Acetylcholine
 | |
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | approximately 2 minutes |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
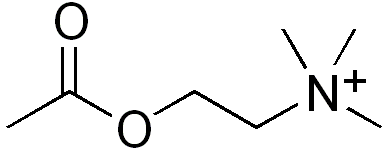
| Formula | C7H16NO2 |
| Molar mass | 146.21 g/mol |
| 3D model (JSmol) | |
| |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
The chemical compound acetylcholine (often abbreviated ACh) is a neurotransmitter in both the peripheral nervous system (PNS) and central nervous system (CNS) in many organisms including humans. Acetylcholine is one of many neurotransmitters in the autonomic nervous system (ANS) and the only neurotransmitter used in the somatic nervous system. It is also the neurotransmitter in all autonomic ganglia.
Chemistry
Acetylcholine is an ester of acetic acid and choline with chemical formula CH3COOCH2CH2N+(CH3)3. This structure is reflected in the systematic name, 2-acetoxy-N,N,N-trimethylethanaminium.
Function
Acetylcholine has functions both in the peripheral nervous system (PNS) and in the central nervous system (CNS) as a neuromodulator.
In the PNS, acetylcholine activates muscles, and is a major neurotransmitter in the autonomic nervous system.
In the CNS, acetylcholine and the associated neurons form a neurotransmitter system, the cholinergic system, which tends to cause excitatory actions.
In PNS
In the PNS, acetylcholine activates muscles, and is a major neurotransmitter in the autonomic nervous system.
When acetylcholine binds to acetylcholine receptors on skeletal muscle fibers, it opens ligand gated sodium channels in the cell membrane. Sodium ions then enter the muscle cell, stimulating muscle contraction. Acetylcholine, while inducing contraction of skeletal muscles, instead induces decreased contraction in cardiac muscle fibers. This distinction is attributed to differences in receptor structure between skeletal and cardiac fibers.
In the autonomic nervous system, acetylcholine is released in the following sites:
- all pre and post-ganglionic parasympathetic neurons
- all preganglionic sympathetic neurons
- preganglionic sympathetic fibers to suprarenal medulla, the modified sympathetic ganglion. On stimulation by acetylcholine, it releases adrenaline and noradrenaline.
- some postganglionic sympathetic fibers : )
- sudomotor neurons to sweat glands
In CNS
In the central nervous system, ACh has a variety of effects as a neuromodulator, e.g. for plasticity and excitability. Other effects are arousal and reward.
Structure
Acetylcholine and the associated neurons form a neurotransmitter system, the cholinergic system. It originates mainly in pontomesencephalotegmental complex, basal optic nucleus of Meynert and medial septal nucleus, and projects axons to vast areas of the brain:
- The pontomesencephalotegmental complex acts mainly on M1 receptors in the brainstem .
- basal optic nucleus of Meynert acts mainly on M1 receptors in the neocortex.
- medial septal nucleus acts mainly on M1 receptors in the hippocampus and neocortex.
Plasticity
ACh is involved with synaptic plasticity, specifically in learning and short-term memory
It has been shown to enhance the amplitude of synaptic potentials following long-term potentiation in many regions, including the dentate gyrus, CA1, piriform cortex, and neocortex. This effect most likely occurs either through enhancing currents through NMDA receptors or indirectly by suppressing adaptation. The suppression of adaptation has been shown in brain slices of regions CA1, cingulate cortex, and piriform cortex as well as in vivo in cat somatosensory and motor cortex by decreasing the conductance of voltage-dependent M currents and Ca2+-dependent K+ currents.
Excitability
Acetylcholine also has other effects on excitability of neurons. Its presence causes a slow depolarization by blocking a tonically active K+ current, which increases neuronal excitability. Paradoxically, it increases spiking activity in inhibitory interneurons while decreasing strength of synaptic transmission from those cells. This decrease in synaptic transmission also occurs selectively at some excitatory cells: for instance, it has an effect on intrinsic and associational fibers in layer Ib of piriform cortex, but has no effect on afferent fibers in layer Ia. Similar laminar selectivity has been shown in dentate gyrus and region CA1 of the hippocampus. One theory to explain this paradox interprets acetylcholine neuromodulation in the neocortex as modulating the estimate of expected uncertainty, acting counter to norepinephrine (NE) signals for unexpected uncertainty. Both would then decrease synaptic transition strength, but ACh would then be needed to counter the effects of NE in learning a signal understood to be noisy.
