Aortic stenosis pathophysiology: Difference between revisions
| (77 intermediate revisions by 8 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{| class="infobox" style="float:right;" | |||
|- | |||
| [[File:Siren.gif|30px|link=Aortic stenosis resident survival guide]]|| <br> || <br> | |||
| [[Aortic stenosis resident survival guide|'''Resident'''<br>'''Survival'''<br>'''Guide''']] | |||
|} | |||
{{Aortic stenosis}} | {{Aortic stenosis}} | ||
{{CMG}}; '''Associate Editor(s)-In-Chief:''' [[User:Mohammed Sbeih|Mohammed A. Sbeih, M.D.]] [mailto:msbeih@wikidoc.org], {{LG}} '''Assistant Editor-In-Chief:''' [[Kristin Feeney|Kristin Feeney, B.S.]] [mailto:kfeeney@elon.edu]; [[User:Rim Halaby|Rim Halaby]] | {{CMG}}; '''Associate Editor(s)-In-Chief:''' [[User:Mohammed Sbeih|Mohammed A. Sbeih, M.D.]] [mailto:msbeih@wikidoc.org], {{LG}}; {{USAMA}} , Claudia P. Hochberg, M.D. [mailto:chochber@bidmc.harvard.edu], [[User:Abdarabi|Abdul-Rahman Arabi, M.D.]] [mailto:abdarabi@yahoo.com], [[User:KeriShafer|Keri Shafer, M.D.]] [mailto:kshafer@bidmc.harvard.edu], [[Priyamvada Singh|Priyamvada Singh, MBBS]] [mailto:psingh13579@gmail.com], {{AA}} Cafer Zorkun, M.D., {{AA}} {{MC}}''' Assistant Editor-In-Chief:''' [[Kristin Feeney|Kristin Feeney, B.S.]] [mailto:kfeeney@elon.edu]; [[User:Rim Halaby|Rim Halaby]] | ||
==Overview== | ==Overview== | ||
[[Aortic stenosis]] is the progressive narrowing of the [[aortic valve]]. Calcific aortic stenosis, in particular, is an active atherosclerotic pathology where inflammation, fibrosis and calcification are involved in the progressive narrowing of the effective aortic valve area in the absence of any commissural fusion. In contrast, rheumatic aortic stenosis is due to fusion of the commissures with valvular scarring and calcification. | [[Aortic stenosis]] is the progressive narrowing of the [[aortic valve]]. Calcific aortic stenosis, in particular, is an active atherosclerotic pathology where inflammation, fibrosis, and calcification are involved in the progressive narrowing of the effective aortic valve area in the absence of any commissural fusion. In contrast, rheumatic aortic stenosis is due to the fusion of the commissures with valvular scarring and calcification. [[Aortic stenosis]] causes an impedance to the antegrade blood flow not only at the level of the aortic valve itself but also at the [[subvalvular aortic stenosis|subvalvular]] ([[subvalvular aortic stenosis|below the aortic valve]]) or [[Supravalvular aortic stenosis|supravalvular]] ([[Supravalvular aortic stenosis|above the aortic valve]]) levels. As a result, chronic pressure overload develops in the [[left ventricle]]. The [[left ventricle]] undergoes hypertrophy as an initial adaptive mechanism to overcome the increased afterload. This compensatory mechanism ends up being maladaptive by causing apoptosis of the hypertrophied [[myocyte]]s and subsequent [[heart failure]]. Hence, [[aortic stenosis]] is a progressive valvular disease which progression depends mainly on the degree of the narrowing of the aortic valve as well as on the maladaptive ventricular wall response. Gross anatomy dissection may be used as a diagnostic tool in the evaluation of aortic stenosis. Common findings associated with aortic stenosis include [[left ventricular hypertrophy]] and [[heart block]]. | ||
==Pathophysiology== | ==Pathophysiology== | ||
*Aortic stenosis in the progressive narrowing of the aortic valve.<ref name="pmid27866029">{{cite journal| author=Galli D, Manuguerra R, Monaco R, Manotti L, Goldoni M, Becchi G et al.| title=Understanding the structural features of symptomatic calcific aortic valve stenosis: A broad-spectrum clinicopathologic study in 236 consecutive surgical cases. | journal=Int J Cardiol | year= 2016 | volume= 228 | issue= | pages= 364-374 | pmid=27866029 | doi=10.1016/j.ijcard.2016.11.180 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27866029 }} </ref> | |||
*The decrease in aortic valve area does not cause a change in the antegrade velocity unless the area is decreased by at least half. [[Aortic stenosis]] causes an impedance to the antegrade blood flow not only at the level of the aortic valve itself but also at the [[Subvalvular aortic stenosis|subvalvular]] ([[Subvalvular aortic stenosis|below the aortic valve]]) or [[Supravalvular aortic stenosis|supravalvular]] ([[Supravalvular aortic stenosis|above the aortic valve]]) levels. As a result, chronic pressure overload develops in the left ventricle. As a result, chronic pressure overload develops in the [[left ventricle]].<ref name="pmid27810479">{{cite journal| author=Joseph J, Naqvi SY, Giri J, Goldberg S| title=Aortic stenosis: pathophysiology, diagnosis and therapy. | journal=Am J Med | year= 2016 | volume= | issue= | pages= | pmid=27810479 | doi=10.1016/j.amjmed.2016.10.005 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27810479 }} </ref> | |||
*Slow compensatory mechanisms occur in the heart to adapt to the pressure changes caused by aortic stenosis. The most prominent adaptive mechanism is ventricular hypertrophy which leads early on to diastolic dysfunction and later on to systolic dysfunction.<ref name="pmid25140960">{{cite journal| author=Otto CM, Prendergast B| title=Aortic-valve stenosis--from patients at risk to severe valve obstruction. | journal=N Engl J Med | year= 2014 | volume= 371 | issue= 8 | pages= 744-56 | pmid=25140960 | doi=10.1056/NEJMra1313875 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=25140960 }} </ref><ref name="pmid23062541">{{cite journal| author=Dweck MR, Boon NA, Newby DE| title=Calcific aortic stenosis: a disease of the valve and the myocardium. | journal=J Am Coll Cardiol | year= 2012 | volume= 60 | issue= 19 | pages= 1854-63 | pmid=23062541 | doi=10.1016/j.jacc.2012.02.093 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23062541 }} </ref> | |||
Shown below is an image summarizing the pathophysiology of aortic stenosis: | Shown below is an image summarizing the pathophysiology of aortic stenosis:<ref name="pmid23062541">{{cite journal| author=Dweck MR, Boon NA, Newby DE| title=Calcific aortic stenosis: a disease of the valve and the myocardium. | journal=J Am Coll Cardiol | year= 2012 | volume= 60 | issue= 19 | pages= 1854-63 | pmid=23062541 | doi=10.1016/j.jacc.2012.02.093 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23062541 }} </ref> | ||
[[ Image:Pathophysiology_of_aortic_stenosis.png|center|500px|Pathophysiology of aortic stenosis]] | [[ Image:Pathophysiology_of_aortic_stenosis.png|center|500px|Pathophysiology of aortic stenosis]] | ||
==Associated Conditions== | |||
====Heyde's Syndrome==== | ====Heyde's Syndrome==== | ||
