | ||
=====Musculoskeletal===== | =====Musculoskeletal===== | ||
*myalgia | *[[myalgia]] | ||
=====Neurologic===== | =====Neurologic===== | ||
*dizziness | *[[dizziness]] | ||
* insomnia | *[[insomnia]] | ||
* somnolence | *[[somnolence]] | ||
* anxiety | *[[anxiety]] | ||
*shakiness | *shakiness | ||
* nervousness | *[[nervousness]] | ||
* cerebral infarction | *[[cerebral infarction]] | ||
*change in dreams | *change in dreams | ||
*impaired concentration | *[[impaired concentration]] | ||
*confusion | *[[confusion]] | ||
*nightmares | *[[nightmares]] | ||
*paresthesia | *[[paresthesia]] | ||
*vertigo | *[[vertigo]] | ||
*Hallucination | *[[Hallucination]] | ||
*hyperkinesia | *[[hyperkinesia]] | ||
=====Respiratory===== | =====Respiratory===== | ||
*asthma | *[[asthma]] | ||
*cough | *[[cough]] | ||
*epistaxis | *[[epistaxis]] | ||
*rhinitis | *[[rhinitis]] | ||
*wheezing | *[[wheezing]] | ||
*bronchitis | *[[bronchitis]] | ||
*dyspnea | *[[dyspnea]] | ||
*pleural effusion | *[[pleural effusion]] | ||
*pneumonia | *[[pneumonia]] | ||
*sinusitis | *[[sinusitis]] | ||
=====Skin and Hypersensitivy Reactions===== | =====Skin and Hypersensitivy Reactions===== | ||
*urticaria | *[[urticaria]] | ||
*rash | *[[rash]] | ||
*pruritus | *[[pruritus]] | ||
*diaphoresis | *[[diaphoresis]] | ||
*maculopapular rash | *[[maculopapular rash]] | ||
*fungal dermatitis | *[[fungal dermatitis]] | ||
*desquamation | *desquamation | ||
*dry skin non-application site | *dry skin non-application site | ||
* hair loss | *[[hair loss]] | ||
*vesiculobullous rash | *[[vesiculobullous rash]] | ||
*sunburn | *[[sunburn]] | ||
=====Special Senses===== | =====Special Senses===== | ||
*taste alterations | *[[taste alterations]] | ||
*eye irritation | *[[eye irritation]] | ||
*taste loss | *taste loss | ||
*tinnitus | *[[tinnitus]] | ||
=====Urogenital===== | =====Urogenital===== | ||
*hematuria | *[[hematuria]] | ||
*urinary tract infections | *[[urinary tract infections]] | ||
*metrorrhagia | *[[metrorrhagia]] | ||
*dysuria | *[[dysuria]] | ||
*urinary frequency | *urinary frequency | ||
*nocturia | *[[nocturia]] | ||
*penile infection | *[[penile infection]] | ||
*proteinuria | *[[proteinuria]] | ||
*vaginal pain | *[[vaginal pain]] | ||
=====Miscellaneous===== | =====Miscellaneous===== | ||
*fungal infections | *[[fungal infections]] | ||
*abdominal distention | *[[abdominal distention]] | ||
*malaise | *[[malaise]] | ||
*asthenia | *[[fatigue]] | ||
*fever | *[[asthenia]] | ||
*chest pain | *[[fever]] | ||
*back pain | *[[chest pain]] | ||
*chills | *[[back pain]] | ||
*generalized pain | *[[chills]] | ||
*[[generalized pain]] | |||
*abnormal microbiological test | *abnormal microbiological test | ||
* moniliasis | *[[moniliasis]] | ||
*abscess | *[[abscess]] | ||
*allergic reaction | *[[allergic reaction]] | ||
*facial edema | *[[facial edema]] | ||
*bacterial infections | *[[bacterial infections]] | ||
*parasitic infections | *parasitic infections | ||
*localized edema | *localized edema | ||
*localized pain | *localized pain | ||
====Laboratory Changes==== | ====Laboratory Changes==== | ||
*Hepatic: Transient increases in AST (SGOT), ALT (SGPT), GGT, alkaline phosphatase, bilirubin, and LDH. | *Hepatic: Transient increases in [[AST]] (SGOT), [[ALT]] (SGPT), [[GGT]], [[alkaline phosphatase]], [[bilirubin]], and [[LDH]]. | ||
*Hematologic: Eosinophilia, leukocytosis, lymphocytosis, granulocytosis, basophilia, monocytosis, thrombocytosis, decreased hemoglobin, decreased hematocrit, leukopenia, neutropenia, lymphocytopenia, thrombocytopenia, thrombocythemia, positive Coombs’ test, and prolonged PT, and PTT. | *Hematologic: [[Eosinophilia]], [[leukocytosis]], [[lymphocytosis]], [[granulocytosis]], [[basophilia]], [[monocytosis]], [[thrombocytosis]], decreased [[hemoglobin]], decreased [[hematocrit]], [[leukopenia]], [[neutropenia]], [[lymphocytopenia]], [[thrombocytopenia]], [[thrombocythemia]], positive [[Coombs’ test]], and prolonged [[PT]], and [[PTT]]. | ||
*Serum Chemistry: Hyperglycemia, hypoglycemia, hypoalbuminemia, hypoproteinemia, hyperkalemia, and hyponatremia. | *Serum Chemistry: [[Hyperglycemia]], [[hypoglycemia]], [[hypoalbuminemia]], [[hypoproteinemia]], [[hyperkalemia]], and [[hyponatremia]]. | ||
*Renal: Increases in BUN and creatinine. | *Renal: Increases in BUN and [[creatinine]]. | ||
====Cephalosporin Class Labeling==== | ====Cephalosporin Class Labeling==== | ||
*In addition to the adverse reactions listed above which have been observed in patients treated with cefpodoxime proxetil, the following adverse reactions and altered laboratory tests have been reported for cephalosporin class antibiotics. | *In addition to the adverse reactions listed above which have been observed in patients treated with cefpodoxime proxetil, the following adverse reactions and altered laboratory tests have been reported for cephalosporin class antibiotics. | ||
*Adverse Reactions and Abnormal Laboratory Tests: Renal dysfunction, toxic nephropathy, hepatic dysfunction including cholestasis, aplastic anemia, hemolytic anemia, serum sickness-like reaction, hemorrhage, agranulocytosis, pancytopenia and seizures. | *Adverse Reactions and Abnormal Laboratory Tests: [[Renal dysfunction]], toxic [[nephropathy]], [[hepatic dysfunction]] including [[cholestasis]], [[aplastic anemia]], [[hemolytic anemia]], [[serum sickness]]-like reaction, [[hemorrhage]], [[agranulocytosis]], [[pancytopenia]] and [[seizures]]. | ||
|postmarketing=*The following serious adverse experiences have been reported: allergic reactions including Stevens-Johnson syndrome, toxic epidermal necrolysis, erythema multiforme and serum sickness-like reactions, pseudomembranous colitis, bloody diarrhea with abdominal pain, ulcerative colitis, rectorrhagia with hypotension, anaphylactic shock, acute liver injury, in utero exposure with miscarriage, purpuric nephritis, pulmonary infiltrate with eosinophilia, and eyelid dermatitis. | |postmarketing=*The following serious adverse experiences have been reported: allergic reactions including [[Stevens-Johnson syndrome]], [[toxic epidermal necrolysis]], [[erythema multiforme]] and [[serum sickness-like reactions]], [[pseudomembranous colitis]], bloody diarrhea with abdominal pain,[[ ulcerative colitis]], [[rectorrhagia]] with [[hypotension]], [[anaphylactic shock]], acute [[liver injury]], in utero exposure with miscarriage, [[purpuric nephritis]], pulmonary infiltrate with eosinophilia, and [[eyelid dermatitis]]. | ||
*One death was attributed to pseudomembranous colitis and disseminated intravascular coagulation. | *One death was attributed to pseudomembranous colitis and [[disseminated intravascular coagulation]]. | ||
|drugInteractions=*Antacids: Concomitant administration of high doses of antacids (sodium bicarbonate and aluminum hydroxide) or H2 blockers reduces peak plasma levels by 24% to 42% and the extent of absorption by 27% to 32%, respectively. The rate of absorption is not altered by these concomitant medications. Oral anti-cholinergics (e.g., propantheline) delay peak plasma levels (47% increase in Tmax), but do not affect the extent of absorption (AUC). | |drugInteractions=*Antacids: Concomitant administration of high doses of antacids ([[sodium bicarbonate]] and [[aluminum hydroxide]]) or H2 blockers reduces peak plasma levels by 24% to 42% and the extent of absorption by 27% to 32%, respectively. The rate of absorption is not altered by these concomitant medications. Oral [[anti-cholinergics]] (e.g., [[propantheline]]) delay peak plasma levels (47% increase in Tmax), but do not affect the extent of absorption (AUC). | ||
*Probenecid: As with other beta-lactam antibiotics, renal excretion of cefpodoxime was inhibited by probenecid and resulted in an approximately 31% increase in AUC and 20% increase in peak cefpodoxime plasma levels. | *[[Probenecid]]: As with other [[beta-lactam antibiotics]], renal excretion of cefpodoxime was inhibited by probenecid and resulted in an approximately 31% increase in AUC and 20% increase in peak cefpodoxime plasma levels. | ||
*Nephrotoxic drugs: Although nephrotoxicity has not been noted when cefpodoxime proxetil was given alone, close monitoring of renal function is advised when cefpodoxime proxetil is administered concomitantly with compounds of known nephrotoxic potential. | *Nephrotoxic drugs: Although nephrotoxicity has not been noted when cefpodoxime proxetil was given alone, close monitoring of renal function is advised when cefpodoxime proxetil is administered concomitantly with compounds of known nephrotoxic potential. | ||
|FDAPregCat=B | |FDAPregCat=B | ||
|useInPregnancyFDA=======Teratogenic Effects====== | |useInPregnancyFDA=======Teratogenic Effects====== | ||
Cefpodoxime proxetil was neither teratogenic nor embryocidal when administered to rats during organogenesis at doses up to 100 mg/kg/day (2 times the human dose based on mg/m2) or to rabbits at doses up to 30 mg/kg/day (1 to 2 times the human dose based on mg/m2). | * Cefpodoxime proxetil was neither teratogenic nor embryocidal when administered to rats during organogenesis at doses up to 100 mg/kg/day (2 times the human dose based on mg/m2) or to rabbits at doses up to 30 mg/kg/day (1 to 2 times the human dose based on mg/m2). | ||
