Probenecid
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Probenecid is an antigout, uricosuric and renal tubular transport blocking agent. that is FDA approved for the treatment of hyperuricemia associated with gout and gouty arthritis. Common adverse reactions include headache, dizziness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Gout
- Therapy with probenecid should not be started until an acute gouty attack has subsided. However, if an acute attack is precipitated during therapy, probenecid may be continued without changing the dosage, and full therapeutic dosage of colchicine, or other appropriate therapy, should be given to control the acute attack.
- Dosage: The recommended adult dosage is 250 mg (½ probenecid tablet), twice a day for one week, followed by 500 mg (1 tablet) twice a day thereafter.
- Some degree of renal impairment may be present in patients with gout. A daily dosage of 1000 mg may be adequate. However, if necessary, the daily dosage may be increased by 500 mg increments every 4 weeks within tolerance (and usually not above 2000 mg per day) if symptoms of gouty arthritis are not controlled or the 24 hour uric acid excretion is not above 700 mg. As noted, probenecid may not be effective in chronic renal insufficiency particularly when the glomerular filtration rate is 30 mg mL/minute or less.
- Gastric intolerance may be indicative of overdosage, and may be corrected by decreasing the dosage.
- As uric acid tends to crystallize out of an acid urine, a liberal fluid intake is recommended, as well as sufficient sodium bicarbonate (3 to 7.5 g daily ), or potassium citrate (7.5 g daily) to maintain an alkaline urine.
- Alkalization of the urine is recommended until the serum urate level returns to normal limits and tophaceous deposits disappear, i.e., during the period when urinary excretion of uric acid is at high level. Thereafter, alkalization of the urine and the usual restriction of purine-producing foods may be somewhat relaxed.
Probenecid should be continued at the dosage that will maintain normal serum urate levels. When acute attacks have been absent for 6 months or more and serum urate levels remain within normal limits, the daily dosage may be decreased by 500 mg every 6 months. The maintenance dosage should not be reduced to the point where serum urate levels tend to rise.
Gonnorrhea
- Recommended by the Center of Disease Control, U.S Department of Health and Human Services, Public Health Service (Morbidity and Mortality Weekly Report Supplement, Volume 34, Number 4S, October 18, 1985). In uncomplicated gonococcal infections in men and women (urethral, cervical, rectal), 1 g of probenecid should be given orally with 4.8 million units of aqueous procaine penicillin G² (given IM), or 3 g of amoxicillin² (given orally), or 3.5 g of ampicillin² (given orally).
Probenecid and Penicillin Therapy (In General)
- Adults: The recommended dosage is 2000 mg (4 tablets of probenecid) daily in divided doses. This dosage should be reduced in older patients in whom renal impairment may be present.
- Children: 2-14 years of age:
- Initial dose: 25 mg/kg body weight (or 0.7 g/square meter body surface).
- Maintenance Dose: 40 mg/kg body weight (or 1.2 g/square meter body surface) per day, divided into 4 doses.
- For children weighing more than 50 kg (110 lb) the adult dosage is recommended.
- Probenecid is contraindicated in children under 2 years of age.
The PSP excretion test may be used to determine the effectiveness of probenecid in retarding penicillin excretion and maintaining therapeutic levels. The renal clearance of PSP is reduced to about one-fifth the normal rate when dosage of probenecid is adequate.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Probenecid in adult patients.
Non–Guideline-Supported Use
Prophylaxis Against Nephrotoxic Effects of Other Drugs
- Cidofovir: 2g PO administered 3 hours before administering cidofovir; probenecid 1g PO should be given at 2 and 8 hours after cidofovir has been infused. In total, 4g PO probenecid per cidofovir infusion.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Gout
- Therapy with probenecid should not be started until an acute gouty attack has subsided. However, if an acute attack is precipitated during therapy, probenecid may be continued without changing the dosage, and full therapeutic dosage of colchicine, or other appropriate therapy, should be given to control the acute attack.
- Dosage: The recommended adult dosage is 250 mg (½ probenecid tablet), twice a day for one week, followed by 500 mg (1 tablet) twice a day thereafter.