*In [[Heyde's syndrome]], [[aortic stenosis]] is associated with [[angiodysplasia]] of the [[colon (anatomy)|colon]]. | *In [[Heyde's syndrome]], [[aortic stenosis]] is associated with [[angiodysplasia]] of the [[colon (anatomy)|colon]]. Presenting symptoms include:<ref name="pmid23605838">{{cite journal| author=Ledingham D| title=Heyde's syndrome: exploring the link between aortic stenosis and an acquired bleeding disorder. | journal=BMJ Case Rep | year= 2013 | volume= 2013 | issue= | pages= | pmid=23605838 | doi=10.1136/bcr-2013-009306 | pmc=3645074 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23605838 }} </ref> | ||
**[[Hematemesis]] | **[[Hematemesis]] | ||
**[[Melena]] | **[[Melena]] | ||
====Von Willebrand Disease==== | ====Von Willebrand Disease==== | ||
*[[Aortic stenosis]] may result in a form of [[von Willebrand disease]] due to an increased turbulence around the stenosed aortic valve which subsequently triggers the break down of [[coagulation]] [[factor VIII]]-associated antigen, (also called [[von Willebrand factor]]) and thus results in a variant of [[von Willebrand disease]]. | *[[Aortic stenosis]] may result in a form of [[von Willebrand disease]] due to an increased turbulence around the stenosed aortic valve which subsequently triggers the break down of [[coagulation]] [[factor VIII]]-associated antigen, (also called [[von Willebrand factor]]) and thus results in a variant of [[von Willebrand disease]].<ref name="pmid12878741">{{cite journal| author=Vincentelli A, Susen S, Le Tourneau T, Six I, Fabre O, Juthier F et al.| title=Acquired von Willebrand syndrome in aortic stenosis. | journal=N Engl J Med | year= 2003 | volume= 349 | issue= 4 | pages= 343-9 | pmid=12878741 | doi=10.1056/NEJMoa022831 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12878741 }} </ref><ref name="pmid26269004">{{cite journal| author=Tamura T, Horiuchi H, Imai M, Tada T, Shiomi H, Kuroda M et al.| title=Unexpectedly High Prevalence of Acquired von Willebrand Syndrome in Patients with Severe Aortic Stenosis as Evaluated with a Novel Large Multimer Index. | journal=J Atheroscler Thromb | year= 2015 | volume= 22 | issue= 11 | pages= 1115-23 | pmid=26269004 | doi=10.5551/jat.30809 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26269004 }} </ref> | ||
===Narrowing of the Aortic Valve=== | ===Narrowing of the Aortic Valve=== | ||
====Calcific Aortic Stenosis==== | ====Calcific Aortic Stenosis==== | ||
Calcific aortic stenosis has long been thought to be the result of | Calcific aortic stenosis has long been thought to be the result of prolonged wear and tear mechanism and age-related degeneration of the valvular cusps as opposed to cusp fusion of the aortic valve in rheumatic heart disease. However, recent studies have revealed that the underlying pathophysiology of calcific aortic stenosis is active inflammation, fibrosis, and calcification. Genetic predisposition plays a role in the rate of progression of aortic stenosis. The stages of the formation of the calcific aortic stenosis are the following:<ref name="pmid23062541">{{cite journal| author=Dweck MR, Boon NA, Newby DE| title=Calcific aortic stenosis: a disease of the valve and the myocardium. | journal=J Am Coll Cardiol | year= 2012 | volume= 60 | issue= 19 | pages= 1854-63 | pmid=23062541 | doi=10.1016/j.jacc.2012.02.093 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23062541 }} </ref> | ||
*Endothelial Damage: | *Endothelial Damage: | ||
:* Endothelial damage is the initial event starting the cascade of events in calcific aortic stenosis. | :* Endothelial damage is the initial event starting the cascade of events in calcific aortic stenosis. | ||
:* It results from increased mechanical stress and decreased shear stress. | :* It results from increased mechanical stress and decreased shear stress. | ||
| Line 34: | Line 39: | ||
*Inflammation: | *Inflammation: | ||
:* Endothelial damage triggers accumulation of lipids (LDL and lipoprotens) that subsequently undergo oxidative changes. | :* Endothelial damage triggers accumulation of lipids (LDL and lipoprotens) that subsequently undergo oxidative changes. | ||
:* As a result, inflammation is accentuated by: | :* As a result, inflammation is accentuated by: | ||
| Line 44: | Line 50: | ||
*Fibrosis: | *Fibrosis: | ||
:* Inflammation eventually leads to activation of fibroblasts and remodeling of the extracellular matrix<ref name="pmid21123617">{{cite journal| author=Frantz C, Stewart KM, Weaver VM| title=The extracellular matrix at a glance. | journal=J Cell Sci | year= 2010 | volume= 123 | issue= Pt 24 | pages= 4195-200 | pmid=21123617 | doi=10.1242/jcs.023820 | pmc=2995612 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21123617 }} </ref> | |||
*Calcification: | *Calcification: | ||
:* Calcification is induced by long standing [[inflammation]] and [[fibrosis]]. | :* Calcification is induced by long standing [[inflammation]] and [[fibrosis]]. | ||
:* The following disorders of mineral metabolism accelerates calcification: | :* The following disorders of mineral metabolism accelerates calcification:<ref name="pmid23062541">{{cite journal| author=Dweck MR, Boon NA, Newby DE| title=Calcific aortic stenosis: a disease of the valve and the myocardium. | journal=J Am Coll Cardiol | year= 2012 | volume= 60 | issue= 19 | pages= 1854-63 | pmid=23062541 | doi=10.1016/j.jacc.2012.02.093 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23062541 }} </ref><ref name="pmid10480155">{{cite journal| author=Ureña P, Malergue MC, Goldfarb B, Prieur P, Guédon-Rapoud C, Pétrover M| title=Evolutive aortic stenosis in hemodialysis patients: analysis of risk factors. | journal=Nephrologie | year= 1999 | volume= 20 | issue= 4 | pages= 217-25 | pmid=10480155 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10480155 }} </ref><ref name="pmid16118543">{{cite journal| author=Aksoy Y, Yagmur C, Tekin GO, Yagmur J, Topal E, Kekilli E et al.| title=Aortic valve calcification: association with bone mineral density and cardiovascular risk factors. | journal=Coron Artery Dis | year= 2005 | volume= 16 | issue= 6 | pages= 379-83 | pmid=16118543 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16118543 }} </ref><ref name="pmid3578364">{{cite journal| author=Strickberger SA, Schulman SP, Hutchins GM| title=Association of Paget's disease of bone with calcific aortic valve disease. | journal=Am J Med | year= 1987 | volume= 82 | issue= 5 | pages= 953-6 | pmid=3578364 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3578364 }} </ref><ref name="pmid11359741">{{cite journal| author=Ortlepp JR, Hoffmann R, Ohme F, Lauscher J, Bleckmann F, Hanrath P| title=The vitamin D receptor genotype predisposes to the development of calcific aortic valve stenosis. | journal=Heart | year= 2001 | volume= 85 | issue= 6 | pages= 635-8 | pmid=11359741 | doi= | pmc=PMC1729782 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11359741 }} </ref> | ||