There are, however, no adequate and well-controlled studies of cefpodoxime proxetil use in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. | There are, however, no adequate and well-controlled studies of cefpodoxime proxetil use in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. | ||
|useInPregnancyAUS=There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Cefpodoxime proxetil in women who are pregnant. | |useInPregnancyAUS=There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Cefpodoxime proxetil in women who are pregnant. | ||
|useInLaborDelivery=Cefpodoxime proxetil has not been studied for use during labor and delivery. Treatment should only be given if clearly needed | |useInLaborDelivery=Cefpodoxime proxetil has not been studied for use during labor and delivery. Treatment should only be given if clearly needed | ||
|useInNursing=Cefpodoxime is excreted in human milk. In a study of 3 lactating women, levels of cefpodoxime in human milk were 0%, 2% and 6% of concomitant serum levels at 4 hours following a 200 mg oral dose of cefpodoxime proxetil. At 6 hours post-dosing, levels were 0%, 9% and 16% of concomitant serum levels. Because of the potential for serious reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. | |useInNursing=* Cefpodoxime is excreted in human milk. In a study of 3 lactating women, levels of cefpodoxime in human milk were 0%, 2% and 6% of concomitant serum levels at 4 hours following a 200 mg oral dose of cefpodoxime proxetil. At 6 hours post-dosing, levels were 0%, 9% and 16% of concomitant serum levels. Because of the potential for serious reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. | ||
|useInPed=Safety and efficacy in infants less than 2 months of age have not been established. | |useInPed=Safety and efficacy in infants less than 2 months of age have not been established. | ||
|useInGeri=Of the 3338 patients in multiple-dose clinical studies of cefpodoxime proxetil film-coated tablets, 521 (16%) were 65 and over, while 214 (6%) were 75 and over. No overall differences in effectiveness or safety were observed between the elderly and younger patients. In healthy geriatric subjects with normal renal function, cefpodoxime half-life in plasma averaged 4.2 hours and urinary recovery averaged 21% after a 400 mg dose was given every 12 hours for 15 days. Other pharmacokinetic parameters were unchanged relative to those observed in healthy younger subjects. | |useInGeri=* Of the 3338 patients in multiple-dose clinical studies of cefpodoxime proxetil film-coated tablets, 521 (16%) were 65 and over, while 214 (6%) were 75 and over. No overall differences in effectiveness or safety were observed between the elderly and younger patients. In healthy geriatric subjects with normal renal function, cefpodoxime half-life in plasma averaged 4.2 hours and urinary recovery averaged 21% after a 400 mg dose was given every 12 hours for 15 days. Other pharmacokinetic parameters were unchanged relative to those observed in healthy younger subjects. | ||
Dose adjustment in elderly patients with normal renal function is not necessary. | Dose adjustment in elderly patients with normal renal function is not necessary. | ||
|useInGender=There is no FDA guidance on the use of Cefpodoxime proxetil with respect to specific gender populations. | |useInGender=There is no FDA guidance on the use of Cefpodoxime proxetil with respect to specific gender populations. | ||
|useInRace=There is no FDA guidance on the use of Cefpodoxime proxetil with respect to specific racial populations. | |useInRace=There is no FDA guidance on the use of Cefpodoxime proxetil with respect to specific racial populations. | ||
|useInRenalImpair=In patients with transient or persistent reduction in urinary output due to renal insufficiency, the total daily dose of cefpodoxime proxetil should be reduced because high and prolonged serum antibiotic concentrations can occur in such individuals following usual doses. Cefpodoxime, like other cephalosporins, should be administered with caution to patients receiving concurrent treatment with potent diuretics. | |useInRenalImpair=In patients with transient or persistent reduction in urinary output due to [[renal insufficiency]], the total daily dose of cefpodoxime proxetil should be reduced because high and prolonged serum antibiotic concentrations can occur in such individuals following usual doses. Cefpodoxime, like other [[cephalosporins]], should be administered with caution to patients receiving concurrent treatment with potent diuretics. | ||
|useInHepaticImpair=There is no FDA guidance on the use of Cefpodoxime proxetil in patients with hepatic impairment. | |useInHepaticImpair=There is no FDA guidance on the use of Cefpodoxime proxetil in patients with hepatic impairment. | ||
|useInReproPotential=There is no FDA guidance on the use of Cefpodoxime proxetil in women of reproductive potentials and males. | |useInReproPotential=There is no FDA guidance on the use of Cefpodoxime proxetil in women of reproductive potentials and males. | ||
| Line 223: | Line 229: | ||
|administration=* Oral Tablets : administer tablets orally with food to enhance absorption | |administration=* Oral Tablets : administer tablets orally with food to enhance absorption | ||
* Oral Suspension : give without regard to food | * Oral Suspension : give without regard to food | ||
|monitoring=There is limited information regarding < | |monitoring=*Monitor for [[C. difficile]] in cefpodoxime-treated patients with diarrhea because of an increased incidence of diarrhea associated with C. difficile in early trials in normal subjects. C. difficile organisms or toxin was reported in 10% of the cefpodoxime-treated adult patients with diarrhea; however, no specific diagnosis of pseudomembranous colitis was made in these patients. | ||
*Close monitoring of renal function is advised when cefpodoxime proxetil is administered concomitantly with compounds of known nephrotoxic potential | |||
|IVCompat=There is limited information regarding IV Compatibility of Cefpodoxime Proxetil in the drug label. | |||
|overdose=*In acute rodent toxicity studies, a single 5 g/kg oral dose produced no adverse effects. | |||
*In the event of serious toxic reaction from overdosage, [[hemodialysis]] or peritoneal dialysis may aid in the removal of cefpodoxime from the body, particularly if renal function is compromised. | |||
*The toxic symptoms following an overdose of beta-lactam antibiotics may include [[nausea]], [[vomiting]], [[epigastric distress]], and [[diarrhea]]. | |||
|drugBox={{Drugbox2 | |||
| Verifiedfields = changed | |||
| verifiedrevid = 414053518 | |||
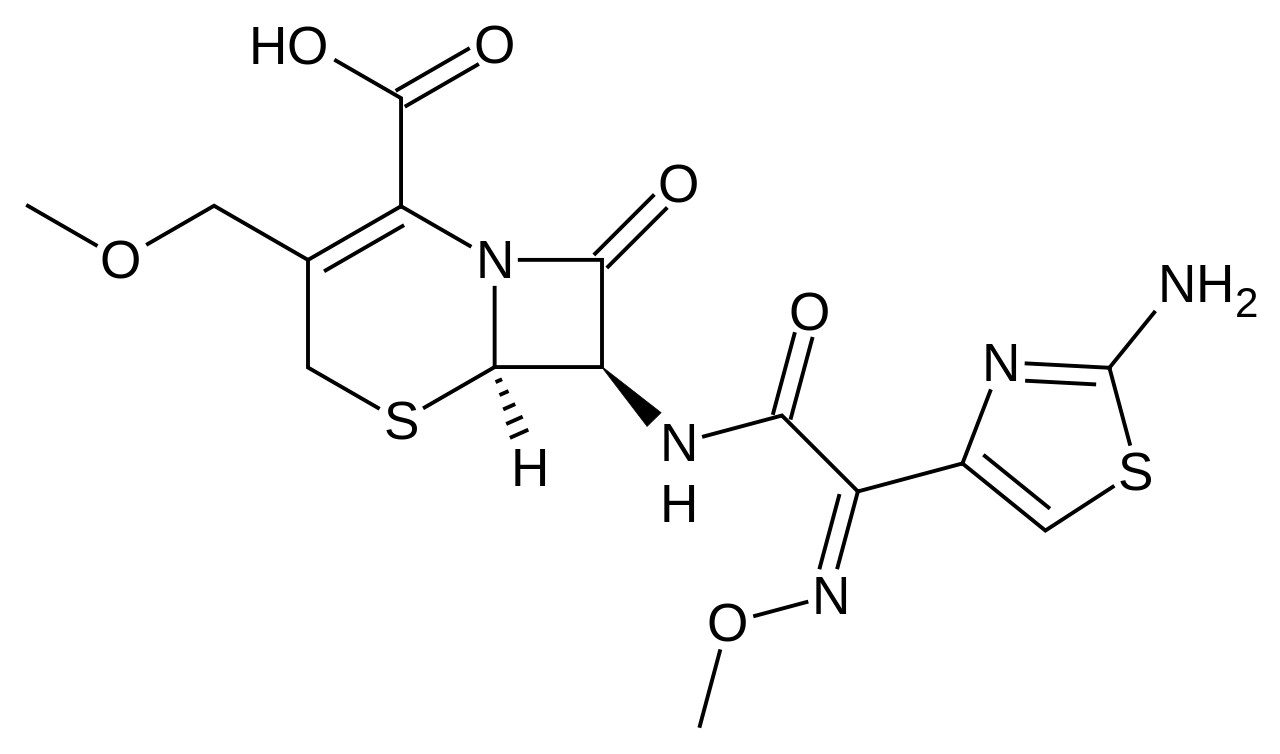
| IUPAC_name = (6''R'',7''R'')-7-{[(2''Z'')-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyimino-acetyl]amino}-3-(methoxymethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid | |||
| image = Cefpodoxime structure.png | |||
| width = 200 | |||
<!--Clinical data--> | |||
| tradename = Generic (formerly ''Vantin'') | |||
| Drugs.com = {{drugs.com|monograph|vantin}} | |||
| MedlinePlus = a698024 | |||
| pregnancy_AU = B1 | |||
| pregnancy_US = B | |||
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S8 --> | |||
| legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> | |||
| legal_US = Rx-only | |||
| routes_of_administration = Oral | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = 50% | |||
| protein_bound = 21% to 29% | |||
| metabolism = Negligible. Cefpodoxime proxetil is metabolized to cefpodoxime by the [[liver]] | |||
| elimination_half-life = 2 hours | |||
| excretion = [[Kidney|Renal]], unchanged | |||
<!-- | <!--Identifiers--> | ||
| | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | | CAS_number_Ref = {{cascite|correct|??}} | ||
| CAS_number = 82619-04-3 | |||
| ATC_prefix = J01 | |||
| ATC_suffix = DD13 | |||
| PubChem = 6335986 | |||
| DrugBank_Ref = {{drugbankcite|changed|drugbank}} | |||
| DrugBank = DB01416 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 4891496 | |||
| UNII_Ref = {{fdacite|changed|FDA}} | |||
| UNII = 7R4F94TVGY | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D07650 | |||
| ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| ChEBI = 3504 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 1672 | |||
<!--Chemical data--> | |||
| C=15 | H=17 | N=5 | O=6 | S=2 | |||
| molecular_weight = 427.458 g/mol | |||
| smiles = O=C2N1/C(=C(\CS[C@@H]1[C@@H]2NC(=O)C(=N\OC)/c3nc(sc3)N)COC)C(=O)O | |||
| InChI = 1/C15H17N5O6S2/c1-25-3-6-4-27-13-9(12(22)20(13)10(6)14(23)24)18-11(21)8(19-26-2)7-5-28-15(16)17-7/h5,9,13H,3-4H2,1-2H3,(H2,16,17)(H,18,21)(H,23,24)/b19-8-/t9-,13-/m1/s1 | |||