Synthesis and Degradation
Acetylcholine is synthesized in certain neurons by the enzyme choline acetyltransferase from the compounds choline and acetyl-CoA.
Normally, the enzyme acetylcholinesterase converts acetylcholine into the inactive metabolites choline and acetate. This enzyme is abundant in the synaptic cleft, and its role in rapidly clearing free acetylcholine from the synapse is essential for proper muscle function.
Receptors
There are two main classes of acetylcholine receptor (AChR), nicotinic acetylcholine receptors (nAChR) and muscarinic acetylcholine receptors (mAChR). They are named for the ligands used to discover the receptors.
Nicotinic
Nicotinic AChRs are ionotropic receptors permeable to sodium, potassium, and chloride ions. They are stimulated by nicotine and acetylcholine. They are of two main types, muscle type and neuronal type. The former can be selectively blocked by curare and the latter by hexamethonium. The main location of nicotinic AChRs are on muscle end plates, autonomic ganglia (both sympathetic and parasympathetic), and in the CNS.[1]
Muscarinic
Muscarinic receptors are metabotropic and affect neurons over a longer time frame. They are stimulated by muscarine and acetylcholine, and blocked by atropine. Muscarinic receptors are found in both the central nervous system and the peripheral nervous system, in heart, lungs, upper GI tract and sweat glands. Extracts from the plant Deadly nightshade included this compound, and its action on muscarinic AChRs that increased pupil size was used for attractiveness in many European cultures in the past. Now, ACh is sometimes used during cataract surgery to produce rapid constriction of the pupil. It must be administered intraocularly because corneal cholinesterase metabolizes topically administered ACh before it can diffuse into the eye. It is sold by the trade name Miochol-E (CIBA Vision). Similar drugs are used to induce mydriasis (dilation of the pupil) in cardiopulmonary resuscitation and many other situations.
Drugs Acting on the ACh System
Blocking, hindering or mimicking the action of acetylcholine has many uses in medicine. Drugs acting on the acetylcholine system are either agonists to the receptors, stimulating the system, or antagonists, inhibiting it.
ACh Receptor Agonists
Acetylcholine receptor agonists can either have an effect directly on the receptors, or exert their effects indirectly, e.g. by affecting the enzyme acetylcholinesterase, which degrades the receptor ligand.
Associated disorders
ACh Receptor Agonists are used to treat myasthenia gravis and Alzheimer's disease.
Myasthenia gravis
The disease myasthenia gravis, characterized by muscle weakness and fatigue, occurs when the body inappropriately produces antibodies against acetylcholine receptors, and thus inhibits proper acetylcholine signal transmission. Over time the motor end plate is destroyed. Drugs that competitively inhibit acetylcholinesterase (e.g., neostigmine or physostigmine) are effective in treating this disorder. They allow endogenously released acetylcholine more time to interact with its respective receptor before being inactivated by acetylcholinesterase in the gap junction.
Alzheimer's disease
Since a shortage of acetylcholine in the brain has been associated with Alzheimer's disease, some drugs that inhibit acetylcholinesterase are used in the treatment of that disease. A recent study has shown that THC is one such drug, effective at reducing the formation of characteristic neurofibrillary tangles and amyloid beta plaques[2].
Direct Acting
- Acetylcholine
- Bethanechol
- Carbachol
- Cevimeline
- Pilocarpine
- Suberylcholine
- Nicotine (in small doses)
Cholinesterase inhibitors
Most indirect acting ACh receptor agonists work by inhibiting the enzyme acetylcholinesterase. The resulting accumulation of acetylcholine causes continuous stimulation of the muscles, glands and central nervous system.
They are examples of enzyme inhibitors, and increase the action of acetylcholine by delaying its degradation; some have been used as nerve agents (Sarin and VX nerve gas) or pesticides (organophosphates and the carbamates). Clinically they are used to reverse the action of muscle relaxants, to treat myasthenia gravis and in Alzheimer's disease (rivastigmine, which increases cholinergic activity in the brain).
