Acoustic neuroma diagnostic study of choice: Difference between revisions
(Created page with "__NOTOC__ {{Acoustic neuroma}} {{CMG}}; {{AE}} == Overview == == Diagnostic Study of Choice == === Study of choice === [Name of the investigation] is the gold standard test...") |
No edit summary |
||
| (7 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Acoustic neuroma}} | {{Acoustic neuroma}} | ||
{{CMG}}; {{AE}} | {{CMG}}; {{AE}}{{M.B}} | ||
== Overview == | == Overview == | ||
[[Gadolinium]]-enhanced [[Magnetic resonance imaging|MRI scan]] is the definitive [[diagnostic test]] for acoustic neuroma and can identify [[tumors]] as small as 1-2 millimeter in [[diameter]]. On [[brain]] [[Magnetic resonance imaging|MRI]], acoustic neuroma characterized by hypointense mass on T1-weighted [[Magnetic resonance imaging|MRI]], and hyperintense mass on T2-weighted [[Magnetic resonance imaging|MRI]]. | |||
== Diagnostic Study of Choice == | == Diagnostic Study of Choice == | ||
*[[Gadolinium]]-enhanced [[magnetic resonance imaging|magnetic resonance imaging (MRI)]] is the preferred [[diagnostic test]] for identifying acoustic neuroma and can identify [[tumors]] as small as 1-2 millimeter in [[diameter]].<ref>{{Cite journal | |||
| author = [[E. P. Lin]] & [[B. T. Crane]] | |||
| title = The Management and Imaging of Vestibular Schwannomas | |||
| journal = [[AJNR. American journal of neuroradiology]] | |||
| volume = 38 | |||
| issue = 11 | |||
| pages = 2034–2043 | |||
| year = 2017 | |||
| month = November | |||
| doi = 10.3174/ajnr.A5213 | |||
| pmid = 28546250 | |||
}}</ref><ref>{{Cite journal | |||
| author = [[D. F. Wilson]], [[R. S. Hodgson]], [[M. F. Gustafson]], [[S. Hogue]] & [[L. Mills]] | |||
| title = The sensitivity of auditory brainstem response testing in small acoustic neuromas | |||
| journal = [[The Laryngoscope]] | |||
| volume = 102 | |||
| issue = 9 | |||
| pages = 961–964 | |||
| year = 1992 | |||
| month = September | |||
| doi = 10.1288/00005537-199209000-00001 | |||
| pmid = 1518359 | |||
}}</ref> | |||
= | {| style="border: 0px; font-size: 90%; margin: 3px; width: 600px" align="center" | ||
| valign="top" | | |||
|+ | |||
! style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|MRI component}} | |||
! style="background: #4479BA; width: 400px;" | {{fontcolor|#FFF|Features}} | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" | | |||
:T1 | |||
| style="padding: 5px 5px; background: #F5F5F5;" | | |||
*Slightly hypointense to the adjacent [[brain]] (63%) | |||
*Isointense to the adjacent [[brain]] (37%) | |||
*May contain hypointense [[Cyst|cystic]] areas | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC;font-weight: bold" | | |||
:T2 | |||
| style="padding: 5px 5px; background: #F5F5F5;" | | |||
*[[Heterogeneous|Heterogeneously]] hyperintense to the adjacent [[brain]] | |||
*[[Cyst|Cystic]] areas [[fluid]] intensity | |||
*May have associated peritumoral [[Arachnoid mater|arachnoid]] [[Cyst|cysts]] | |||
! style="background: #4479BA; | |||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC;font-weight: bold" | | |||
| style=" | :T1 C+ (Gd) | ||
| | | style="padding: 5px 5px; background: #F5F5F5;" | | ||
*[[Contrast medium|Contrast]] enhancement is vivid | |||
*But [[heterogeneous]] in larger [[Tumor|tumors]] | |||
|- | |- | ||
|} | |} | ||
Post-op [[Magnetic resonance imaging|MRI]]: Linear enhancement may not indicate [[tumor]]. But if there is [[Nodule (medicine)|nodular]] enhancement, suspect [[tumor]] recurrence which will necessitate follow-up [[Magnetic resonance imaging|MRI]].<ref name="radio">Acoustic Schwannoma. Radiopedia(2015) http://radiopaedia.org/articles/acoustic-schwannoma Accessed on October 2 2015</ref> | |||
[[File:Intracanalicular_acoustic_schwannoma.jpg|thumb|none|200px|[[Magnetic resonance imaging|MRI]] showing Intracanalicular acoustic neuroma<ref>Image courtesy of Dr Frank Gaillard. [http://www.radiopaedia.org Radiopaedia] (original file [http://radiopaedia.org/cases/intracanalicular-acoustic-schwannoma here]).[http://radiopaedia.org/licence Creative Commons BY-SA-NC</ref>]] | |||
[[File:Intrameatal vestibularis schwannoma.jpg|thumb|none|200px|[[Magnetic resonance imaging|MRI]] showing [[Internal auditory meatus|Intrameatal]] vestibular schwannoma<ref>Image courtesy of Dr. Roberto Schubert [http://www.radiopaedia.org Radiopaedia] (original file [http://radiopaedia.org/cases/intrameatal-vestibularis-schwannoma here]).[http://radiopaedia.org/licence Creative Commons BY-SA-NC</ref>]] | |||
==References== | ==References== | ||
Latest revision as of 18:05, 26 April 2019
|
Acoustic neuroma Microchapters | |
|
Diagnosis | |
|---|---|
|
Treatment | |
|
Case Studies | |
|
Acoustic neuroma diagnostic study of choice On the Web | |
|
American Roentgen Ray Society Images of Acoustic neuroma diagnostic study of choice | |
|
Risk calculators and risk factors for Acoustic neuroma diagnostic study of choice | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mohsen Basiri M.D.