| InChIKey = WYUSVOMTXWRGEK-HBWVYFAYBO | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C15H17N5O6S2/c1-25-3-6-4-27-13-9(12(22)20(13)10(6)14(23)24)18-11(21)8(19-26-2)7-5-28-15(16)17-7/h5,9,13H,3-4H2,1-2H3,(H2,16,17)(H,18,21)(H,23,24)/b19-8-/t9-,13-/m1/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = WYUSVOMTXWRGEK-HBWVYFAYSA-N | |||
}} | |||
|mechAction=Cefpodoxime is a bactericidal agent that acts by inhibition of bacterial cell wall synthesis. Cefpodoxime has activity in the presence of some beta-lactamases, both penicillinases and cephalosporinases, of Gram-negative and Gram-positive bacteria. | |mechAction=Cefpodoxime is a bactericidal agent that acts by inhibition of bacterial cell wall synthesis. Cefpodoxime has activity in the presence of some beta-lactamases, both penicillinases and cephalosporinases, of Gram-negative and Gram-positive bacteria. | ||
|structure=*The chemical name is (RS)-1(isopropoxycarbonyloxy) ethyl (+)-(6R,7R)-7-[2-(2-amino-4-thiazolyl)-2-{(Z)methoxyimino}acetamido]-3-methoxymethyl-8-oxo-5-thia-1-azabicyclo [4.2.0]oct-2-ene- 2-carboxylate. | |structure=*The chemical name is (RS)-1(isopropoxycarbonyloxy) ethyl (+)-(6R,7R)-7-[2-(2-amino-4-thiazolyl)-2-{(Z)methoxyimino}acetamido]-3-methoxymethyl-8-oxo-5-thia-1-azabicyclo [4.2.0]oct-2-ene- 2-carboxylate. | ||
| Line 240: | Line 300: | ||
|PD=There is limited information regarding Pharmacodynamics of Cefpodoxime Proxetil in the drug label. | |PD=There is limited information regarding Pharmacodynamics of Cefpodoxime Proxetil in the drug label. | ||
|PK=====Absorption and Excretion==== | |PK=====Absorption and Excretion==== | ||
Cefpodoxime proxetil is a prodrug that is absorbed from the gastrointestinal tract and de-esterified to its active metabolite, cefpodoxime. Following oral administration of 100 mg of cefpodoxime proxetil to fasting subjects, approximately 50% of the administered cefpodoxime dose was absorbed systemically. Over the recommended dosing range (100 to 400 mg), approximately 29 to 33% of the administered cefpodoxime dose was excreted unchanged in the urine in 12 hours. There is minimal metabolism of cefpodoxime in vivo. | * Cefpodoxime proxetil is a prodrug that is absorbed from the gastrointestinal tract and de-esterified to its active metabolite, cefpodoxime. Following oral administration of 100 mg of cefpodoxime proxetil to fasting subjects, approximately 50% of the administered cefpodoxime dose was absorbed systemically. Over the recommended dosing range (100 to 400 mg), approximately 29 to 33% of the administered cefpodoxime dose was excreted unchanged in the urine in 12 hours. There is minimal metabolism of cefpodoxime in vivo. | ||
====Effects of Food==== | ====Effects of Food==== | ||
*The extent of absorption (mean AUC) and the mean peak plasma concentration increased when film-coated tablets were administered with food. Following a 200 mg tablet dose taken with food, the AUC was 21 to 33% higher than under fasting conditions, and the peak plasma concentration averaged 3.1 mcg/mL in fed subjects versus 2.6 mcg/mL in fasted subjects. Time to peak concentration was not significantly different between fed and fasted subjects. | *The extent of absorption (mean AUC) and the mean peak plasma concentration increased when film-coated tablets were administered with food. Following a 200 mg tablet dose taken with food, the AUC was 21 to 33% higher than under fasting conditions, and the peak plasma concentration averaged 3.1 mcg/mL in fed subjects versus 2.6 mcg/mL in fasted subjects. Time to peak concentration was not significantly different between fed and fasted subjects. | ||
*When a 200 mg dose of the suspension was taken with food, the extent of absorption (mean AUC) and mean peak plasma concentration in fed subjects were not significantly different from fasted subjects, but the rate of absorption was slower with food 48% increase in Tmax ). | *When a 200 mg dose of the suspension was taken with food, the extent of absorption (mean AUC) and mean peak plasma concentration in fed subjects were not significantly different from fasted subjects, but the rate of absorption was slower with food 48% increase in Tmax ). | ||
====Pharmacokinetics of Cefpodoxime Proxetil Film-coated Tablets==== | ====Pharmacokinetics of Cefpodoxime Proxetil Film-coated Tablets==== | ||
Over the recommended dosing range, (100 to 400 mg), the rate and extent of cefpodoxime absorption exhibited dose-dependency; dose-normalized Cmax and AUC decreased by up to 32% with increasing dose. Over the recommended dosing range, the Tmax was approximately 2 to 3 hours and the T1/2 ranged from 2.09 to 2.84 hours. Mean Cmax was 1.4 mcg/mL for the 100 mg dose, 2.3 mcg/mL for the 200 mg dose, and 3.9 mcg/mL for the 400 mg dose. In patients with normal renal function, neither accumulation nor significant changes in other pharmacokinetic parameters were noted following multiple oral doses of up to 400 mg Q 12 hours. | * Over the recommended dosing range, (100 to 400 mg), the rate and extent of cefpodoxime absorption exhibited dose-dependency; dose-normalized Cmax and AUC decreased by up to 32% with increasing dose. Over the recommended dosing range, the Tmax was approximately 2 to 3 hours and the T1/2 ranged from 2.09 to 2.84 hours. Mean Cmax was 1.4 mcg/mL for the 100 mg dose, 2.3 mcg/mL for the 200 mg dose, and 3.9 mcg/mL for the 400 mg dose. In patients with normal renal function, neither accumulation nor significant changes in other pharmacokinetic parameters were noted following multiple oral doses of up to 400 mg Q 12 hours. | ||
[[File:Cefpodoxime Tablets PK.png|none|600px]] | [[File:Cefpodoxime Tablets PK.png|none|600px]] | ||
====Pharmacokinetics of Cefpodoxime Proxetil Suspension==== | ====Pharmacokinetics of Cefpodoxime Proxetil Suspension==== | ||
| Line 252: | Line 312: | ||
[[File:Cefpodoxime Susp PK.png|none|600px]] | [[File:Cefpodoxime Susp PK.png|none|600px]] | ||
====Distribution==== | ====Distribution==== | ||
Protein binding of cefpodoxime ranges from 22 to 33% in serum and from 21 to 29% in plasma. | * Protein binding of cefpodoxime ranges from 22 to 33% in serum and from 21 to 29% in plasma. | ||
=====Skin Blister===== | =====Skin Blister===== | ||
Following multiple-dose administration every 12 hours for 5 days of 200 mg or 400 mg cefpodoxime proxetil, the mean maximum cefpodoxime concentration in skin blister fluid averaged 1.6 and 2.8 mcg/mL, respectively. Skin blister fluid cefpodoxime levels at 12 hours after dosing averaged 0.2 and 0.4 mcg/mL for the 200 mg and 400 mg multiple-dose regimens, respectively. | * Following multiple-dose administration every 12 hours for 5 days of 200 mg or 400 mg cefpodoxime proxetil, the mean maximum cefpodoxime concentration in skin blister fluid averaged 1.6 and 2.8 mcg/mL, respectively. Skin blister fluid cefpodoxime levels at 12 hours after dosing averaged 0.2 and 0.4 mcg/mL for the 200 mg and 400 mg multiple-dose regimens, respectively. | ||
=====Tonsil Tissue===== | =====Tonsil Tissue===== | ||
Following a single, oral 100 mg cefpodoxime proxetil film-coated tablet, the mean maximum cefpodoxime concentration in tonsil tissue averaged 0.24 mcg/g at 4 hours post-dosing and 0.09 mcg/g at 7 hours post-dosing. Equilibrium was achieved between plasma and tonsil tissue within 4 hours of dosing. No detection of cefpodoxime in tonsillar tissue was reported 12 hours after dosing. These results demonstrated that concentrations of cefpodoxime exceeded the MIC90 of S. pyogenes for at least 7 hours after dosing of 100 mg of cefpodoxime proxetil. | * Following a single, oral 100 mg cefpodoxime proxetil film-coated tablet, the mean maximum cefpodoxime concentration in tonsil tissue averaged 0.24 mcg/g at 4 hours post-dosing and 0.09 mcg/g at 7 hours post-dosing. Equilibrium was achieved between plasma and tonsil tissue within 4 hours of dosing. No detection of cefpodoxime in tonsillar tissue was reported 12 hours after dosing. These results demonstrated that concentrations of cefpodoxime exceeded the MIC90 of S. pyogenes for at least 7 hours after dosing of 100 mg of cefpodoxime proxetil. | ||
=====Lung Tissue===== | =====Lung Tissue===== | ||
Following a single, oral 200 mg cefpodoxime proxetil film-coated tablet, the mean maximum cefpodoxime concentration in lung tissue averaged 0.63 mcg/g at 3 hours post-dosing, 0.52 mcg/g at 6 hours post-dosing, and 0.19 mcg/g at 12 hours post-dosing. The results of this study indicated that cefpodoxime penetrated into lung tissue and produced sustained drug concentrations for at least 12 hours after dosing at levels that exceeded the MIC90 for S. pneumoniae and H. influenzae. | * Following a single, oral 200 mg cefpodoxime proxetil film-coated tablet, the mean maximum cefpodoxime concentration in lung tissue averaged 0.63 mcg/g at 3 hours post-dosing, 0.52 mcg/g at 6 hours post-dosing, and 0.19 mcg/g at 12 hours post-dosing. The results of this study indicated that cefpodoxime penetrated into lung tissue and produced sustained drug concentrations for at least 12 hours after dosing at levels that exceeded the MIC90 for [[S. pneumoniae]] and [[H. influenzae]]. | ||
=====CSF===== | =====CSF===== | ||
