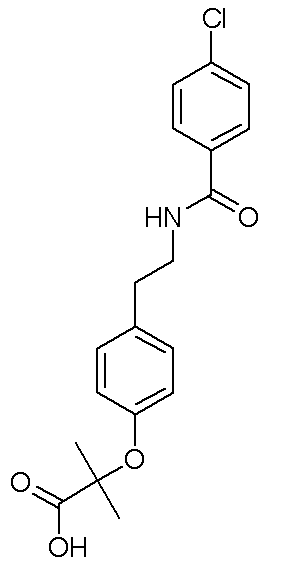
Bezafibrate
 | |
| Clinical data | |
|---|---|
| Dependence liability | N/A |
| Routes of administration | oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C19H20ClNO4 |
| Molar mass | 361.819 |
|
WikiDoc Resources for Bezafibrate |
|
Articles |
|---|
|
Most recent articles on Bezafibrate Most cited articles on Bezafibrate |
|
Media |
|
Powerpoint slides on Bezafibrate |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Bezafibrate at Clinical Trials.gov Clinical Trials on Bezafibrate at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Bezafibrate
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Bezafibrate Discussion groups on Bezafibrate Patient Handouts on Bezafibrate Directions to Hospitals Treating Bezafibrate Risk calculators and risk factors for Bezafibrate
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Bezafibrate |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Cafer Zorkun, M.D., Ph.D. [2]
For patient information, click here
Overview
Bezafibrate (Bezalip® and various other brand names) is a fibrate drug used for the treatment of hyperlipidaemia. It helps to lower LDL cholesterol and triglyceride in the blood, and increase HDL.
History
Bezafibrate was first introduced by Boehringer Mannheim in 1977.
Use
Adjunct to diet and other therapeutic measures for treatment of type IIa and IIb mixed [[hyperlipidemia, to regulate lipid and apoprotein levels; treatment of adult patients with high to very high triglyceride levels (Fredrickson classification type IV and V hyperlipidemias), who are at high risk of sequelae and complications from their dyslipidemia.
Bezafibrate improves markers of combined hyperlipidemia, effectively reducing LDL and triglycerides and improving HDL levels.[1] The main effect on cardiovascular morbidity is in patients with the metabolic syndrome, the features of which are attenuated by bezafibrate.[2] Studies show that in patients with impaired glucose tolerance, bezafibrate may delay progress to diabetes[3], and in those with insulin resistance it slowed progress in the HOMA severity marker.[4]
Administration
Immediate release: Do not crush or chew; tablet should be swallowed whole and with sufficient fluid, with or after meals
Sustained release: Do not crush or chew; tablet should be swallowed whole with sufficient fluid. Take in morning or evening with or after meals
Pregnancy Risk Factor
Not available; not recommended (per manufacturer)
Pregnancy Implications
Embryotoxicity has occurred in animals at toxic doses. Women planning pregnancy should discontinue bezafibrate several months before conception; strict birth control procedures must be exercised.
Lactation
Excretion in breast milk unknown/contraindicated
Contraindications
Hypersensitivity to bezafibrate, fibrates, or any component of the formulation; hepatic or renal dysfunction; primary biliary cirrhosis; preexisting gallbladder disease; concurrent use of MAO inhibitors; pregnancy or breast-feeding; not indicated for the treatment of type I hyperlipoproteinemia
Warnings/Precautions
Has been shown to be hepatotoxic and possibly tumorigenic (animal models); discontinue if response is not obtained in 3 months. Use caution in patients with history of jaundice or hepatic disorder; abnormal liver function tests have been observed (reversible when discontinued). Bezafibrate has been associated with rare myositis or rhabdomyolysis; risk may be increased by concurrent therapy with HMG CoA reductase inhibitors or cyclosporine. Use caution in renal impairment (dosage reduction required). Use with caution in patients with hypoalbuminemia or nephrotic syndrome. Limited experience is available in children; therefore, caution should be used when treating children.