:** [[Hemodialysis]] | :**[[Hemodialysis]] | ||
:** [[Osteoporosis]] | :** [[Osteoporosis]] | ||
:** [[Paget's disease]] | :** [[Paget's disease]] | ||
:** [[Vitamin D]] | :** [[Vitamin D]] receptor polymorphism | ||
:* [[Calcification]] starts at the base of the cusps and then reaches the leaflets. Calcification results in a decrease in the leflet motion and hence decrease in the effective valve area in the absence of any fusion of the commissures.<ref name="pmid18820172">{{cite journal| author=Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD et al.| title=2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. | journal=Circulation | year= 2008 | volume= 118 | issue= 15 | pages= e523-661 | pmid=18820172 | doi=10.1161/CIRCULATIONAHA.108.190748 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18820172 }} </ref> | :* [[Calcification]] starts at the base of the cusps and then reaches the leaflets. Calcification results in a decrease in the leflet motion and hence decrease in the effective valve area in the absence of any fusion of the commissures.<ref name="pmid18820172">{{cite journal| author=Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD et al.| title=2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. | journal=Circulation | year= 2008 | volume= 118 | issue= 15 | pages= e523-661 | pmid=18820172 | doi=10.1161/CIRCULATIONAHA.108.190748 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18820172 }} </ref> | ||
:*Calcification is coordinated by osteoblasts in the context of a highly regulated mechanism similar to bone formation.<ref name="pmid23062541">{{cite journal| author=Dweck MR, Boon NA, Newby DE| title=Calcific aortic stenosis: a disease of the valve and the myocardium. | journal=J Am Coll Cardiol | year= 2012 | volume= 60 | issue= 19 | pages= 1854-63 | pmid=23062541 | doi=10.1016/j.jacc.2012.02.093 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23062541 }} </ref> | :*Calcification is coordinated by osteoblasts in the context of a highly regulated mechanism similar to bone formation.<ref name="pmid23062541">{{cite journal| author=Dweck MR, Boon NA, Newby DE| title=Calcific aortic stenosis: a disease of the valve and the myocardium. | journal=J Am Coll Cardiol | year= 2012 | volume= 60 | issue= 19 | pages= 1854-63 | pmid=23062541 | doi=10.1016/j.jacc.2012.02.093 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23062541 }} </ref> | ||
====Rheumatic Aortic Stenosis==== | ====Rheumatic Aortic Stenosis==== | ||
* | *[[Rheumatic aortic stenosis]] is due to the fusion of the valve commissures and subsequent scarring and calcification. Rheumatic aortic stenosis is always accompanied by [[mitral regurgitation]].<ref name="pmid18820172">{{cite journal| author=Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD et al.| title=2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. | journal=Circulation | year= 2008 | volume= 118 | issue= 15 | pages= e523-661 | pmid=18820172 | doi=10.1161/CIRCULATIONAHA.108.190748 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18820172 }} </ref> | ||
* [[Rheumatic fever]] which depends on a prior autoimmunological stimulation, is a systemic disease affecting the peri-arteriolar connective tissue and can occur after an untreated Group A streptococcal pharyngeal infection. It is believed to be caused by antibody [[cross-reactivity]] which is a [[Hypersensitivity#Type 2 - antibody-dependent| | * [[Rheumatic fever]] which depends on a prior autoimmunological stimulation, is a systemic disease affecting the peri-arteriolar connective tissue and can occur after an untreated Group A streptococcal pharyngeal infection. It is believed to be caused by antibody [[cross-reactivity]] which is a [[Hypersensitivity#Type 2 - antibody-dependent|type II hypersensitivity reaction]] and is termed 'molecular mimicry'. | ||
* Chronic [[rheumatic heart disease]] is characterized by repeated inflammation with fibrinous resolution. The cardinal anatomic changes of the valve include leaflet thickening, commissural fusion and shortening and thickening of the tendinous cords. Rheumatic heart disease | * Chronic [[rheumatic heart disease]] is characterized by repeated inflammation with fibrinous resolution. The cardinal anatomic changes of the valve include leaflet thickening, commissural fusion and shortening and thickening of the tendinous cords.<ref name="pmid23703332">{{cite journal| author=Kumar RK, Tandon R| title=Rheumatic fever & rheumatic heart disease: the last 50 years. | journal=Indian J Med Res | year= 2013 | volume= 137 | issue= 4 | pages= 643-58 | pmid=23703332 | doi= | pmc=3724245 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23703332 }} </ref>Rheumatic heart disease may lead to up to 99% stenosis of the [[aortic valve]] often resulting in a “fish mouth” gross appearance. | ||
====Congenital Aortic Stenosis==== | ====Congenital Aortic Stenosis==== | ||
Congenital malformation of the [[aortic valve]] is one of the less common causes of [[aortic stenosis]]. Congenital [[aortic stenosis]] is detected in young adults. | Congenital malformation of the [[aortic valve]] is one of the less common causes of [[aortic stenosis]].<ref name="pmid22685681">{{cite journal| author=Mordi I, Tzemos N| title=Bicuspid aortic valve disease: a comprehensive review. | journal=Cardiol Res Pract | year= 2012 | volume= 2012 | issue= | pages= 196037 | pmid=22685681 | doi=10.1155/2012/196037 | pmc=3368178 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=22685681 }} </ref>Congenital [[aortic stenosis]] is detected in young adults. | ||
===Left Ventricular Hypertrophy=== | ===Left Ventricular Hypertrophy=== | ||