Adequate data on CSF levels of cefpodoxime are not available. | * Adequate data on CSF levels of cefpodoxime are not available. | ||
====Effects of Decreased Renal Function==== | ====Effects of Decreased Renal Function==== | ||
Elimination of cefpodoxime is reduced in patients with moderate to severe renal impairment (<50 mL/min creatinine clearance).In subjects with mild impairment of renal function (50 to 80 mL/min creatinine clearance), the average plasma half-life of cefpodoxime was 3.5 hours. In subjects with moderate (30 to 49 mL/min creatinine clearance) or severe renal impairment (5 to 29 mL/min creatinine clearance), the half-life increased to 5.9 and 9.8 hours, respectively. Approximately 23% of the administered dose was cleared from the body during a standard 3-hour hemodialysis procedure. | * Elimination of cefpodoxime is reduced in patients with moderate to severe renal impairment (<50 mL/min creatinine clearance).In subjects with mild impairment of renal function (50 to 80 mL/min creatinine clearance), the average plasma half-life of cefpodoxime was 3.5 hours. In subjects with moderate (30 to 49 mL/min creatinine clearance) or severe renal impairment (5 to 29 mL/min creatinine clearance), the half-life increased to 5.9 and 9.8 hours, respectively. Approximately 23% of the administered dose was cleared from the body during a standard 3-hour [[hemodialysis]] procedure. | ||
====Effect of Hepatic Impairment (cirrhosis)==== | ====Effect of Hepatic Impairment (cirrhosis)==== | ||
Absorption was somewhat diminished and elimination unchanged in patients with cirrhosis. The mean cefpodoxime T1/2 and renal clearance in cirrhotic patients were similar to those derived in studies of healthy subjects. Ascites did not appear to affect values in cirrhotic subjects. No dosage adjustment is recommended in this patient population. | * Absorption was somewhat diminished and elimination unchanged in patients with [[cirrhosis]]. The mean cefpodoxime T1/2 and renal clearance in cirrhotic patients were similar to those derived in studies of healthy subjects. [[Ascites]] did not appear to affect values in cirrhotic subjects. No dosage adjustment is recommended in this patient population. | ||
====Pharmacokinetics in Elderly Subjects==== | ====Pharmacokinetics in Elderly Subjects==== | ||
Elderly subjects do not require dosage adjustments unless they have diminished renal function.In healthy geriatric subjects, cefpodoxime half-life in plasma averaged 4.2 hours (vs 3.3 in younger subjects) and urinary recovery averaged 21% after a 400 mg dose was administered every 12 hours. Other pharmacokinetic parameters (Cmax , AUC, and Tmax ) were unchanged relative to those observed in healthy young subjects. | * Elderly subjects do not require dosage adjustments unless they have diminished renal function.In healthy geriatric subjects, cefpodoxime half-life in plasma averaged 4.2 hours (vs 3.3 in younger subjects) and urinary recovery averaged 21% after a 400 mg dose was administered every 12 hours. Other pharmacokinetic parameters (Cmax , AUC, and Tmax ) were unchanged relative to those observed in healthy young subjects. | ||
====Microbiology==== | ====Microbiology==== | ||
=====Mechanism of Resistance===== | =====Mechanism of Resistance===== | ||
| Line 272: | Line 332: | ||
*Cefpodoxime has been shown to be active against most isolates of the following bacteria, both in vitro and in clinical infections: | *Cefpodoxime has been shown to be active against most isolates of the following bacteria, both in vitro and in clinical infections: | ||
======Gram-positive bacteria====== | ======Gram-positive bacteria====== | ||
*Staphylococcus aureus (methicillin-susceptible strains, including those producing penicillinases) | *[[Staphylococcus aureus]] (methicillin-susceptible strains, including those producing [[penicillinases]]) | ||
*Staphylococcus saprophyticus | *[[Staphylococcus saprophyticus]] | ||
*Streptococcus pneumoniae (excluding penicillin-resistant isolates) | *[[Streptococcus pneumoniae]] (excluding penicillin-resistant isolates) | ||
*Streptococcus pyogenes | *[[Streptococcus pyogenes]] | ||
======Gram-negative bacteria====== | ======Gram-negative bacteria====== | ||
*Escherichia coli | *[[Escherichia coli]] | ||
*Klebsiella pneumonia | *[[Klebsiella pneumonia]] | ||
*Proteus mirabilis | *[[Proteus mirabilis]] | ||
*Haemophilus influenzae (including beta-lactamase producing isolates) | *[[Haemophilus influenzae]] (including beta-lactamase producing isolates) | ||
*Moraxella catarrhalis | *[[Moraxella catarrhalis]] | ||
*Neisseria gonorrhoeae (including penicillinase-producing isolates) | *[[Neisseria gonorrhoeae]] (including penicillinase-producing isolates) | ||
*The following in vitro data are available, but their clinical significance is unknown. At least 90 percent of the following microorganisms exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for cefpodoxime. However, the efficacy of cefpodoxime in treating clinical infections due to these microorganisms has not been established in adequate and well-controlled clinical trials. | *The following in vitro data are available, but their clinical significance is unknown. At least 90 percent of the following microorganisms exhibit an in vitro [[minimum inhibitory concentration]] (MIC) less than or equal to the susceptible breakpoint for cefpodoxime. However, the efficacy of cefpodoxime in treating clinical infections due to these microorganisms has not been established in adequate and well-controlled clinical trials. | ||
======Gram-positive bacteria====== | ======Gram-positive bacteria====== | ||
*Streptococcus agalactiae | *[[Streptococcus agalactiae]] | ||
*Streptococcus spp. (Groups C, F, G) | *[[Streptococcus spp. (Groups C, F, G)]] | ||
======Gram-negative bacteria====== | ======Gram-negative bacteria====== | ||
*Citrobacter diversus | *[[Citrobacter diversus]] | ||
*Klebsiella oxytoca | *[[Klebsiella oxytoca]] | ||
*Proteus vulgaris | *[[Proteus vulgaris]] | ||
*Providencia rettgeri | *[[Providencia rettgeri]] | ||
*Haemophilus parainfluenzae | *[[Haemophilus parainfluenzae]] | ||
======Anaerobic Gram-positive bacteria====== | ======Anaerobic Gram-positive bacteria====== | ||
*Peptostreptococcus magnus | *[[Peptostreptococcus magnus]] | ||
=====Susceptibility Test Methods===== | =====Susceptibility Test Methods===== | ||
When available, the clinical microbiology laboratory should provide the results of in vitro susceptibility test results for antimicrobial drug products used in resident hospitals to the physician as periodic reports that describe the susceptibility profile of nosocomial and community-acquired pathogens. These reports should aid the physician in selecting an antibacterial drug product for treatment. | * When available, the clinical microbiology laboratory should provide the results of in vitro susceptibility test results for antimicrobial drug products used in resident hospitals to the physician as periodic reports that describe the susceptibility profile of nosocomial and community-acquired pathogens. These reports should aid the physician in selecting an antibacterial drug product for treatment. | ||
=====Dilution techniques===== | =====Dilution techniques===== | ||
Quantitative methods are used to determine antimicrobial minimal inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized test method. The MIC values should be interpreted according to criteria provided in Table 1. | * Quantitative methods are used to determine antimicrobial [[minimal inhibitory concentrations]] (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized test method. The MIC values should be interpreted according to criteria provided in Table 1. | ||
=====Diffusion techniques===== | =====Diffusion techniques===== | ||
*Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. The zone size provides an estimate of the susceptibility of bacteria to antimicrobial compounds. The zone size should be determined using a standardized test method. This procedure uses paper disks impregnated with 10 mcg cefpodoxime to test the susceptibility of microorganisms to cefpodoxime. The disk diffusion interpretive criteria are provided in Table 1. | *Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. The zone size provides an estimate of the susceptibility of bacteria to antimicrobial compounds. The zone size should be determined using a standardized test method. This procedure uses paper disks impregnated with 10 mcg cefpodoxime to test the susceptibility of microorganisms to cefpodoxime. The disk diffusion interpretive criteria are provided in Table 1. | ||
| Line 304: | Line 364: | ||