Reversible
The following substances reversibly inhibit the enzyme acetylcholinesterase (which breaks down acetylcholine), thereby increasing acetylcholine levels.
- Many medications in Alzheimer's disease
- Edrophonium (differs myasthenic and cholinergic crisis)
- Neostigmine (in myasthenia gravis)
- Physostigmine (in glaucoma and anticholinergic drug overdoses)
- Pyridostigmine (in myasthenia gravis
- Carbamate insecticides (e.g. Aldicarb)
Irreversible
Semi-permanently inhibit the enzyme acetylcholinesterase.
- Echothiophate
- Isoflurophate
- Organophosphate Insecticides (Malathion, Parathion, Azinphos Methyl, Chlorpyrifos, among others)
- Organophosphate-containing nerve agents (e.g. Sarin gas)
Victims of sarin gas, commonly die of suffocation as they cannot relax their diaphragm.
Reactivation of Acetylcholine Esterase
ACh Receptor Antagonists
Antimuscarinic Agents
Ganglionic Blockers
- Mecamylamine
- Hexamethonium
- Nicotine (in high doses)
- Trimethaphan
Neuromuscular Blockers
- Atracurium
- Cisatracurium
- Doxacurium
- Metocurine
- Mivacurium
- Pancuronium
- Rocuronium
- Succinylcholine
- Tubocurarine
- Vecuronium
- HemiCholine
Synthesis inhibitors
Organic mercurial compounds have a high affinity for sulfhydryl groups, which causes dysfunction of the enzyme choline acetyl transferase. This inhibition may lead to acetylcholine deficiency, and can have consequences on motor function.
Release inhibitors
Botulin acts by suppressing the release of acetylcholine; where the venom from a black widow spider has the reverse effect.
Other / Uncategorized / Unknown
History
Acetylcholine (ACh) was first identified in 1914 by Henry Hallett Dale for its actions on heart tissue. It was confirmed as a neurotransmitter by Otto Loewi who initially gave it the name vagusstoff because it was released from the vagus nerve. Both received the 1936 Nobel Prize in Physiology or Medicine for their work.
Acetylcholine was the first neurotransmitter to be identified.
References
- ↑ Katzung, B.G. (2003). Basic and Clinical Pharmacology (9th ed.). McGraw-Hill Medical. ISBN 0-07-141092-9
- ↑ Eubanks LM, Rogers CJ, Beuscher AE 4th, Koob GF, Olson AJ, Dickerson TJ, Janda KD. "A molecular link between the active component of marijuana and Alzheimer's disease pathology." Molecular Pharmaceutics. 2006 Nov-Dec; 3(6):773-7. PMID 17140265
- Brenner, G. M. and Stevens, C. W. (2006). Pharmacology (2nd ed.). Philadelphia, PA: W.B. Saunders Company (Elsevier). ISBN 1-4160-2984-2
- Canadian Pharmacists Association (2000). Compendium of Pharmaceuticals and Specialties (25th ed.). Toronto, ON: Webcom. ISBN 0-919115-76-4
- Carlson, NR (2001). Physiology of Behavior (7th ed.). Needham Heights, MA: Allyn and Bacon. ISBN 0-205-30840-6
- Gershon, Michael D. (1998). The Second Brain. New York, NY: HarperCollins. ISBN 0-06-018252-0
- Hasselmo, ME. "Neuromodulation and cortical function: Modeling the physiological basis of behavior." Behavioral Brain Research. 1995 Feb; 67(1):1-27. PMID 7748496
- Yu, AJ & Dayan, P. "Uncertainty, neuromodulation, and attention." Neuron. 2005 May 19; 46(4):681-92. PMID 15944135
External links
ar:أستيل كولين ceb:Acetylcholine de:Acetylcholin hr:Acetilkolin it:Acetilcolina he:אצטילכולין lt:Acetilcholinas hu:Acetilkolin nl:Acetylcholine no:Acetylkolin sl:Acetilholin sr:Ацетилхолин fi:Asetyylikoliini sv:Acetylkolin tl:Acetylcholine uk:Ацетилхолін ur:اسی ٹائل کولین Template:Jb1 Template:WH Template:WS
- Pages with script errors
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Choline esters
- Acetates
- Neurotransmitters
- Quaternary ammonium compounds