* Long-standing [[aortic stenosis]] exposes the left ventricle to prolonged pressure overload which leads to [[left ventricular hypertrophy|concentric hypertrophy]] as a compensatory mechanism to preserve left ventricular function.<ref name="pmid129304">{{cite journal |author=Sasayama S, Ross J, Franklin D, Bloor CM, Bishop S, Dilley RB |title=Adaptations of the left ventricle to chronic pressure overload |journal=[[Circulation Research]] |volume=38 |issue=3 |pages=172–8 |year=1976 |month=March |pmid=129304 |doi= |url=http://circres.ahajournals.org/cgi/pmidlookup?view=long&pmid=129304 |accessdate=2012-04-10}}</ref><ref name="pmid155986">{{cite journal |author=Gaasch WH |title=Left ventricular radius to wall thickness ratio |journal=[[The American Journal of Cardiology]] |volume=43 |issue=6 |pages=1189–94 |year=1979 |month=June |pmid=155986 |doi= |url= |accessdate=2012-04-10}}</ref><ref name="pmid6446989">{{cite journal |author=Spann JF, Bove AA, Natarajan G, Kreulen T |title=Ventricular performance, pump function and compensatory mechanisms in patients with aortic stenosis |journal=[[Circulation]] |volume=62 |issue=3 |pages=576–82 |year=1980 |month=September |pmid=6446989 |doi= |url=http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=6446989 |accessdate=2012-04-10}}</ref> The left ventricular wall increases in thickness (i.e. [[concentric hypertrophy]] occurs) as a result of the parallel replication of the [[sarcomeres]] | * Long-standing [[aortic stenosis]] exposes the left ventricle to prolonged pressure overload which leads to [[left ventricular hypertrophy|concentric hypertrophy]] as a compensatory mechanism to preserve left ventricular function.<ref name="pmid129304">{{cite journal |author=Sasayama S, Ross J, Franklin D, Bloor CM, Bishop S, Dilley RB |title=Adaptations of the left ventricle to chronic pressure overload |journal=[[Circulation Research]] |volume=38 |issue=3 |pages=172–8 |year=1976 |month=March |pmid=129304 |doi= |url=http://circres.ahajournals.org/cgi/pmidlookup?view=long&pmid=129304 |accessdate=2012-04-10}}</ref><ref name="pmid155986">{{cite journal |author=Gaasch WH |title=Left ventricular radius to wall thickness ratio |journal=[[The American Journal of Cardiology]] |volume=43 |issue=6 |pages=1189–94 |year=1979 |month=June |pmid=155986 |doi= |url= |accessdate=2012-04-10}}</ref><ref name="pmid6446989">{{cite journal |author=Spann JF, Bove AA, Natarajan G, Kreulen T |title=Ventricular performance, pump function and compensatory mechanisms in patients with aortic stenosis |journal=[[Circulation]] |volume=62 |issue=3 |pages=576–82 |year=1980 |month=September |pmid=6446989 |doi= |url=http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=6446989 |accessdate=2012-04-10}}</ref> The left ventricular wall increases in thickness (i.e. [[concentric hypertrophy]] occurs) as a result of the parallel replication of the [[sarcomeres]] | ||
*However, [[left ventricle]] hypertrophy ends up being a maladpative mechanism and a marker of a bad prognosis. Hypertrophied myocardial cells undergo apoptosis at a rate larger to that of regeneration.<ref name="pmid23062541">{{cite journal| author=Dweck MR, Boon NA, Newby DE| title=Calcific aortic stenosis: a disease of the valve and the myocardium. | journal=J Am Coll Cardiol | year= 2012 | volume= 60 | issue= 19 | pages= 1854-63 | pmid=23062541 | doi=10.1016/j.jacc.2012.02.093 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23062541 }} </ref> | *However, [[left ventricle]] hypertrophy ends up being a maladpative mechanism and a marker of a bad prognosis. Hypertrophied myocardial cells undergo apoptosis at a rate larger to that of regeneration.<ref name="pmid23062541">{{cite journal| author=Dweck MR, Boon NA, Newby DE| title=Calcific aortic stenosis: a disease of the valve and the myocardium. | journal=J Am Coll Cardiol | year= 2012 | volume= 60 | issue= 19 | pages= 1854-63 | pmid=23062541 | doi=10.1016/j.jacc.2012.02.093 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23062541 }} </ref> In fact, angiotensin II promotes the apoptosis of the hypertrophied vessels, thus angiotensin receptor blockers (ARB) paly a role in the treatment of heart failure secondary to aortic stenosis even without having a great role in decreasing the blood pressure.<ref name="pmid11799082">{{cite journal| author=González A, López B, Ravassa S, Querejeta R, Larman M, Díez J et al.| title=Stimulation of cardiac apoptosis in essential hypertension: potential role of angiotensin II. | journal=Hypertension | year= 2002 | volume= 39 | issue= 1 | pages= 75-80 | pmid=11799082 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11799082 }} </ref> | ||
* Left ventricular hypertrophy weakly relates to the degree of stenosis itself but rather to advanced age, male sex and obesity. Other factors that participate in the [[hypertrophy]] of the left ventricle are [[hypertension]] and increased [[arterial stiffness]]. | * Left ventricular hypertrophy weakly relates to the degree of stenosis itself but rather to advanced age, male sex and obesity. Other factors that participate in the [[hypertrophy]] of the left ventricle are [[hypertension]] and increased [[arterial stiffness]]. | ||
* Fibrosis is an integral component of the hypertrophy.<ref name="pmid23062541">{{cite journal| author=Dweck MR, Boon NA, Newby DE| title=Calcific aortic stenosis: a disease of the valve and the myocardium. | journal=J Am Coll Cardiol | year= 2012 | volume= 60 | issue= 19 | pages= 1854-63 | pmid=23062541 | doi=10.1016/j.jacc.2012.02.093 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23062541 }} </ref> | * Fibrosis is an integral component of the myocardial hypertrophy.<ref name="pmid23062541">{{cite journal| author=Dweck MR, Boon NA, Newby DE| title=Calcific aortic stenosis: a disease of the valve and the myocardium. | journal=J Am Coll Cardiol | year= 2012 | volume= 60 | issue= 19 | pages= 1854-63 | pmid=23062541 | doi=10.1016/j.jacc.2012.02.093 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23062541 }} </ref> | ||
===Early Diastolic Dysfunction=== | ===Early Diastolic Dysfunction=== | ||
During the initial period of [[concentric hypertrophy]], the left ventricle is not dilated and there is preservation of the left ventricular systolic function. Diastolic function, however, may be reduced due to a reduction in diastolic compliance. <ref name="pmid136186">{{cite journal |author=Gaasch WH, Levine HJ, Quinones MA, Alexander JK |title=Left ventricular compliance: mechanisms and clinical implications |journal=[[The American Journal of Cardiology]] |volume=38 |issue=5 |pages=645–53 |year=1976 |month=November |pmid=136186 |doi= |url= | During the initial period of [[concentric hypertrophy]], the left ventricle is not dilated and there is preservation of the left ventricular systolic function. Diastolic function, however, may be reduced due to a reduction in diastolic compliance.<ref name="pmid136186">{{cite journal |author=Gaasch WH, Levine HJ, Quinones MA, Alexander JK |title=Left ventricular compliance: mechanisms and clinical implications |journal=[[The American Journal of Cardiology]] |volume=38 |issue=5 |pages=645–53 |year=1976 |month=November |pmid=136186 |doi= |url= |accessdate=2012-04-10}}</ref><ref name="pmid">{{cite journal |author=Hess OM, Ritter M, Schneider J, Grimm J, Turina M, Krayenbuehl HP |title=Diastolic stiffness and myocardial structure in aortic valve disease before and after valve replacement |journal=[[]] |volume= |issue= |pages=855–65 |year=1984 |month=May |pmid= |doi= |url=http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=6231136 |accessdate=2012-04-10}}</ref><ref name="pmid8151903">{{cite journal |author=Gaasch WH |title=Diagnosis and treatment of heart failure based on left ventricular systolic or diastolic dysfunction |journal=[[JAMA : the Journal of the American Medical Association]] |volume=271 |issue=16 |pages=1276–80 |year=1994 |month=April |pmid=8151903 |doi= |url= |accessdate=2012-04-10}}</ref> | ||