*A report of Susceptible indicates that the antimicrobial is likely to inhibit growth of the pathogen if the antimicrobial compound reaches the concentration at the infection site necessary to inhibit growth of the pathogen. A report of Intermediate indicates that the result should be considered equivocal, and if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where a high dosage of drug can be used. This category also provides a buffer zone that prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of Resistant indicates that the antimicrobial is not likely to inhibit growth of the pathogen if the antimicrobial compound reaches the concentrations usually achievable at the infection site; other therapy should be selected. | *A report of Susceptible indicates that the antimicrobial is likely to inhibit growth of the pathogen if the antimicrobial compound reaches the concentration at the infection site necessary to inhibit growth of the pathogen. A report of Intermediate indicates that the result should be considered equivocal, and if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where a high dosage of drug can be used. This category also provides a buffer zone that prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of Resistant indicates that the antimicrobial is not likely to inhibit growth of the pathogen if the antimicrobial compound reaches the concentrations usually achievable at the infection site; other therapy should be selected. | ||
=====Quality Control===== | =====Quality Control===== | ||
Standardized susceptibility test procedures require the use of laboratory controls to monitor and ensure the accuracy and precision of supplies and reagents used in the assay, and the techniques of the individual performing the test. Standard Cefpodoxime powder should provide the following range of MIC values noted in Table 2. For the diffusion technique using the 10 mcg disk, the criteria in Table 2 should be achieved. | * Standardized susceptibility test procedures require the use of laboratory controls to monitor and ensure the accuracy and precision of supplies and reagents used in the assay, and the techniques of the individual performing the test. Standard Cefpodoxime powder should provide the following range of MIC values noted in Table 2. For the diffusion technique using the 10 mcg disk, the criteria in Table 2 should be achieved. | ||
[[File:Quality Control 2.png|none|600px]] | [[File:Quality Control 2.png|none|600px]] | ||
<!--Nonclinical Toxicology--> | <!--Nonclinical Toxicology--> | ||
|nonClinToxic=There is limited information regarding Nonclinical Toxicology of Cefpodoxime Proxetil in the drug label. | |nonClinToxic=There is limited information regarding Nonclinical Toxicology of Cefpodoxime Proxetil in the drug label. | ||
|clinicalStudies======Cystitis===== | |clinicalStudies======Cystitis===== | ||
*In two double-blind, 2:1 randomized, comparative trials performed in adults in the United States, cefpodoxime proxetil was compared to other beta-lactam antibiotics. In these studies, the following bacterial eradication rates were obtained at 5 to 9 days after therapy: | *In two double-blind, 2:1 randomized, comparative trials performed in adults in the United States, cefpodoxime proxetil was compared to other [[beta-lactam antibiotics]]. In these studies, the following bacterial eradication rates were obtained at 5 to 9 days after therapy: | ||
[[File:Cystitis Clinical Trials.png|none|600px]] | [[File:Cystitis Clinical Trials.png|none|600px]] | ||
*In these studies, clinical cure rates and bacterial eradication rates for cefpodoxime proxetil were comparable to the comparator agents; however, the clinical cure rates and bacteriologic eradication rates were lower than those observed with some other classes of approved agents for cystitis. | *In these studies, clinical cure rates and bacterial eradication rates for cefpodoxime proxetil were comparable to the comparator agents; however, the clinical cure rates and bacteriologic eradication rates were lower than those observed with some other classes of approved agents for [[cystitis]]. | ||
=====Acute Otitis Media Studies===== | =====Acute Otitis Media Studies===== | ||
In controlled studies of acute otitis media performed in the United States, where significant rates of beta-lactamase-producing organisms were found, cefpodoxime proxetil was compared to cefixime. In these studies, using very strict evaluability criteria and microbiologic and clinical response criteria at the 4 to 21 day post-therapy follow-up, the following presumptive bacterial eradication/clinical success outcomes (cured and improved) were obtained. | In controlled studies of acute otitis media performed in the United States, where significant rates of beta-lactamase-producing organisms were found, cefpodoxime proxetil was compared to [[cefixime]]. In these studies, using very strict evaluability criteria and microbiologic and clinical response criteria at the 4 to 21 day post-therapy follow-up, the following presumptive bacterial eradication/clinical success outcomes (cured and improved) were obtained. | ||
[[File:Acute OM studies.png|none|600px]] | [[File:Acute OM studies.png|none|600px]] | ||
|howSupplied=*Cefpodoxime Proxetil Tablets, USP 100 mg are light yellowish-orange, elliptical, film-coated tablets debossed with ‘C’ on one side and ‘61’ on the other side. | |howSupplied=*Cefpodoxime Proxetil Tablets, USP 100 mg are light yellowish-orange, elliptical, film-coated tablets debossed with ‘C’ on one side and ‘61’ on the other side. | ||
| Line 319: | Line 379: | ||
:* Bottles of 100 (NDC 65862-095-01) | :* Bottles of 100 (NDC 65862-095-01) | ||
:*Bottles of 1000 (NDC 65862-095-99) | :*Bottles of 1000 (NDC 65862-095-99) | ||
*Cefpodoxime Proxetil Tablets, USP 200 mg are coral red, elliptical, film-coated tablets debossed with ‘C’ on one side and ‘62’ on the other side. | * Cefpodoxime Proxetil Tablets, USP 200 mg are coral red, elliptical, film-coated tablets debossed with ‘C’ on one side and ‘62’ on the other side. | ||
:*Bottles of 20 (NDC 65862-096-20) | :*Bottles of 20 (NDC 65862-096-20) | ||
:*Bottles of 100 (NDC 65862-096-01) | :*Bottles of 100 (NDC 65862-096-01) | ||
| Line 326: | Line 386: | ||
*Dispense in tight, light-resistant container. | *Dispense in tight, light-resistant container. | ||
*Replace cap securely after each opening. | *Replace cap securely after each opening. | ||
|packLabel= | |packLabel=====Package Label-principal display panel - 100 MG (20 tablet bottle)==== | ||
|fdaPatientInfo= | [[File:Package Label.png|none|600px]] | ||
====Package Label-principal display panel - 200 MG (20 tablet bottle)==== | |||
[[File:Package Label2.png|none|600px]] | |||
|alcohol=* Alcohol- | ====Ingredients and Appearance==== | ||
[[File:CefpodoximeIngredients and App.png|none|600px]] | |||
[[File:CefpodoximeIngredients and App2.png|none|600px]] | |||
|fdaPatientInfo=*Patients should be counseled that antibacterial drugs including cefpodoxime proxetil should only be used to treat bacterial infections. They do not treat [[viral infections]] (e.g., the [[common cold]]). When cefpodoxime proxetil is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by cefpodoxime proxetil or other antibacterial drugs in the future. | |||
*[[Diarrhea]] is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible. | |||
|alcohol=* Alcohol-Cefpodoxime interaction has not been established. | |||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames=*Vantin | |brandNames=*Vantin <ref>{{cite web|url=http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c36f2137-98bb-4583-89f7-6bce9582c465|title=CEFPODOXIME PROXETIL - cefpodoxime proxetil tablet, film coated }}</ref> | ||
}} | }} | ||
<!--Pill Image--> | <!--Pill Image--> | ||
{{PillImage|fileName=Cefpodoxime_Proxetil_NDC_07815439.jpg|drugName=Cefpodoxime Proxetil|NDC=07815439|drugAuthor=Sandoz Inc|ingredients=CEFPODOXIME PROXETIL[CEFPODOXIME]|pillImprint=SZ;439|dosageValue=200|dosageUnit=mg|pillColor=Orange|pillShape=Oval|pillSize=16|pillScore=1}} | |||
{{PillImage|fileName=CEFPODOXIME_PROXETIL_NDC_658620095.jpg|drugName=CEFPODOXIME PROXETIL|NDC=658620095|drugAuthor=Aurobindo Pharma Limited|ingredients=CEFPODOXIME PROXETIL[CEFPODOXIME]|pillImprint=C;61|dosageValue=100|dosageUnit=mg|pillColor=Yellow|pillShape=Oval|pillSize=11|pillScore=1}} | |||
Latest revision as of 02:23, 17 September 2015
{{DrugProjectFormSinglePage |authorTag=Rabin Bista, M.B.B.S. [1] |genericName=Cefpodoxime proxetil |aOrAn=a |drugClass=orally administered, extended spectrum, semi-synthetic antibiotic of the cephalosporin class |indicationType=treatment |indication=acute otitis media,pharyngitis,tonsillitis,community-acquired pneumonia,acute bacterial exacerbation of chronic bronchitis,acute uncomplicated urethral and cervical gonorrhea,acute maxillary sinusitis,uncomplicated urinary tract infections and uncomplicated skin and skin structure infections |adverseReactions=Diarrhea,Nausea,Vaginal Fungal Infections,Vulvovaginal Infections,Abdominal pain, Headache |blackBoxWarningTitle=ConditionName: |fdaLIADAdult====Indications===
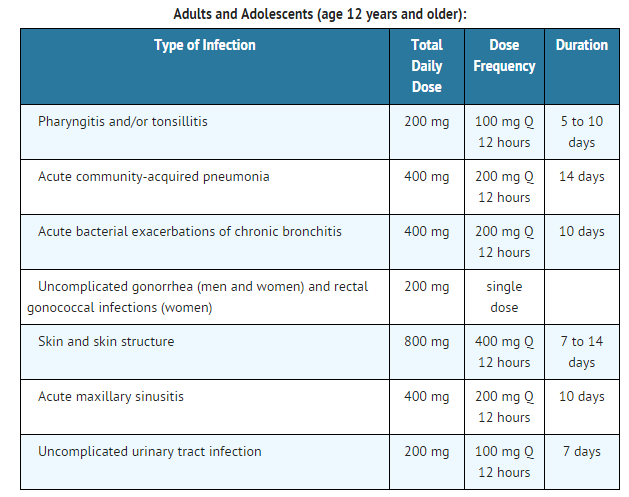
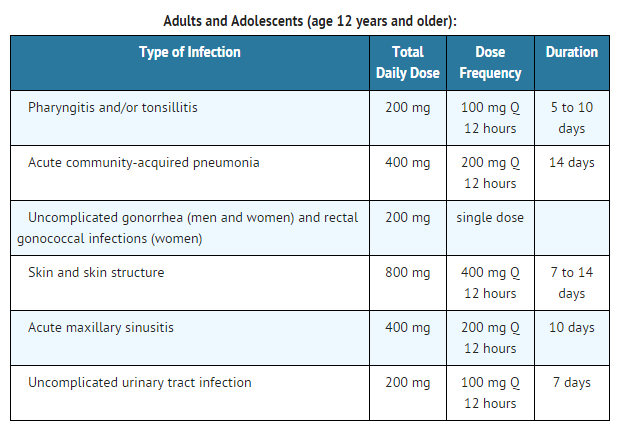
- Acute otitis media caused by Streptococcus pneumoniae (excluding penicillin-resistant strains), Streptococcus pyogenes, Haemophilus influenzae (including beta-lactamase-producing strains), or Moraxella (Branhamella) catarrhalis (including beta-lactamase-producing strains).