- Some degree of renal impairment may be present in patients with gout. A daily dosage of 1000 mg may be adequate. However, if necessary, the daily dosage may be increased by 500 mg increments every 4 weeks within tolerance (and usually not above 2000 mg per day) if symptoms of gouty arthritis are not controlled or the 24 hour uric acid excretion is not above 700 mg. As noted, probenecid may not be effective in chronic renal insufficiency particularly when the glomerular filtration rate is 30 mg mL/minute or less.
- Gastric intolerance may be indicative of overdosage, and may be corrected by decreasing the dosage.
- As uric acid tends to crystallize out of an acid urine, a liberal fluid intake is recommended, as well as sufficient sodium bicarbonate (3 to 7.5 g daily ), or potassium citrate (7.5 g daily) to maintain an alkaline urine.
- Alkalization of the urine is recommended until the serum urate level returns to normal limits and tophaceous deposits disappear, i.e., during the period when urinary excretion of uric acid is at high level. Thereafter, alkalization of the urine and the usual restriction of purine-producing foods may be somewhat relaxed.
Probenecid should be continued at the dosage that will maintain normal serum urate levels. When acute attacks have been absent for 6 months or more and serum urate levels remain within normal limits, the daily dosage may be decreased by 500 mg every 6 months. The maintenance dosage should not be reduced to the point where serum urate levels tend to rise.
Gonnorrhea
- Recommended by the Center of Disease Control, U.S Department of Health and Human Services, Public Health Service (Morbidity and Mortality Weekly Report Supplement, Volume 34, Number 4S, October 18, 1985). In uncomplicated gonococcal infections in men and women (urethral, cervical, rectal), 1 g of probenecid should be given orally with 4.8 million units of aqueous procaine penicillin G² (given IM), or 3 g of amoxicillin² (given orally), or 3.5 g of ampicillin² (given orally).
Probenecid and Penicillin Therapy (In General)
- Adults: The recommended dosage is 2000 mg (4 tablets of probenecid) daily in divided doses. This dosage should be reduced in older patients in whom renal impairment may be present.
- Children: 2-14 years of age:
- Initial dose: 25 mg/kg body weight (or 0.7 g/square meter body surface).
- Maintenance Dose: 40 mg/kg body weight (or 1.2 g/square meter body surface) per day, divided into 4 doses.
- For children weighing more than 50 kg (110 lb) the adult dosage is recommended.
- Probenecid is contraindicated in children under 2 years of age.
The PSP excretion test may be used to determine the effectiveness of probenecid in retarding penicillin excretion and maintaining therapeutic levels. The renal clearance of PSP is reduced to about one-fifth the normal rate when dosage of probenecid is adequate.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Probenecid in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Probenecid in pediatric patients.
Contraindications
- Hypersensitivity to probenecid.
- Children under 2 years of age.
- Not recommended in persons with known blood dyscrasias or uric acid kidney stones.
- Therapy with probenecid should not be started until an acute gouty attack has subsided.
Warnings
- Exacerbation of gout following therapy with probenecid may occur; in such cases colchicine or other appropriate therapy is advisable.
- Probenecid increases plasma concentrations of methotrexate in both animals and humans. In animal studies, increased methotrexate toxicity has been reported. If probenecid is given with methotrexate, the dosage of methotrexate should be reduced and serum levels may need to be monitored.
- In patients on probenecid the use of salicylates in either small or large doses is contraindicated because it antagonizes the uricosuric action of probenecid. The biphasic action of salicylates in the renal tubules accounts for the so-called “paradoxical effect” of uricosuric agents. In patients on probenecid who require a mild analgesic agent the use of acetaminophen rather than small doses of salicylates would be preferred.
- Rarely, severe allergic reactions and anaphylaxis have been reported with the use of probenecid. Most of these have been reported to occur within several hours after readministration following prior usage of the drug.
- The appearance of hypersensitivity reactions requires cessation of therapy with probenecid.
- Use in Pregnancy:
- Probenecid crosses the placenta barrier and appears in cord blood. The use of any drug in women of childbearing potential requires that the anticipated benefit be weighed against the possible hazards.
Adverse Reactions
Clinical Trials Experience
The following adverse reactions have been observed and within each category are listed in order of decreasing severity.