Adverse Reactions
1% to 10%
Central nervous system: Dizziness (2%), insomnia (1%), migraine (1%), pain (1%)
Dermatologic: Pruritus (3%), eczema (1%), rash
Gastrointestinal: Gastritis (6%), flatulence (5%), dyspepsia (3%), nausea, diarrhea, constipation
Hematologic: Anemia (1%)
Hepatic: Transaminases increased
Miscellaneous: Allergic reaction (1%)
Neuromuscular and skeletal: CPK increased
Renal: Creatinine increased
<1%: Alkaline phosphatase increased, alopecia, asthenia, cholelithiasis, epigastric distress, erythema, headache, impotence, muscle pain, muscle cramps, myopathy, rhabdomyolysis, urticaria
Overdosage/Toxicology
Limited information. Symptomatic and supportive measures should be taken; hemodialysis should not be considered. In patients with existing renal dysfunction (if dosage recommendations are not followed), over dosage may occur and severe rhabdomyolysis may develop.
Drug Interactions
- Substrate of CYP3A4 (minor)
- Cholestyramine and colestipol: May impair bezafibrate absorption (separate doses by at least 2 hours).
- Cyclosporine: Severe myositis and rhabdomyolysis have been reported with concurrent use.
- Furosemide: Blood levels of furosemide and fibric acid derivatives (i.e., clofibrate and fenofibrate) may be increased during concurrent dosing (particularly in hypoalbuminemia). Limited documentation; monitor for increased effect/toxicity.
- HMG-CoA reductase inhibitors: Severe myositis and rhabdomyolysis have been reported with concurrent use; use extreme caution.
- MAO inhibitors: May increase the risk of hepatotoxicity.
- Warfarin (oral anticoagulants): May increase prothrombin time; dosage of oral anticoagulant should be reduced by 50%.
Stability
Store at room temperature of 15°C to 30°C (59°F to 86°F); protect from high humidity.
Mechanism of Action
Mechanism not definitely established; may increase VLDL catabolism by increasing lipoprotein and hepatic triglyceride lipase activities; attenuation of triglyceride biosynthesis by inhibition of acetyl-CoA carboxylase; decreased cholesterol biosynthesis by inhibition of 3-hydroxy-3-methyglutaryl-coenzyme A reductase
Pharmacodynamics/Kinetics
Absorption: Immediate release: Almost completely; Sustained release: 70%
Distribution: 17 L
Protein binding: 94% to 96%
Half-life elimination: 1-2 hours
Time to peak, serum: Immediate release: 1-2 hours; Sustained release: 3-4 hours
Excretion: Urine (95%); feces (3%)
Like the other fibrates, bezafibrate is an agonist of PPARα; some studies suggest it may have some activity on PPARγ and PPARδ as well.
Dosage
Oral: Adults:
Immediate release: 200 mg 2-3 times/day; may reduce to 200 mg twice daily in patients with good response
Sustained release: 400 mg once daily
Dosing interval in renal impairment:
Clcr >60 mL/minute: 200 mg 3 times/day
Clcr 60-40 mL/minute: 200 mg 2 times/day
Clcr 40-15 mL/minute: 200 mg every 1-2 days
Clcr<15 mL/minute: 200 mg every 3 days
Hemodialysis: 200 mg every 3 days
Monitoring Parameters
Periodic evaluation of serum lipids, cholesterol, and triglycerides (especially in the first few months of therapy). LFTs after 3-6 months; then annually. CBC (periodically during the first 12 months). Fasting glucose, creatinine, and CPK periodically.
Test Interactions
Glucose, creatinine, ALT, CGT, and CPK
Dietary Considerations
Should be taken with or after meals. Before initiation of therapy, patients should be placed on a standard lipid-lowering diet for 6 weeks and the diet should be continued during drug therapy.
Patient Education
Comply exactly to the terms of the prescription; do not change the dose or stop prescription without your prescriber's advice. Medication is meant to supplement an appropriate diet. Inform prescriber if you suffer from liver or kidney disease or are taking other medications (especially warfarin or cyclosporine). Swallow tablets without chewing and with sufficient fluid, with or after meals. If you are also taking cholestyramine or a bile acid resin, separate doses by at least 2 hours. Call prescriber if you experience abdominal pain, constipation, diarrhea, nausea, headache, dizziness, skin reactions, muscular pain or cramps, and fatigue. You will need regular check-ups and laboratory monitoring as recommended by your prescriber. Pregnancy / breast-feeding precautions: Inform prescriber if you are or intend to become pregnant. Do not breast-feed.