- Pharyngitis and/or tonsillitis caused by Streptococcus pyogenes.
- Community-acquired pneumonia caused by S. pneumoniae or H. Influenzae (including beta-lactamase-producing strains).
- Acute bacterial exacerbation of chronic bronchitis caused by S. pneumoniae, H. influenzae (non-beta-lactamase-producing strains only), or M. catarrhalis.
- Acute, uncomplicated urethral and cervical gonorrhea caused by Neisseria gonorrhoeae (including penicillinase-producing strains).
- Acute, uncomplicated ano-rectal infections in women due to Neisseria gonorrhoeae (including penicillinase-producing strains).
- Uncomplicated skin and skin structure infections caused by Staphylococcus aureus (including penicillinase-producing strains) or Streptococcus pyogenes. Abscesses should be surgically drained as clinically indicated.
- Acute maxillary sinusitis caused by Haemophilus influenzae (including beta-lactamase-producing strains), Streptococcus pneumoniae, and Moraxella catarrhalis.
- Uncomplicated urinary tract infections (cystitis) caused by Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, or Staphylococcus saprophyticus.
Dosage
Film Coated Tablets
Granules for oral suspension
Patients with Renal Dysfunction
- For patients with severe renal impairment (<30 mL/min creatinine clearance), the dosing intervals should be increased to Q 24 hours. In patients maintained on hemodialysis, the dose frequency should be 3 times/week after hemodialysis.
- When only the serum creatinine level is available, the following formula (based on sex, weight, and age of the patient) may be used to estimate creatinine clearance (mL/min). For this estimate to be valid, the serum creatinine level should represent a steady state of renal function.
- Males: Weight (kg) x (140 - age)
(mL/min) 72 x serum creatinine (mg/100 mL)
- Females: 0.85 x above value
(mL/min)
Patients with Cirrhosis
- Cefpodoxime pharmacokinetics in cirrhotic patients (with or without ascites) are similar to those in healthy subjects. Dose adjustment is not necessary in this population
|offLabelAdultGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Cefpodoxime proxetil in adult patients |offLabelAdultNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Cefpodoxime proxetil in adult patients.
|fdaLIADPed=
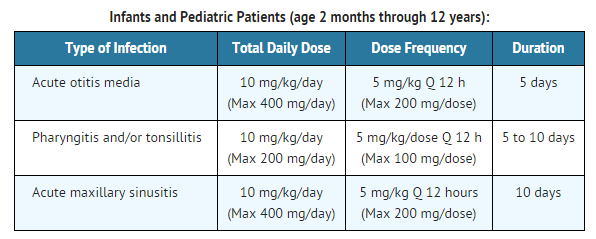
|offLabelPedGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Cefpodoxime proxetil in pediatric patients.
|offLabelPedNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Cefpodoxime proxetil in pediatric patients.
|contraindications=* Cefpodoxime proxetil is contraindicated in patients with a known allergy to cefpodoxime or to the cephalosporin group of antibiotics. |warnings=* BEFORE THERAPY WITH CEFPODOXIME PROXETIL IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFPODOXIME, OTHER CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF CEFPODOXIME IS TO BE ADMINISTERED TO PENICILLIN SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS HYPERSENSITIVITY AMONG BETA-LACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY. IF AN ALLERGIC REACTION TO CEFPODOXIME PROXETIL OCCURS, DISCONTINUE THE DRUG. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, INTRAVENOUS FLUIDS, INTRAVENOUS ANTIHISTAMINE, AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED.
- Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including cefpodoxime proxetil, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
- C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
- If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
- A concerted effort to monitor for C. difficile in cefpodoxime-treated patients with diarrhea was undertaken because of an increased incidence of diarrhea associated with C. difficile in early trials in normal subjects. C. difficile organisms or toxin was reported in 10% of the cefpodoxime-treated adult patients with diarrhea; however, no specific diagnosis of pseudomembranous colitis was made in these patients.
- In post-marketing experience outside the United States, reports of pseudomembranous colitis associated with the use of cefpodoxime proxetil have been received.
Precautions
- In patients with transient or persistent reduction in urinary output due to renal insufficiency, the total daily dose of cefpodoxime proxetil should be reduced because high and prolonged serum antibiotic concentrations can occur in such individuals following usual doses. Cefpodoxime, like other cephalosporins, should be administered with caution to patients receiving concurrent treatment with potent diuretics.
- As with other antibiotics, prolonged use of cefpodoxime proxetil may result in overgrowth of non-susceptible organisms. Repeated evaluation of the patient’s condition is essential. If superinfection occurs during therapy, appropriate measures should be taken.
- Prescribing cefpodoxime proxetil in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
|clinicalTrials======Body as a Whole=====
Cardiovascular
Digestive
- vomiting
- dyspepsia
- dry mouth
- flatulence
- decreased appetite
- constipation
- oral moniliasis
- anorexia
- eructation
- gastritis
- mouth ulcer
- gastrointestinal disorders
- rectal disorders
- tongue disorders
- tooth disorders
- increased thirst
- oral lesions
- tenesmus
- dry throat
- toothache
Hematologic and Lymphatic
- anemia
- Thrombocythemia
- positive direct Coombs’ test
- eosinophilia
- leukocytosis
- leukopenia
- prolonged partial thromboplastin time
- thrombocytopenic purpura
Metabolic and Nutritional
- dehydration
- gout
- peripheral edema
- weight increase
- Increased SGPT
Musculoskeletal
Neurologic
- dizziness
- insomnia
- somnolence
- anxiety
- shakiness
- nervousness
- cerebral infarction
- change in dreams
- impaired concentration
- confusion
- nightmares
- paresthesia
- vertigo
- Hallucination
- hyperkinesia
Respiratory
Skin and Hypersensitivy Reactions
- urticaria
- rash
- pruritus
- diaphoresis
- maculopapular rash
- fungal dermatitis
- desquamation
- dry skin non-application site
- hair loss
- vesiculobullous rash
- sunburn
Special Senses
- taste alterations
- eye irritation
- taste loss
- tinnitus
Urogenital
- hematuria
- urinary tract infections
- metrorrhagia
- dysuria
- urinary frequency
- nocturia
- penile infection
- proteinuria
- vaginal pain
Miscellaneous
- fungal infections
- abdominal distention
- malaise
- fatigue
- asthenia
- fever
- chest pain
- back pain
- chills
- generalized pain
- abnormal microbiological test
- moniliasis
- abscess
- allergic reaction
- facial edema
- bacterial infections
- parasitic infections
- localized edema
- localized pain
Laboratory Changes
- Hepatic: Transient increases in AST (SGOT), ALT (SGPT), GGT, alkaline phosphatase, bilirubin, and LDH.
- Hematologic: Eosinophilia, leukocytosis, lymphocytosis, granulocytosis, basophilia, monocytosis, thrombocytosis, decreased hemoglobin, decreased hematocrit, leukopenia, neutropenia, lymphocytopenia, thrombocytopenia, thrombocythemia, positive Coombs’ test, and prolonged PT, and PTT.
- Serum Chemistry: Hyperglycemia, hypoglycemia, hypoalbuminemia, hypoproteinemia, hyperkalemia, and hyponatremia.
- Renal: Increases in BUN and creatinine.
Cephalosporin Class Labeling
- In addition to the adverse reactions listed above which have been observed in patients treated with cefpodoxime proxetil, the following adverse reactions and altered laboratory tests have been reported for cephalosporin class antibiotics.
- Adverse Reactions and Abnormal Laboratory Tests: Renal dysfunction, toxic nephropathy, hepatic dysfunction including cholestasis, aplastic anemia, hemolytic anemia, serum sickness-like reaction, hemorrhage, agranulocytosis, pancytopenia and seizures.
|postmarketing=*The following serious adverse experiences have been reported: allergic reactions including Stevens-Johnson syndrome, toxic epidermal necrolysis, erythema multiforme and serum sickness-like reactions, pseudomembranous colitis, bloody diarrhea with abdominal pain,ulcerative colitis, rectorrhagia with hypotension, anaphylactic shock, acute liver injury, in utero exposure with miscarriage, purpuric nephritis, pulmonary infiltrate with eosinophilia, and eyelid dermatitis.
- One death was attributed to pseudomembranous colitis and disseminated intravascular coagulation.
|drugInteractions=*Antacids: Concomitant administration of high doses of antacids (sodium bicarbonate and aluminum hydroxide) or H2 blockers reduces peak plasma levels by 24% to 42% and the extent of absorption by 27% to 32%, respectively. The rate of absorption is not altered by these concomitant medications. Oral anti-cholinergics (e.g., propantheline) delay peak plasma levels (47% increase in Tmax), but do not affect the extent of absorption (AUC).
- Probenecid: As with other beta-lactam antibiotics, renal excretion of cefpodoxime was inhibited by probenecid and resulted in an approximately 31% increase in AUC and 20% increase in peak cefpodoxime plasma levels.
- Nephrotoxic drugs: Although nephrotoxicity has not been noted when cefpodoxime proxetil was given alone, close monitoring of renal function is advised when cefpodoxime proxetil is administered concomitantly with compounds of known nephrotoxic potential.
|FDAPregCat=B |useInPregnancyFDA=======Teratogenic Effects======
- Cefpodoxime proxetil was neither teratogenic nor embryocidal when administered to rats during organogenesis at doses up to 100 mg/kg/day (2 times the human dose based on mg/m2) or to rabbits at doses up to 30 mg/kg/day (1 to 2 times the human dose based on mg/m2).