Central Nervous System
Metabolic
- Precipitation of acute gouty arthritis.
Gastrointestinal
Genitourinary
- Nephrotic syndrome
- Uric acid stones with or without hematuria
- Renal colic
- Costovertebral pain
- Urinary frequency
Hypersensitivity
Hematologic
- Aplastic anemia
- Leukopenia
- Hemolytic anemia which in some patients could be related to genetic deficiency of glucose-6-phosphate dehydrogenase in red blood cells
- Anemia
Integumentary
Postmarketing Experience
There is limited information regarding Probenecid Postmarketing Experience in the drug label.
Drug Interactions
- When probenecid is used to elevate plasma concentrations of penicillin or other beta-lactams, or when such drugs are given to patients taking probenecid therapeutically, high plasma concentrations of the other drug may increase the incidence of adverse reactions associated with that drug. In the case of penicillin or other beta-lactams, psychic disturbances have been reported.
- The use of salicylates antagonizes the uricosuric action of probenecid. The uricosuric action of probenecid is also antagonized by pyrazinamide.
- Probenecid produces an insignificant increase in free sulfonamide plasma concentrations, but a significant increase in total sulfonamide plasma levels. Since probenecid decreases the renal excretion of conjugated sulfonamides, plasma concentrations of the latter should be determined from time to time when sulfonamide and probenecid are coadministered for prolonged periods. Probenecid may prolong or enhance the action of oral sulfonylureas and thereby increase the risk of hypoglycemia.
- It has been reported that patients receiving probenecid require significantly less thiopental for induction of anesthesia. In addition, ketamine and thiopental anesthesia were significantly prolonged in rats receiving probenecid.
- The concomitant administration of probenecid increases the mean plasma elimination half-life of a number of drugs which can lead to increased plasma concentrations. These include agents such as indomethacin, acetaminophen, naproxen, ketoprofen, meclofenamate, lorazepam, and rifampin. Although the clinical significance of this observation has not been established, a lower dosage of the drug may be required to produce a therapeutic effect, and increases a dosage of the drug in question should be made cautiously and in small increments when probenecid is being coadministered. Although specific instances of toxicity due to this potential interaction have not been observed to date, physicians should be alert to this possibility.
- Probenecid given concomitantly with sulindac had only a slight effect on plasma sulfide levels, while plasma levels of sulindac and sulfone were increased. Sulindac was shown to produce a modest reduction in the uricosuric action of probenecid, which probably is not significant under most circumstances.
- In animals and in humans, probenecid has been reported to increase plasma concentrations of methotrexate
- Falsely high readings for theophylline have been reported in an in vitro study, using the Schack and Waxler technique, when therapeutic concentrations of theophylline and probenecid were added to human plasma.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B
Probenecid crosses the placenta barrier and appears in cord blood. The use of any drug in women of childbearing potential requires that the anticipated benefit be weighed against the possible hazards.
Pregnancy Category (AUS): B2
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Probenecid in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Probenecid during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Probenecid in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Probenecid in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Probenecid in geriatric settings.
Gender
There is no FDA guidance on the use of Probenecid with respect to specific gender populations.
Race
There is no FDA guidance on the use of Probenecid with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Probenecid in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Probenecid in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Probenecid in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Probenecid in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Probenecid Administration in the drug label.
Monitoring
There is limited information regarding Probenecid Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Probenecid and IV administrations.
Overdosage
There is limited information regarding Probenecid overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology

| |
| Template:Px | |
Probenecid
| |
| Systematic (IUPAC) name | |
| 4-(dipropylsulfamoyl)benzoic acid | |
| Identifiers | |
| CAS number | |
| ATC code | M04 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 285.36 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 75-95% |
| Metabolism | ? |
| Half life | 2-6 hours (dose: 0.5-1 g) |
| Excretion | renal (77-88%) |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | oral |
Mechanism of Action
- Probenecid is a uricosuric and renal tubular blocking agent. It inhibits the tubular reabsorption of urate, thus increasing the urinary excretion of uric acid and decreasing serum urate levels. Effective uricosuria reduces the miscible urate pool, retards urate deposition, and promotes resorption of urate deposits.