This [[diastolic dysfunction]] may in turn lead to a rise in [[pulmonary capillary wedge pressure]] and consequently lead to [[dyspnea]]. | This [[diastolic dysfunction]] may in turn lead to a rise in [[pulmonary capillary wedge pressure]] and consequently lead to [[dyspnea]]. [[Cardiac output]] may also be reduced as a result of [[diastolic dysfunction]] and impaired filling of the [[left ventricle]]. Early in the course of aortic stenosis, there may be a failure to augment [[cardiac output]] during exercise resulting in [[dyspnea on exertion]]. | ||
===Late Systolic Dysfunction=== | ===Late Systolic Dysfunction=== | ||
| Line 86: | Line 92: | ||
====Myocardial Fibrosis==== | ====Myocardial Fibrosis==== | ||
Myocardial scarring or fibrosis may develop with prolonged aortic stenosis, probably due to chronic [[subendocardial ischemia]] or increased wall stress. | Myocardial scarring or fibrosis may develop with prolonged aortic stenosis, probably due to chronic [[subendocardial ischemia]] or increased wall stress. <ref name="pmid21762938">{{cite journal| author=Yarbrough WM, Mukherjee R, Ikonomidis JS, Zile MR, Spinale FG| title=Myocardial remodeling with aortic stenosis and after aortic valve replacement: mechanisms and future prognostic implications. | journal=J Thorac Cardiovasc Surg | year= 2012 | volume= 143 | issue= 3 | pages= 656-64 | pmid=21762938 | doi=10.1016/j.jtcvs.2011.04.044 | pmc=3210937 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21762938 }} </ref> | ||
====Dyssynchronous Contraction==== | ====Dyssynchronous Contraction==== | ||
| Line 92: | Line 98: | ||
===Atrial Fibrillation=== | ===Atrial Fibrillation=== | ||
The stiff non-compliant left ventricle can become increasingly dependent on the [[left atrium]] for filling. The presence of atrial fibrillation and the loss of atrial contractility can result in reduced left ventricular filling and reduced [[cardiac output]]. | The stiff non-compliant left ventricle can become increasingly dependent on the [[left atrium]] for filling, which predisposes to [[atrial fibrillation]]. The presence of [[atrial fibrillation]] and the associated loss of atrial contractility can result in reduced left ventricular filling and reduced [[cardiac output]].<ref name="pmid23006976">{{cite journal| author=Burup Kristensen C, Jensen JS, Sogaard P, Carstensen HG, Mogelvang R| title=Atrial fibrillation in aortic stenosis--echocardiographic assessment and prognostic importance. | journal=Cardiovasc Ultrasound | year= 2012 | volume= 10 | issue= | pages= 38 | pmid=23006976 | doi=10.1186/1476-7120-10-38 | pmc=3517318 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23006976 }} </ref> | ||
==Hemodynamics of Aortic Stenosis== | ==Hemodynamics of Aortic Stenosis== | ||
===Pressure Gradient and Valve Area=== | ===Pressure Gradient and Valve Area=== | ||
When the aortic valve becomes stenosed, it can result in the formation of a pressure gradient between the [[left ventricle]] ([[LV]]) and the [[aorta]].<ref name=Lilly>{{cite book | author = Lilly LS (editor) | title = Pathophysiology of Heart Disease | edition = 3rd ed. | publisher = Lippincott Williams & Wilkins | year = 2003 | id = ISBN 0-7817-4027-4 }}</ref> The more constricted the valve, the bigger the gradient between the [[LV]] and the [[aorta]]. | When the aortic valve becomes stenosed, it can result in the formation of a pressure gradient between the [[left ventricle]] ([[LV]]) and the [[aorta]].<ref name="Lilly">{{cite book | author = Lilly LS (editor) | title = Pathophysiology of Heart Disease | edition = 3rd ed. | publisher = Lippincott Williams & Wilkins | year = 2003 | id = ISBN 0-7817-4027-4 }}</ref> The more constricted the valve is, the bigger the gradient between the [[LV]] and the [[aorta]] is. | ||
For instance, the pressure gradient in patients with [[mild AS]] might be 20 [[mmHg]]. This means that, at peak systole, while the [[LV]] may generate a pressure of 140 mmHg, the pressure that is transmitted into the aorta will only be 120 mmHg. Therefore, while a [[Sphygmomanometer|blood pressure cuff]] may measure a normal [[systolic blood pressure]] the actual pressure generated by and inside the [[LV]] would be considerably higher. As the left ventricle fails, it may no longer be able to mount the contractility necessary to generate a large gradient across the aortic valve. | For instance, the pressure gradient in patients with [[mild AS]] might be 20 [[mmHg]]. This means that, at peak systole, while the [[LV]] may generate a pressure of 140 mmHg, the pressure that is transmitted into the aorta will only be 120 mmHg. Therefore, while a [[Sphygmomanometer|blood pressure cuff]] may measure a normal [[systolic blood pressure]] the actual pressure generated by and inside the [[LV]] would be considerably higher. As the left ventricle fails, it may no longer be able to mount the contractility necessary to generate a large gradient across the aortic valve. | ||
Therefore, the absence of a large gradient across the aortic valve does not exclude the presence of critical aortic stenosis. The presence of a [[Intravascular pressure gradient|low gradient]] and a [[ejection fraction|low ejection]] results in a [[ | Therefore, the absence of a large gradient across the aortic valve does not exclude the presence of critical aortic stenosis. The presence of a [[Intravascular pressure gradient|low gradient]] and a [[ejection fraction|low ejection]] results in a [[low flow aortic stenosis]]. It is for this reason that the best measure of the severity of aortic stenosis is the [[aortic valve area]] and not the aortic valve gradient. | ||
Read in detail by clicking on the topics: | Read in detail by clicking on the topics: | ||
| Line 110: | Line 116: | ||
===Flow Velocity=== | ===Flow Velocity=== | ||