There are, however, no adequate and well-controlled studies of cefpodoxime proxetil use in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. |useInPregnancyAUS=There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Cefpodoxime proxetil in women who are pregnant. |useInLaborDelivery=Cefpodoxime proxetil has not been studied for use during labor and delivery. Treatment should only be given if clearly needed |useInNursing=* Cefpodoxime is excreted in human milk. In a study of 3 lactating women, levels of cefpodoxime in human milk were 0%, 2% and 6% of concomitant serum levels at 4 hours following a 200 mg oral dose of cefpodoxime proxetil. At 6 hours post-dosing, levels were 0%, 9% and 16% of concomitant serum levels. Because of the potential for serious reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. |useInPed=Safety and efficacy in infants less than 2 months of age have not been established. |useInGeri=* Of the 3338 patients in multiple-dose clinical studies of cefpodoxime proxetil film-coated tablets, 521 (16%) were 65 and over, while 214 (6%) were 75 and over. No overall differences in effectiveness or safety were observed between the elderly and younger patients. In healthy geriatric subjects with normal renal function, cefpodoxime half-life in plasma averaged 4.2 hours and urinary recovery averaged 21% after a 400 mg dose was given every 12 hours for 15 days. Other pharmacokinetic parameters were unchanged relative to those observed in healthy younger subjects. Dose adjustment in elderly patients with normal renal function is not necessary. |useInGender=There is no FDA guidance on the use of Cefpodoxime proxetil with respect to specific gender populations. |useInRace=There is no FDA guidance on the use of Cefpodoxime proxetil with respect to specific racial populations. |useInRenalImpair=In patients with transient or persistent reduction in urinary output due to renal insufficiency, the total daily dose of cefpodoxime proxetil should be reduced because high and prolonged serum antibiotic concentrations can occur in such individuals following usual doses. Cefpodoxime, like other cephalosporins, should be administered with caution to patients receiving concurrent treatment with potent diuretics. |useInHepaticImpair=There is no FDA guidance on the use of Cefpodoxime proxetil in patients with hepatic impairment. |useInReproPotential=There is no FDA guidance on the use of Cefpodoxime proxetil in women of reproductive potentials and males. |useInImmunocomp=There is no FDA guidance one the use of Cefpodoxime proxetil in patients who are immunocompromised.
|administration=* Oral Tablets : administer tablets orally with food to enhance absorption
- Oral Suspension : give without regard to food
|monitoring=*Monitor for C. difficile in cefpodoxime-treated patients with diarrhea because of an increased incidence of diarrhea associated with C. difficile in early trials in normal subjects. C. difficile organisms or toxin was reported in 10% of the cefpodoxime-treated adult patients with diarrhea; however, no specific diagnosis of pseudomembranous colitis was made in these patients.
- Close monitoring of renal function is advised when cefpodoxime proxetil is administered concomitantly with compounds of known nephrotoxic potential
|IVCompat=There is limited information regarding IV Compatibility of Cefpodoxime Proxetil in the drug label. |overdose=*In acute rodent toxicity studies, a single 5 g/kg oral dose produced no adverse effects.
- In the event of serious toxic reaction from overdosage, hemodialysis or peritoneal dialysis may aid in the removal of cefpodoxime from the body, particularly if renal function is compromised.
- The toxic symptoms following an overdose of beta-lactam antibiotics may include nausea, vomiting, epigastric distress, and diarrhea.
|drugBox=
|mechAction=Cefpodoxime is a bactericidal agent that acts by inhibition of bacterial cell wall synthesis. Cefpodoxime has activity in the presence of some beta-lactamases, both penicillinases and cephalosporinases, of Gram-negative and Gram-positive bacteria. |structure=*The chemical name is (RS)-1(isopropoxycarbonyloxy) ethyl (+)-(6R,7R)-7-[2-(2-amino-4-thiazolyl)-2-{(Z)methoxyimino}acetamido]-3-methoxymethyl-8-oxo-5-thia-1-azabicyclo [4.2.0]oct-2-ene- 2-carboxylate.
- Its molecular formula is C21 H27 N5 O9 S2 and its structural formula is represented below:
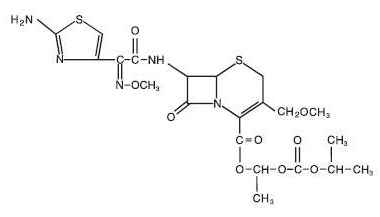
|PD=There is limited information regarding Pharmacodynamics of Cefpodoxime Proxetil in the drug label. |PK=====Absorption and Excretion====
- Cefpodoxime proxetil is a prodrug that is absorbed from the gastrointestinal tract and de-esterified to its active metabolite, cefpodoxime. Following oral administration of 100 mg of cefpodoxime proxetil to fasting subjects, approximately 50% of the administered cefpodoxime dose was absorbed systemically. Over the recommended dosing range (100 to 400 mg), approximately 29 to 33% of the administered cefpodoxime dose was excreted unchanged in the urine in 12 hours. There is minimal metabolism of cefpodoxime in vivo.
Effects of Food
- The extent of absorption (mean AUC) and the mean peak plasma concentration increased when film-coated tablets were administered with food. Following a 200 mg tablet dose taken with food, the AUC was 21 to 33% higher than under fasting conditions, and the peak plasma concentration averaged 3.1 mcg/mL in fed subjects versus 2.6 mcg/mL in fasted subjects. Time to peak concentration was not significantly different between fed and fasted subjects.
- When a 200 mg dose of the suspension was taken with food, the extent of absorption (mean AUC) and mean peak plasma concentration in fed subjects were not significantly different from fasted subjects, but the rate of absorption was slower with food 48% increase in Tmax ).
Pharmacokinetics of Cefpodoxime Proxetil Film-coated Tablets
- Over the recommended dosing range, (100 to 400 mg), the rate and extent of cefpodoxime absorption exhibited dose-dependency; dose-normalized Cmax and AUC decreased by up to 32% with increasing dose. Over the recommended dosing range, the Tmax was approximately 2 to 3 hours and the T1/2 ranged from 2.09 to 2.84 hours. Mean Cmax was 1.4 mcg/mL for the 100 mg dose, 2.3 mcg/mL for the 200 mg dose, and 3.9 mcg/mL for the 400 mg dose. In patients with normal renal function, neither accumulation nor significant changes in other pharmacokinetic parameters were noted following multiple oral doses of up to 400 mg Q 12 hours.
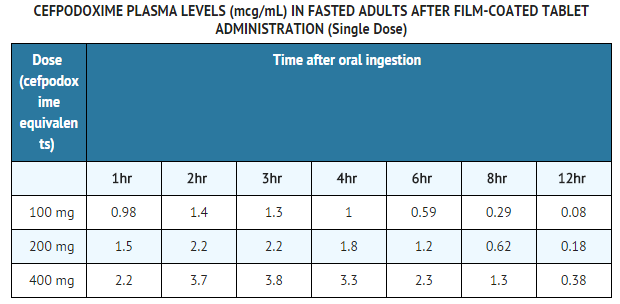
Pharmacokinetics of Cefpodoxime Proxetil Suspension
- In adult subjects, a 100 mg dose of oral suspension produced an average peak cefpodoxime concentration of approximately 1.5 mcg/mL (range: 1.1 to 2.1 mcg/mL), which is equivalent to that reported following administration of the 100 mg tablet. Time to peak plasma concentration and area under the plasma concentration-time curve (AUC) for the oral suspension were also equivalent to those produced with film-coated tablets in adults following a 100 mg oral dose.
- The pharmacokinetics of cefpodoxime were investigated in 29 patients aged 1 to 17 years. Each patient received a single, oral, 5 mg/kg dose of cefpodoxime oral suspension. Plasma and urine samples were collected for 12 hours after dosing. The plasma levels reported from this study are as follows:
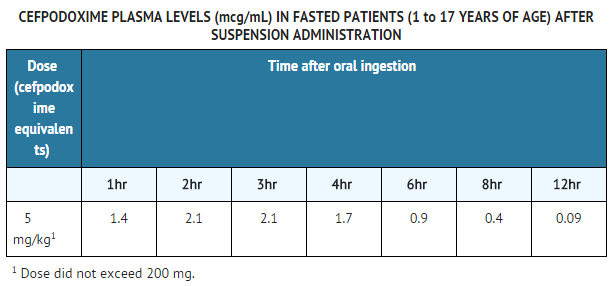
Distribution
- Protein binding of cefpodoxime ranges from 22 to 33% in serum and from 21 to 29% in plasma.
Skin Blister
- Following multiple-dose administration every 12 hours for 5 days of 200 mg or 400 mg cefpodoxime proxetil, the mean maximum cefpodoxime concentration in skin blister fluid averaged 1.6 and 2.8 mcg/mL, respectively. Skin blister fluid cefpodoxime levels at 12 hours after dosing averaged 0.2 and 0.4 mcg/mL for the 200 mg and 400 mg multiple-dose regimens, respectively.
Tonsil Tissue
- Following a single, oral 100 mg cefpodoxime proxetil film-coated tablet, the mean maximum cefpodoxime concentration in tonsil tissue averaged 0.24 mcg/g at 4 hours post-dosing and 0.09 mcg/g at 7 hours post-dosing. Equilibrium was achieved between plasma and tonsil tissue within 4 hours of dosing. No detection of cefpodoxime in tonsillar tissue was reported 12 hours after dosing. These results demonstrated that concentrations of cefpodoxime exceeded the MIC90 of S. pyogenes for at least 7 hours after dosing of 100 mg of cefpodoxime proxetil.
Lung Tissue
- Following a single, oral 200 mg cefpodoxime proxetil film-coated tablet, the mean maximum cefpodoxime concentration in lung tissue averaged 0.63 mcg/g at 3 hours post-dosing, 0.52 mcg/g at 6 hours post-dosing, and 0.19 mcg/g at 12 hours post-dosing. The results of this study indicated that cefpodoxime penetrated into lung tissue and produced sustained drug concentrations for at least 12 hours after dosing at levels that exceeded the MIC90 for S. pneumoniae and H. influenzae.