Overview
Gadolinium-enhanced MRI scan is the definitive diagnostic test for acoustic neuroma and can identify tumors as small as 1-2 millimeter in diameter. On brain MRI, acoustic neuroma characterized by hypointense mass on T1-weighted MRI, and hyperintense mass on T2-weighted MRI.
Diagnostic Study of Choice
- Gadolinium-enhanced magnetic resonance imaging (MRI) is the preferred diagnostic test for identifying acoustic neuroma and can identify tumors as small as 1-2 millimeter in diameter.[1][2]
| MRI component | Features |
|---|---|
|
|
|
|
|
|
Post-op MRI: Linear enhancement may not indicate tumor. But if there is nodular enhancement, suspect tumor recurrence which will necessitate follow-up MRI.[3]
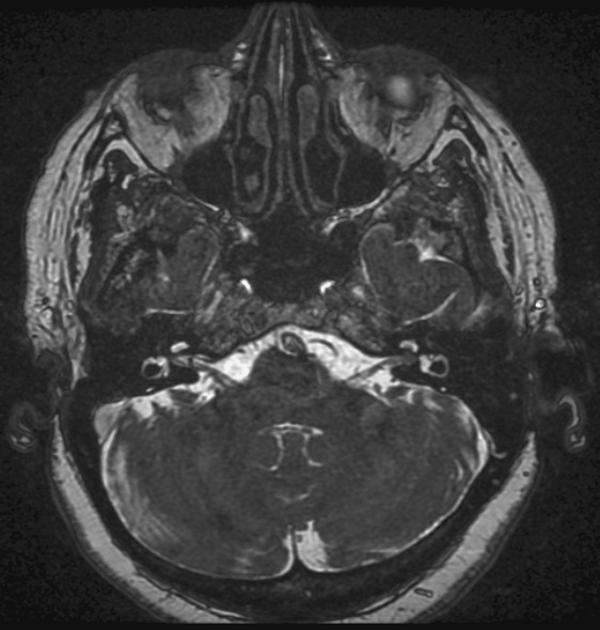
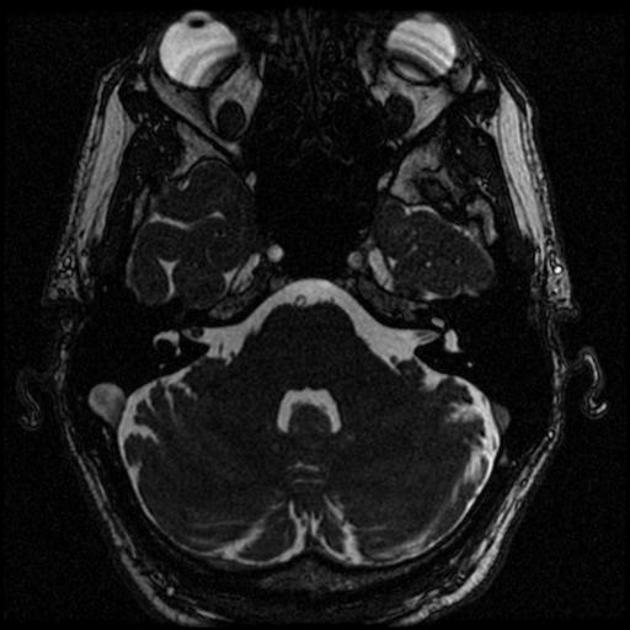
References
- ↑ E. P. Lin & B. T. Crane (2017). "The Management and Imaging of Vestibular Schwannomas". AJNR. American journal of neuroradiology. 38 (11): 2034–2043. doi:10.3174/ajnr.A5213. PMID 28546250. Unknown parameter
|month=ignored (help) - ↑ D. F. Wilson, R. S. Hodgson, M. F. Gustafson, S. Hogue & L. Mills (1992). "The sensitivity of auditory brainstem response testing in small acoustic neuromas". The Laryngoscope. 102 (9): 961–964. doi:10.1288/00005537-199209000-00001. PMID 1518359. Unknown parameter
|month=ignored (help) - ↑ Acoustic Schwannoma. Radiopedia(2015) http://radiopaedia.org/articles/acoustic-schwannoma Accessed on October 2 2015
- ↑ Image courtesy of Dr Frank Gaillard. Radiopaedia (original file here).[http://radiopaedia.org/licence Creative Commons BY-SA-NC
- ↑ Image courtesy of Dr. Roberto Schubert Radiopaedia (original file here).[http://radiopaedia.org/licence Creative Commons BY-SA-NC