- Probenecid inhibits the tubular secretion of penicillin and usually increases penicillin plasma levels by any route the antibiotic is given. A 2-fold to 4-fold elevation has been demonstrated for various penicillins.
- Probenecid also has been reported to inhibit the renal transport of many other compounds including aminohippuric acid (PAH), aminosalicylic acid (PAS), indomethacin, sodium iodomethamate and related iodinated organic acids, 17 –ketosteroids, pantothenic acid, phenolsulfonphthalein (PSP), sulfonamides, and sulfonylureas.
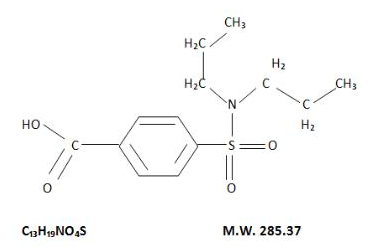
Structure
- The chemical name for probenecid is 4-[(dipropylamino) sulfony1] benzoic acid. It has the following structural formula:
Pharmacodynamics
There is limited information regarding Probenecid Pharmacodynamics in the drug label.
Pharmacokinetics
- Probenecid decreases both hepatic and renal excretion of sulfobromophtalein (BSP). The tubular reabsorption of phosphorus is inhibited in hypoparathyroid but not in euparathyroid individuals. Probenecid does not influence plasma concentrations of salicylates, nor the excretion of streptomycin, chloramphenicol, chlortetracycline, oxytetracycline, or neomycin.
Nonclinical Toxicology
There is limited information regarding Probenecid Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Probenecid Clinical Studies in the drug label.
How Supplied
- Probenecid Tablets, USP are available containing 500 mg of Probenecid, USP.
- The tablets are capsule shaped, film-coated yellow, debossed LCI on one side and 1367 on the other side. They are available as follows:
- NDC 10135-541-10 bottles of 1000 tablets
Storage
Store at 20˚ to 25˚c (68˚-77˚f). Protect from light.
Images
Drug Images
{{#ask: Page Name::Probenecid |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Probenecid |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Probenecid Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Probenecid interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Probenecid Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Probenecid |Label Name=Probenecid Box.png
}}
| File:Probenecid.png | |
| Clinical data | |
|---|---|
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 75-95% |
| Elimination half-life | 6-12 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C13H19NO4S |
| Molar mass | 285.36 g/mol |
Probenecid is a uricosuric drug, primarily used in treating gout or hyperuricemia, that increases uric acid removal in the urine. One of its trade names is 'Benuryl.'
Probenecid also decreases the renal excretion of some drugs.
In one study, probenecid was shown to more than double a patient's exposure to oseltamivir (trade name Tamiflu), an antiviral drug used to combat influenza.[citation needed] This is significant because nations are currently stockpiling oseltamivir in anticipation of an influenza pandemic, and there could be supply shortages.[citation needed] During World War II, probenecid was used to extend limited supplies of penicillin,[1] and is still currently used to increase antibiotic concentrations in serious infections. It has also found use as a masking agent by athletes attempting to get away with using performance enhancing drugs.
In the kidneys it is filtered at the glomerulus, secreted in the proximal tubule and reabsorbed in the distal tubule.
Probenecid's exact mechanism of action in the kidneys' nephrons is unknown. (I wish to question this statement. The text Human Physiology by Dee Unglaub Silverthorn explains that gout is caused by elevated levels of uric acid in the plasma, and that the kidney's organic anion transporter (OAT) reclaims uric acid from the urine and returns it to the plasma. If the organic acid probenecid is administered to a patient, the OAT binds to probenecid instead of to uric acid, preventing the reabsorption of uric acid. As a result, more uric acid leaves the body in the urine, lowering the uric acid concentration in the plasma. This is an example of the way in which competition between substrates transported across cell membranes has been put to use in medicine.)
Probenecid reduces the reabsorption of uric acid.
See also
References
- ↑ Butler D (2005). "Wartime tactic doubles power of scarce bird-flu drug". Nature. 438 (7064): 6. PMID 16267514.
- Pages with script errors
- Pages with broken file links
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- All articles with unsourced statements
- Articles with unsourced statements from May 2007
- Articles with invalid date parameter in template
- Articles with unsourced statements from December 2007
- Antigout agents