If the left ventricular function and contractility are preserved, a flow velocity across the stenosed valve of at least 2.6 m/sec is deemed consistent with aortic stenosis. This is based on [[echocardiographic]] estimation of the aortic jet velocity, the [[aortic valve area]] and the mean transvalvular gradient. The aortic valve becomes calcified in [[aortic sclerosis|aortic valve sclerosis]] (not [[aortic stenosis|stenosis]]); however, the aortic jet velocity is ≤ 2.5 m/sec (without a significant [[Intravascular pressure gradient|gradient]]). [[Aortic valve sclerosis]] is commonly characterized by a focal thickening of the aortic cusps with calcific nodules generally at the base of leaflets and a transvalvular velocity within the normal range (Vmax <2 m/s). Until few years ago, [[aortic valve sclerosis]] was considered to be a physiologic process related to aging without any clinical relevance. However, [[aortic valve sclerosis]] is not observed in about 50% of people over 80 years old. Furthermore, several experimental and clinical studies have demonstrated that aortic valve sclerosis could represent an active phenomenon significantly related to the risk factors of [[atherosclerosis]].<ref name="pmid11827633">{{cite journal| author=Branch KR, O'Brien KD, Otto CM| title=Aortic valve sclerosis as a marker of active atherosclerosis. | journal=Curr Cardiol Rep | year= 2002 | volume= 4 | issue= 2 | pages= 111-7 | pmid=11827633 | doi= | pmc= | url= }} </ref><ref name=> {{Faggiano P, D'Aloia A, Antonini-Canterin F, Pinamonti B, DiLenarda A, Brentana L, Metra M, Nodari S, Dei Cas L. Usefulness of cardiac calcification on two-dimensional echocardiography for distinguishing ischemic from nonischemic dilated cardiomyopathy: a preliminary report. J Cardiovasc Med. 2006.}}</ref> | If the left ventricular function and contractility are preserved, a flow velocity across the stenosed valve of at least 2.6 m/sec is deemed consistent with aortic stenosis. This is based on the [[echocardiographic]] estimation of the aortic jet velocity, the [[aortic valve area]] and the mean transvalvular gradient. The aortic valve becomes calcified in [[aortic sclerosis|aortic valve sclerosis]] (not [[aortic stenosis|stenosis]]); however, the aortic jet velocity is ≤ 2.5 m/sec (without a significant [[Intravascular pressure gradient|gradient]]). [[Aortic valve sclerosis]] is commonly characterized by a focal thickening of the aortic cusps with calcific nodules generally at the base of leaflets and a transvalvular velocity within the normal range (Vmax <2 m/s). Until a few years ago, [[aortic valve sclerosis]] was considered to be a physiologic process related to aging without any clinical relevance. However, [[aortic valve sclerosis]] is not observed in about 50% of people over 80 years old. Furthermore, several experimental and clinical studies have demonstrated that aortic valve sclerosis could represent an active phenomenon significantly related to the risk factors of [[atherosclerosis]].<ref name="pmid11827633">{{cite journal| author=Branch KR, O'Brien KD, Otto CM| title=Aortic valve sclerosis as a marker of active atherosclerosis. | journal=Curr Cardiol Rep | year= 2002 | volume= 4 | issue= 2 | pages= 111-7 | pmid=11827633 | doi= | pmc= | url= }} </ref><ref name=""> <nowiki>{{Faggiano P, D'Aloia A, Antonini-Canterin F, Pinamonti B, DiLenarda A, Brentana L, Metra M, Nodari S, Dei Cas L. Usefulness of cardiac calcification on two-dimensional echocardiography for distinguishing ischemic from nonischemic dilated cardiomyopathy: a preliminary report. J Cardiovasc Med. 2006.}}</nowiki></ref> | ||
===Relationship of Hemodynamic Severity to Symptoms of Aortic Stenosis=== | ===Relationship of Hemodynamic Severity to Symptoms of Aortic Stenosis=== | ||
*Patients with [[aortic stenosis]] become symptomatic when: | *Patients with [[aortic stenosis]] become symptomatic when:<ref name="pmid18848134">{{cite journal| author=Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD et al.| title=2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. | journal=J Am Coll Cardiol | year= 2008 | volume= 52 | issue= 13 | pages= e1-142 | pmid=18848134 | doi=10.1016/j.jacc.2008.05.007 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18848134 }} </ref><ref name="pmid23474606">{{cite journal| author=Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H et al.| title=[Guidelines on the management of valvular heart disease (version 2012). The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)]. | journal=G Ital Cardiol (Rome) | year= 2013 | volume= 14 | issue= 3 | pages= 167-214 | pmid=23474606 | doi=10.1714/1234.13659 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23474606 }} </ref> | ||
:*The [[Aortic valve area calculation|valve area]] is less than 1.0 cm<sup>2</sup>. | :*The [[Aortic valve area calculation|valve area]] is less than 1.0 cm<sup>2</sup>. | ||
:*The jet velocity is over 4.0 m/sec. | :*The jet velocity is over 4.0 m/sec. | ||
:*Mean transvalvular pressure gradient exceeds 40 mm Hg. | :*Mean transvalvular pressure gradient exceeds 40 mm Hg. | ||
*However, many patients develop symptoms only when more severe valve obstruction is present, other patients become symptomatic at less severe degree of stenosis, particularly if there is coexisting [[aortic regurgitation]]. | *However, many patients develop symptoms only when more severe valve obstruction is present, other patients become symptomatic at a less severe degree of stenosis, particularly if there is coexisting [[aortic regurgitation]]. | ||
====ACC/AHA Guidelines - Severity Classification <ref name="pmid18848134">{{cite journal| author=Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD et al.| title=2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. | journal=J Am Coll Cardiol | year= 2008 | volume= 52 | issue= 13 | pages= e1-142 | pmid=18848134 | doi=10.1016/j.jacc.2008.05.007 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18848134 }} </ref>==== | ====ACC/AHA Guidelines - Severity Classification <ref name="pmid18848134">{{cite journal| author=Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD et al.| title=2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. | journal=J Am Coll Cardiol | year= 2008 | volume= 52 | issue= 13 | pages= e1-142 | pmid=18848134 | doi=10.1016/j.jacc.2008.05.007 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18848134 }} </ref>==== | ||
| Line 135: | Line 142: | ||
|} | |} | ||
<sup>†</sup> ''Valve gradients are flow dependent. They should be assessed with knowledge of [[cardiac output]] or antegrade flow across the valve when used to estimate of severity of valve stenosis.'' | <sup>†</sup> ''Valve gradients are flow-dependent. They should be assessed with knowledge of [[cardiac output]] or antegrade flow across the valve when used to estimate of the severity of valve stenosis.'' | ||