CSF
- Adequate data on CSF levels of cefpodoxime are not available.
Effects of Decreased Renal Function
- Elimination of cefpodoxime is reduced in patients with moderate to severe renal impairment (<50 mL/min creatinine clearance).In subjects with mild impairment of renal function (50 to 80 mL/min creatinine clearance), the average plasma half-life of cefpodoxime was 3.5 hours. In subjects with moderate (30 to 49 mL/min creatinine clearance) or severe renal impairment (5 to 29 mL/min creatinine clearance), the half-life increased to 5.9 and 9.8 hours, respectively. Approximately 23% of the administered dose was cleared from the body during a standard 3-hour hemodialysis procedure.
Effect of Hepatic Impairment (cirrhosis)
- Absorption was somewhat diminished and elimination unchanged in patients with cirrhosis. The mean cefpodoxime T1/2 and renal clearance in cirrhotic patients were similar to those derived in studies of healthy subjects. Ascites did not appear to affect values in cirrhotic subjects. No dosage adjustment is recommended in this patient population.
Pharmacokinetics in Elderly Subjects
- Elderly subjects do not require dosage adjustments unless they have diminished renal function.In healthy geriatric subjects, cefpodoxime half-life in plasma averaged 4.2 hours (vs 3.3 in younger subjects) and urinary recovery averaged 21% after a 400 mg dose was administered every 12 hours. Other pharmacokinetic parameters (Cmax , AUC, and Tmax ) were unchanged relative to those observed in healthy young subjects.
Microbiology
Mechanism of Resistance
- Resistance to cefpodoxime is primarily through hydrolysis by beta-lactamase, alteration of penicillin-binding proteins (PBPs), and decreased permeability.
- Cefpodoxime has been shown to be active against most isolates of the following bacteria, both in vitro and in clinical infections:
Gram-positive bacteria
- Staphylococcus aureus (methicillin-susceptible strains, including those producing penicillinases)
- Staphylococcus saprophyticus
- Streptococcus pneumoniae (excluding penicillin-resistant isolates)
- Streptococcus pyogenes
Gram-negative bacteria
- Escherichia coli
- Klebsiella pneumonia
- Proteus mirabilis
- Haemophilus influenzae (including beta-lactamase producing isolates)
- Moraxella catarrhalis
- Neisseria gonorrhoeae (including penicillinase-producing isolates)
- The following in vitro data are available, but their clinical significance is unknown. At least 90 percent of the following microorganisms exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for cefpodoxime. However, the efficacy of cefpodoxime in treating clinical infections due to these microorganisms has not been established in adequate and well-controlled clinical trials.
Gram-positive bacteria
Gram-negative bacteria
- Citrobacter diversus
- Klebsiella oxytoca
- Proteus vulgaris
- Providencia rettgeri
- Haemophilus parainfluenzae
Anaerobic Gram-positive bacteria
Susceptibility Test Methods
- When available, the clinical microbiology laboratory should provide the results of in vitro susceptibility test results for antimicrobial drug products used in resident hospitals to the physician as periodic reports that describe the susceptibility profile of nosocomial and community-acquired pathogens. These reports should aid the physician in selecting an antibacterial drug product for treatment.
Dilution techniques
- Quantitative methods are used to determine antimicrobial minimal inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized test method. The MIC values should be interpreted according to criteria provided in Table 1.
Diffusion techniques
- Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. The zone size provides an estimate of the susceptibility of bacteria to antimicrobial compounds. The zone size should be determined using a standardized test method. This procedure uses paper disks impregnated with 10 mcg cefpodoxime to test the susceptibility of microorganisms to cefpodoxime. The disk diffusion interpretive criteria are provided in Table 1.

- A report of Susceptible indicates that the antimicrobial is likely to inhibit growth of the pathogen if the antimicrobial compound reaches the concentration at the infection site necessary to inhibit growth of the pathogen. A report of Intermediate indicates that the result should be considered equivocal, and if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where a high dosage of drug can be used. This category also provides a buffer zone that prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of Resistant indicates that the antimicrobial is not likely to inhibit growth of the pathogen if the antimicrobial compound reaches the concentrations usually achievable at the infection site; other therapy should be selected.
Quality Control
- Standardized susceptibility test procedures require the use of laboratory controls to monitor and ensure the accuracy and precision of supplies and reagents used in the assay, and the techniques of the individual performing the test. Standard Cefpodoxime powder should provide the following range of MIC values noted in Table 2. For the diffusion technique using the 10 mcg disk, the criteria in Table 2 should be achieved.
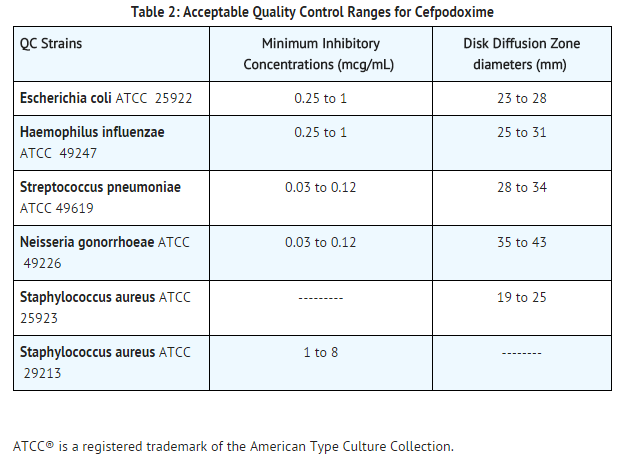
|nonClinToxic=There is limited information regarding Nonclinical Toxicology of Cefpodoxime Proxetil in the drug label. |clinicalStudies======Cystitis=====
- In two double-blind, 2:1 randomized, comparative trials performed in adults in the United States, cefpodoxime proxetil was compared to other beta-lactam antibiotics. In these studies, the following bacterial eradication rates were obtained at 5 to 9 days after therapy:
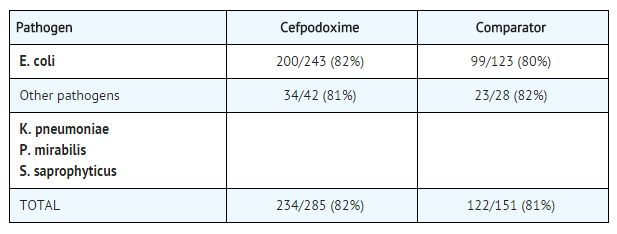
- In these studies, clinical cure rates and bacterial eradication rates for cefpodoxime proxetil were comparable to the comparator agents; however, the clinical cure rates and bacteriologic eradication rates were lower than those observed with some other classes of approved agents for cystitis.
Acute Otitis Media Studies
In controlled studies of acute otitis media performed in the United States, where significant rates of beta-lactamase-producing organisms were found, cefpodoxime proxetil was compared to cefixime. In these studies, using very strict evaluability criteria and microbiologic and clinical response criteria at the 4 to 21 day post-therapy follow-up, the following presumptive bacterial eradication/clinical success outcomes (cured and improved) were obtained.
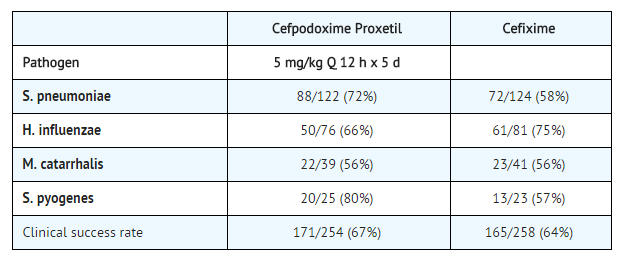
|howSupplied=*Cefpodoxime Proxetil Tablets, USP 100 mg are light yellowish-orange, elliptical, film-coated tablets debossed with ‘C’ on one side and ‘61’ on the other side.
- Bottles of 20 (NDC 65862-095-20)
- Bottles of 100 (NDC 65862-095-01)
- Bottles of 1000 (NDC 65862-095-99)
- Cefpodoxime Proxetil Tablets, USP 200 mg are coral red, elliptical, film-coated tablets debossed with ‘C’ on one side and ‘62’ on the other side.
- Bottles of 20 (NDC 65862-096-20)
- Bottles of 100 (NDC 65862-096-01)
- Bottles of 1000 (NDC 65862-096-99)
|storage=*Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
- Dispense in tight, light-resistant container.
- Replace cap securely after each opening.
|packLabel=====Package Label-principal display panel - 100 MG (20 tablet bottle)====

Package Label-principal display panel - 200 MG (20 tablet bottle)

Ingredients and Appearance
|fdaPatientInfo=*Patients should be counseled that antibacterial drugs including cefpodoxime proxetil should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When cefpodoxime proxetil is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by cefpodoxime proxetil or other antibacterial drugs in the future.
- Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
|alcohol=* Alcohol-Cefpodoxime interaction has not been established.
|brandNames=*Vantin [1] }} {{#subobject:
|Page Name=Cefpodoxime |Pill Name=Cefpodoxime_Proxetil_NDC_07815439.jpg |Drug Name=Cefpodoxime Proxetil |Pill Ingred=CEFPODOXIME PROXETIL[CEFPODOXIME]|+sep=; |Pill Imprint=SZ;439 |Pill Dosage=200 mg |Pill Color=Orange|+sep=; |Pill Shape=Oval |Pill Size (mm)=16 |Pill Scoring=1 |Pill Image= |Drug Author=Sandoz Inc |NDC=07815439
}}
{{#subobject:
|Page Name=Cefpodoxime |Pill Name=CEFPODOXIME_PROXETIL_NDC_658620095.jpg |Drug Name=CEFPODOXIME PROXETIL |Pill Ingred=CEFPODOXIME PROXETIL[CEFPODOXIME]|+sep=; |Pill Imprint=C;61 |Pill Dosage=100 mg |Pill Color=Yellow|+sep=; |Pill Shape=Oval |Pill Size (mm)=11 |Pill Scoring=1 |Pill Image= |Drug Author=Aurobindo Pharma Limited |NDC=658620095
}}