Nursing Implications
Monitor serum lipids, LFTs, CBC
Dental Health: Effects on Dental Treatment
No significant effects or complications reported
Dental Health: Vasoconstrictor/Local Anesthetic Precautions No information available to require special precautions
Mental Health: Effects on Mental Status
May cause dizziness or insomnia
Mental Health: Effects on Psychiatric Treatment Contraindicated with MAO inhibitors
Available Dosage Forms
Tablet, immediate release: 200 mg
Tablet, sustained release: 400 mg
Side-effects
The main toxicity is hepatic (abnormal liver enzymes), and myopathy and rarely rhabdomyolysis have been reported.
Other uses
The Australian biotech company Giaconda combines bezafibrate with chenodeoxycholic acid in an anti-hepatitis C drug combination called Hepaconda.
International Brand Names
Azufibrat® (DE); Befibrat® (DE); Béfizal® (FR); Beza 1A Pharma® (DE); Beza AbZ® (DE); Bezabeta® (DE); Bezacur® (AR, DE, LU); Bezacur® Retard (CL); Bezadoc® (DE); Bezafibrat 1A Pharma® (AT); Bezafibrat AL® (DE); Bezafibrat Arcana® (AT); Bezafibrate® (GB, ID, IL); Bezafibrat Genericon® (AT); Bezafibrat Heumann® (DE); Bezafibrat Lannacher® (AT); Bezafibrato Genfar® (EC); Bezafibrat PB® (DE); Bezafibrat Ratiopharm® (AT); Bezafibrat-ratiopharm® (DE); Bezafibrat Sandoz® (DE); Bezafibrat Stada® (DE); Bezafibrat Stada Retard® (SG); bezafibrat von ct® (DE); Bezafisal® (MX); Bezagamma® (DE); Bezagen® (GB); Bezalip® (AT, BE, BR, CA, CH, CO, CR, CZ, DE, DO, EC, ES, FI, FR, GB, GT, HK, HU, ID, IN, IT, JO, JP, LU, MX, NL, NZ, PA, PH, SE, SG, SV, TH, TW, ZA); Bezalip® Retard (AT, IL, PL, RO, SE, SG, TH); Bezamerck® (DE); Bezamidin® (PL); Bezamil® (TH); Bezapharm® (DE); Bezastad® (AT); Bezatol® (JP); Cedur® (BE, BR, CH, DE, LU); Cholestenorm® (RU); Decolest® (RO); Difaterol® (ES); Elpi Lip® (AR); Eulitop® (BE, ES); Fibalip® (NZ); Hadiel® (IT); Lacromid® (CY); Liparol® (GB); Lipox® (DE); Nebufurd Retard® (AR); Nimus® (CL); Norlip® (IL); Oralipin® (CL); Oralipin Retard® (CL); PMS-Bezafibrate (CA); Polyzalip® (TH); Raset® (TH); Reducterol® (ES); Regadrin B® (CZ, DE, RO); Rolab-Bezafibrate® (ZA); Sklerofibrat® (DE); Solibay® (MX); Verbital® (RO); Zafibral® (SG); Zimbacol® (GB)
References
- ↑ Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) study. Circulation 2000;102:21-7. PMID 10880410.
- ↑ Tenenbaum A, Motro M, Fisman EZ, Tanne D, Boyko V, Behar S. Bezafibrate for the secondary prevention of myocardial infarction in patients with metabolic syndrome. Arch Intern Med 2005;165:1154-60. PMID 15911729.
- ↑ Tenenbaum A, Motro M, Fisman EZ, Schwammenthal E, Adler Y, Goldenberg I, Leor J, Boyko V, Mandelzweig L, Behar S. Peroxisome proliferator-activated receptor ligand bezafibrate for prevention of type 2 diabetes mellitus in patients with coronary artery disease. Circulation 2004;109:2197-202. PMID 15123532
- ↑ Tenenbaum A, Fisman EZ, Boyko V, Benderly M, Tanne D, Haim M, Matas Z, Motro M, Behar S. Attenuation of progression of insulin resistance in patients with coronary artery disease by bezafibrate. Arch Intern Med 2006;166:737-41. PMID 16606809.
- Pages with script errors
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Drug
- Cardiovascular Drugs
- Fibrates