==Low Flow, Low Gradient Aortic Stenosis== | ==Low Flow, Low Gradient Aortic Stenosis== | ||
*In aortic stenosis, as the aortic orifice area decreases the transvalvular gradient increases. In fact, when the aortic valve effective orifice area decreases below 1 cm<sup>2</sup> the mean transvalvular gradient is expected to be larger than 40 mm Hg. The transvalvular gradient is highly | *In aortic stenosis, as the aortic orifice area decreases the transvalvular gradient increases. In fact, when the aortic valve effective orifice area decreases below 1.0 cm<sup>2</sup> the mean transvalvular gradient is expected to be larger than 40 mm Hg. The transvalvular gradient is highly dependent on the flow of blood through the valve. | ||
* However, when severe systolic or/and diastolic myocardial dysfunction coexist with the aortic stenosis, there is a decrease in the flow through the valve leading to a prominent decrease in the transvalvular gradient. This is called [[low flow, low gradient aortic stenosis]] ( | * However, when severe systolic or/and diastolic myocardial dysfunction coexist with the aortic stenosis, there is a decrease in the flow through the valve leading to a prominent decrease in the transvalvular gradient. This is called [[low flow, low gradient aortic stenosis]] (LFLG AS). | ||
* | * LFLG AS is a challenging diagnosis that must be done in order to tailor the management plan. It is important to recognize these entities because they might cause either underestimation or overestimation of the degree of severity of the aortic stenosis. | ||
* Two various scenarios occur in the setting of | * Two various scenarios occur in the setting of LFLG AS depending on the status of the left ventricular ejection fraction: | ||
** | **LFLG AS with low LVEF | ||
** | **LFLG AS with normal LVEF<ref name="pmid23062546">{{cite journal| author=Pibarot P, Dumesnil JG| title=Low-flow, low-gradient aortic stenosis with normal and depressed left ventricular ejection fraction. | journal=J Am Coll Cardiol | year= 2012 | volume= 60 | issue= 19 | pages= 1845-53 | pmid=23062546 | doi=10.1016/j.jacc.2012.06.051 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23062546 }} </ref> | ||
Shown below is a table depicting the differences between | Shown below is a table depicting the differences between LFLG AS with low LVEF and LFLG AS with normal LVEF: | ||
{|class="wikitable" border="1" | {|class="wikitable" border="1" | ||
|- align="center" | |- align="center" | ||
|''' | ! align="center" style="background:#4479BA; color: #FFFFFF;" + |'''LFLG AS''' | ||
|'''Percentage of the Cases of Severe AS Caused by | ! align="center" style="background:#4479BA; color: #FFFFFF;" + |'''Percentage of the Cases of Severe AS Caused by LFLG AS''' | ||
|'''Pathophysiology''' | ! align="center" style="background:#4479BA; color: #FFFFFF;" + |'''Pathophysiology''' | ||
|'''Diagnostic Challenges''' | ! align="center" style="background:#4479BA; color: #FFFFFF;" + |'''Diagnostic Challenges''' | ||
|- align="center" | |- align="center" | ||
| | |LFLG AS with low LVEF | ||
|5-10% of severe aortic stenosis | |5-10% of severe aortic stenosis | ||
|Decreased systolic function: | |Decreased systolic function: | ||
| Line 162: | Line 169: | ||
|Differentiation between severe aortic stenosis and pseudo-severe aortic stenosis | |Differentiation between severe aortic stenosis and pseudo-severe aortic stenosis | ||
|- align="center" | |- align="center" | ||
| | |LFLG AS with normal LVEF | ||
|10-25% of severe aortic stenosis | |10-25% of severe aortic stenosis | ||
|Decreased diastolic function: | |Decreased diastolic function: | ||
| Line 174: | Line 181: | ||
===Low Flow, Low Gradient Aortic Stenosis with Low Ejection Fraction=== | ===Low Flow, Low Gradient Aortic Stenosis with Low Ejection Fraction=== | ||
*When ventricular [[systolic dysfunction]] is present, the [[myocardium]] can not contract strongly enough to pump blood with a lot of pressure. In that case, low flow and subsequent low transvalvular gradient are present and this entity is called | *When ventricular [[systolic dysfunction]] is present, the [[myocardium]] can not contract strongly enough to pump blood with a lot of pressure. In that case, low flow and subsequent low transvalvular gradient are present and this entity is called LFLG AS with low LVEF. | ||
* | *LFLG AS with low LVEF is defined as:<ref name="pmid18848134">{{cite journal| author=Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD et al.| title=2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. | journal=J Am Coll Cardiol | year= 2008 | volume= 52 | issue= 13 | pages= e1-142 | pmid=18848134 | doi=10.1016/j.jacc.2008.05.007 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18848134 }} </ref><ref name="pmid23474606">{{cite journal| author=Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H et al.| title=[Guidelines on the management of valvular heart disease (version 2012). The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)]. | journal=G Ital Cardiol (Rome) | year= 2013 | volume= 14 | issue= 3 | pages= 167-214 | pmid=23474606 | doi=10.1714/1234.13659 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23474606 }} </ref><ref name="pmid23062546">{{cite journal| author=Pibarot P, Dumesnil JG| title=Low-flow, low-gradient aortic stenosis with normal and depressed left ventricular ejection fraction. | journal=J Am Coll Cardiol | year= 2012 | volume= 60 | issue= 19 | pages= 1845-53 | pmid=23062546 | doi=10.1016/j.jacc.2012.06.051 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23062546 }} </ref> | ||
#An [[aortic valve area|aortic valve areas]] < 1.0 cm<sup>2</sup> | #An [[aortic valve area|aortic valve areas]] < 1.0 cm<sup>2</sup> | ||
#A [[ejection fraction|left ventricular ejection fraction]] < 40% | #A [[ejection fraction|left ventricular ejection fraction]] < 40% | ||
#A [[Intravascular pressure gradient|mean pressure difference or gradient]] across the aortic valve of < 30 mm Hg | #A [[Intravascular pressure gradient|mean pressure difference or gradient]] across the aortic valve of < 30 mm Hg | ||
*When low flow, low gradient aortic stenosis is present, the challenge is to differentiate whether the | *When low flow, low gradient aortic stenosis is present, the challenge is to differentiate whether the LFLG AS with low LVEF is a true severe aortic stenosis or a pseudo-severe aortic stenosis. It is very important to differentiate between these two entities as they have different outcomes following aortic valve replacement.<ref name="pmid23062546">{{cite journal| author=Pibarot P, Dumesnil JG| title=Low-flow, low-gradient aortic stenosis with normal and depressed left ventricular ejection fraction. | journal=J Am Coll Cardiol | year= 2012 | volume= 60 | issue= 19 | pages= 1845-53 | pmid=23062546 | doi=10.1016/j.jacc.2012.06.051 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23062546 }} </ref> | ||
====True Severe Aortic Stenosis==== | ====True Severe Aortic Stenosis==== | ||
*The aortic stenosis is so severe that it caused secondary left ventricular dysfunction. This systolic dysfunction causes decreased contractility and hence causes decreased ejection force and low transvalvular flow and gradient. | *The aortic stenosis is so severe that it caused secondary left ventricular dysfunction. This systolic dysfunction causes decreased contractility and hence causes decreased ejection force and low transvalvular flow and gradient.<ref name="pmid23062546">{{cite journal| author=Pibarot P, Dumesnil JG| title=Low-flow, low-gradient aortic stenosis with normal and depressed left ventricular ejection fraction. | journal=J Am Coll Cardiol | year= 2012 | volume= 60 | issue= 19 | pages= 1845-53 | pmid=23062546 | doi=10.1016/j.jacc.2012.06.051 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23062546 }} </ref> | ||
====Pseudo-Severe Aortic Stenosis==== | ====Pseudo-Severe Aortic Stenosis==== | ||
The aortic stenosis in Pseudo-Severe Aortic Stenosis is mild or moderate but it co-exists with another myocardial disease that is independent from the aortic stenosis. Overestimation of the severity of the aortic stenosis happens in this context. | |||
*The presence of [[fibrosis]] in the left ventricle may cause an incomplete recovery after [[aortic valve replacement]]<ref name="pmid">{{cite journal |author=Carabello BA, Green LH, Grossman W, Cohn LH, Koster JK, Collins JJ |title= |journal=[[]] |volume= |issue= |pages=42–8 |year=1980 |month=July |pmid= |doi= |url=http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=7379284 |accessdate=2012-04-10}}</ref>. | *The presence of [[fibrosis]] in the left ventricle may cause an incomplete recovery after [[aortic valve replacement]]<ref name="pmid">{{cite journal |author=Carabello BA, Green LH, Grossman W, Cohn LH, Koster JK, Collins JJ |title= |journal=[[]] |volume= |issue= |pages=42–8 |year=1980 |month=July |pmid= |doi= |url=http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=7379284 |accessdate=2012-04-10}}</ref>. | ||
*This scenario can also occur among patients in whom there is a history of [[myocardial infarction]] due to the absence of sufficient contractility to mount an aortic [[Intravascular pressure gradient|gradient]]. | *This scenario can also occur among patients in whom there is a history of [[myocardial infarction]] due to the absence of sufficient contractility to mount an aortic [[Intravascular pressure gradient|gradient]]. | ||
*It may also occur when myocardial fibrosis develops due to longstanding aortic stenosis.<ref name="pmid23062546">{{cite journal| author=Pibarot P, Dumesnil JG| title=Low-flow, low-gradient aortic stenosis with normal and depressed left ventricular ejection fraction. | journal=J Am Coll Cardiol | year= 2012 | volume= 60 | issue= 19 | pages= 1845-53 | pmid=23062546 | doi=10.1016/j.jacc.2012.06.051 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23062546 }} </ref> | *It may also occur when myocardial fibrosis develops due to longstanding aortic stenosis.<ref name="pmid23062546">{{cite journal| author=Pibarot P, Dumesnil JG| title=Low-flow, low-gradient aortic stenosis with normal and depressed left ventricular ejection fraction. | journal=J Am Coll Cardiol | year= 2012 | volume= 60 | issue= 19 | pages= 1845-53 | pmid=23062546 | doi=10.1016/j.jacc.2012.06.051 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23062546 }} </ref> | ||
===Low Flow, Low Gradient Aortic Stenosis with Normal Ejection Fraction=== | ===Low Flow, Low Gradient Aortic Stenosis with Normal Ejection Fraction=== | ||
LFLG AS with normal ejection fraction has been recently described and it is usually an advanced stage of valvular and myocardial diseases. | |||
* | * LFLG AS with normal ejection fraction has a lot of similarities with normal ejection fraction [[diastolic heart failure]]. In fact, it is usually present in older females in the context of systemic hypertension. The underlying pathophysiology is a restrictive myocardium. | ||
* The characteristics of | * The characteristics of LFLG AS with normal ejection fraction is the presence of extensive remodeling due to predominant diastolic dysfunction as well as systolic dysfunction. However, the decrease in the systolic performance of the left ventricle does not show a decrease in the [[ejection fraction]].<ref name="pmid23062546">{{cite journal| author=Pibarot P, Dumesnil JG| title=Low-flow, low-gradient aortic stenosis with normal and depressed left ventricular ejection fraction. | journal=J Am Coll Cardiol | year= 2012 | volume= 60 | issue= 19 | pages= 1845-53 | pmid=23062546 | doi=10.1016/j.jacc.2012.06.051 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23062546 }} </ref> | ||
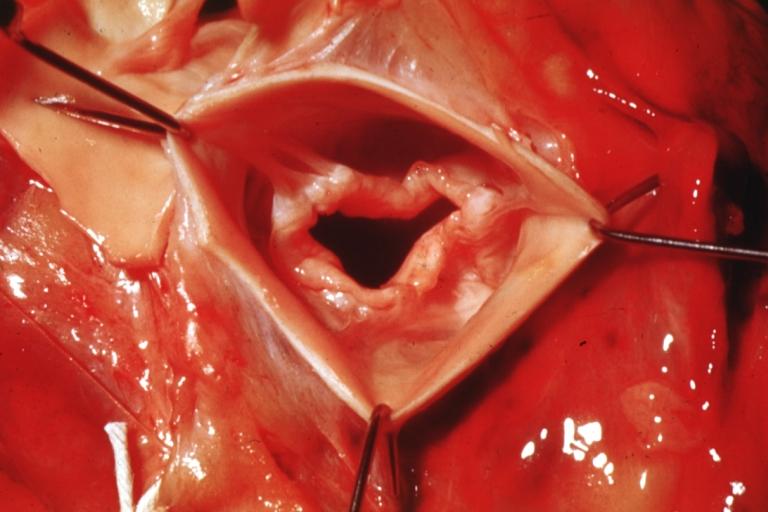
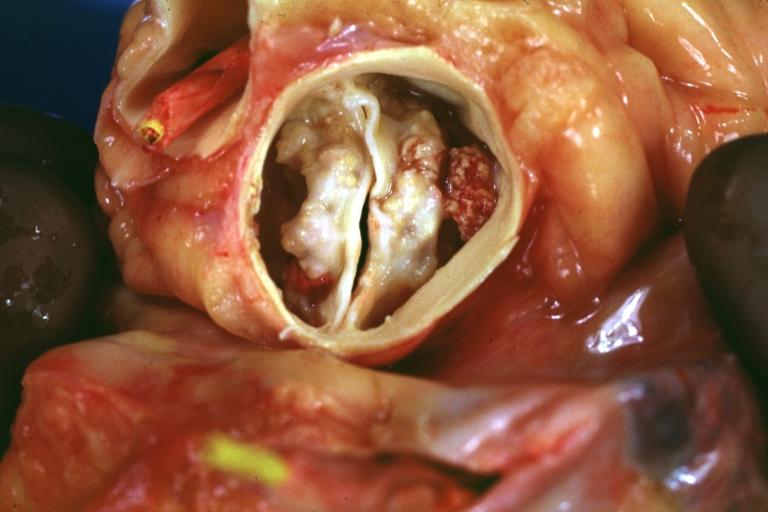
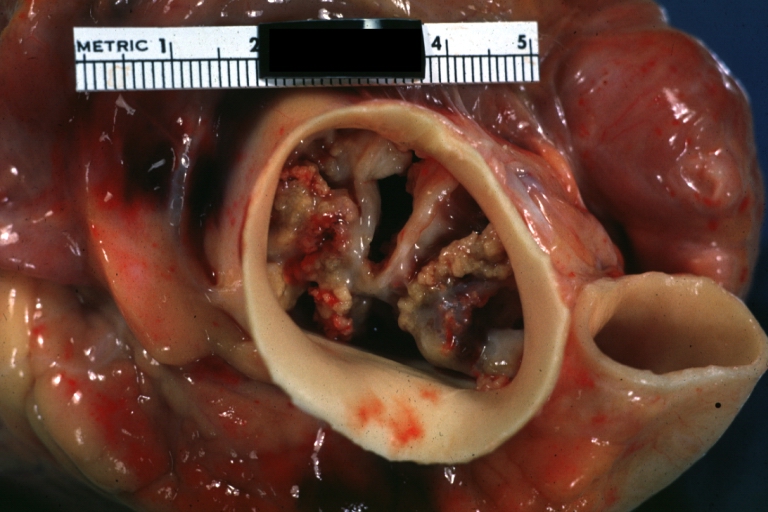
==Gross Pathology== | |||
Pathological findings of congenital or acquired aortic stenosis in adults result in thickening and calcification of aortic valve. The following patterns may be seen:<ref name="pmid3402479">{{cite journal| author=Normand J, Loire R, Zambartas C| title=The anatomical aspects of adult aortic stenosis. | journal=Eur Heart J | year= 1988 | volume= 9 Suppl E | issue= | pages= 31-6 | pmid=3402479 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3402479 }} </ref> | |||
*Calcified bicuspid valve involving anterior or posterior cusps | |||
*Calcified aortic valve cusps with the fusion of commissures seen in post rheumatic cases | |||
*Degenerative calcific aortic stenosis which shows sinuses of Valsalva filled with calcium deposits seen in age >70 | |||
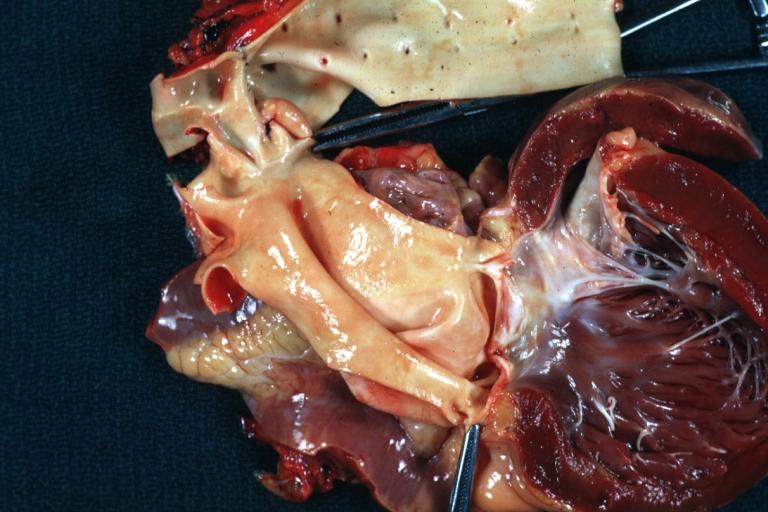
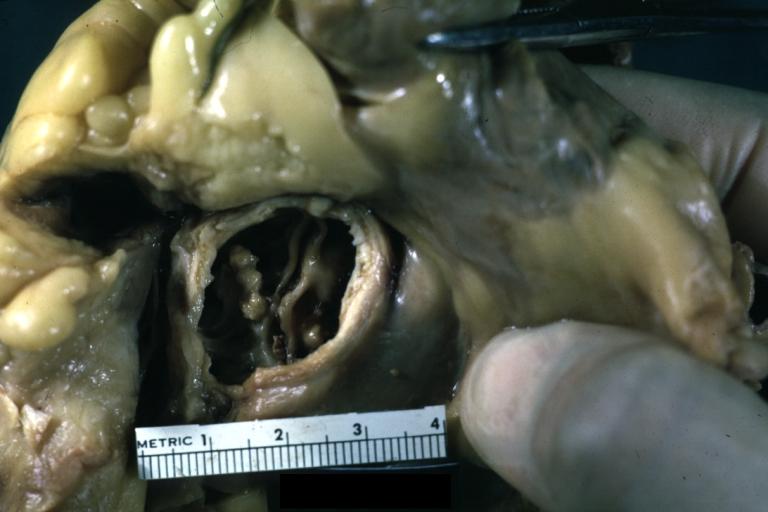
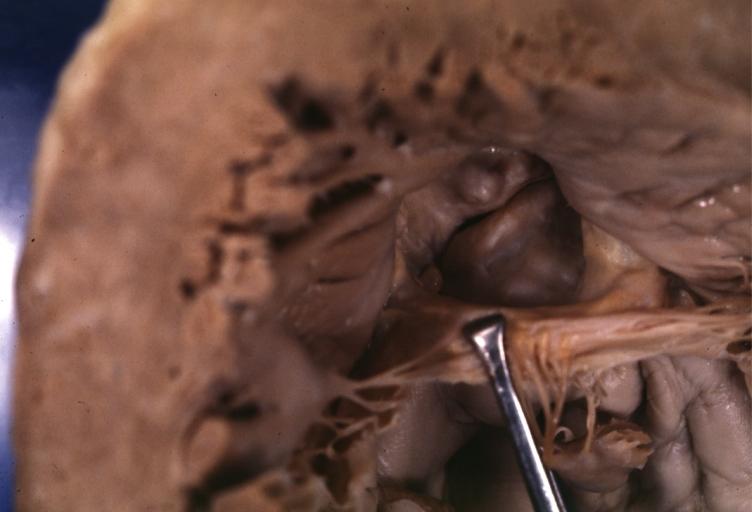
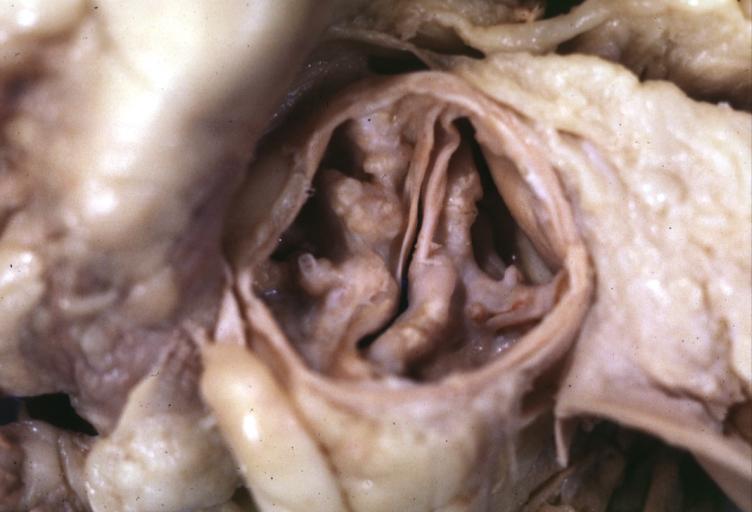
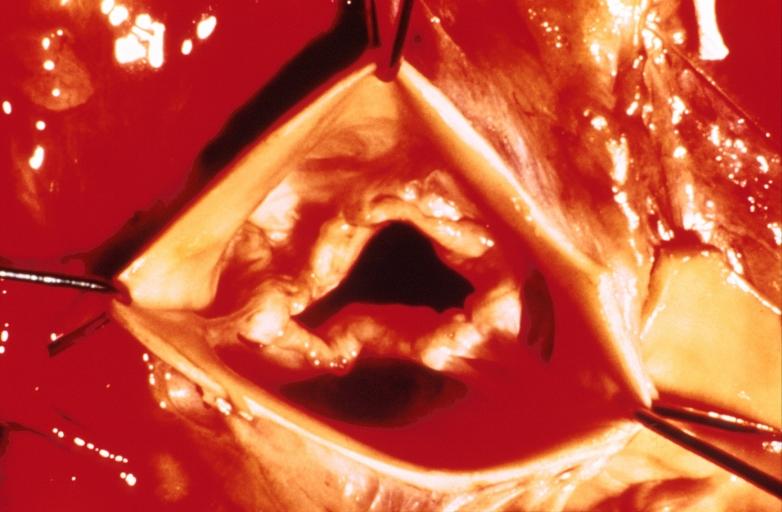
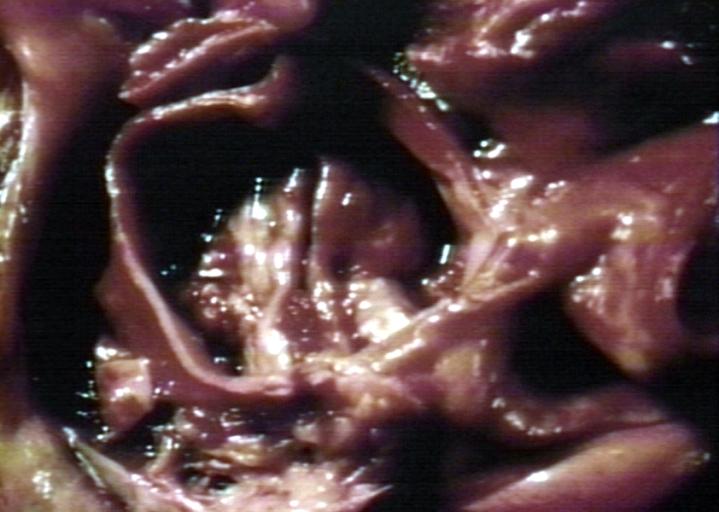
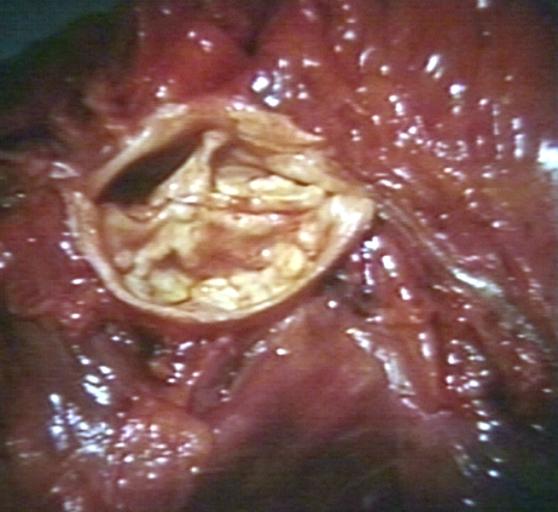
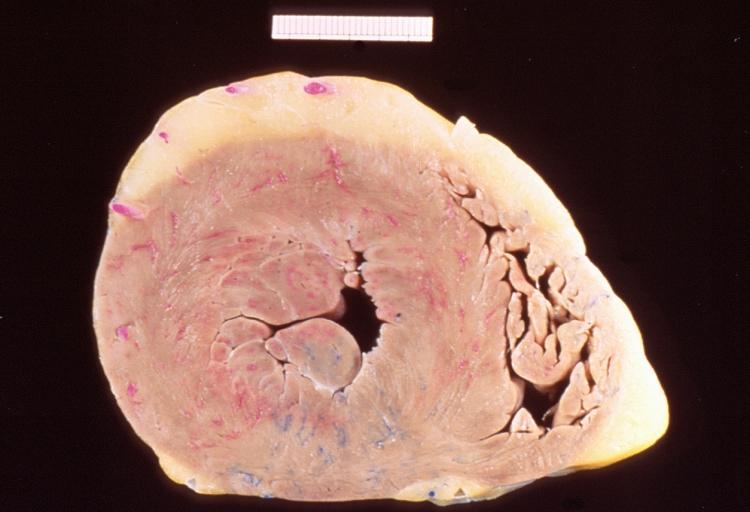
Images shown below are courtesy of Professor Peter Anderson DVM Ph.D. and published with permission. [http://www.peir.net © PEIR, the University of Alabama at Birmingham, Department of Pathology] | |||
<gallery> | |||
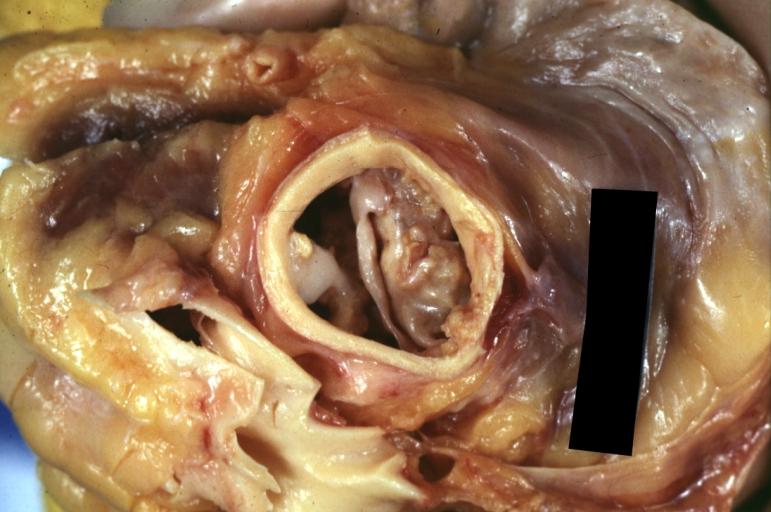
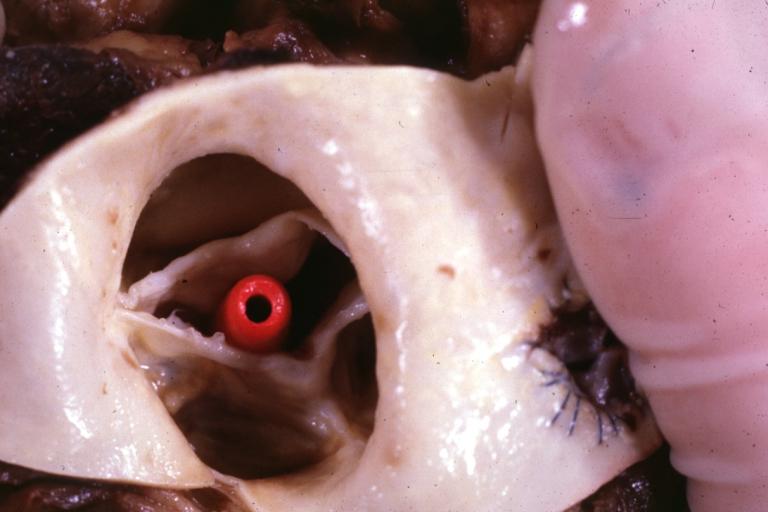
Image:Bicuspid aortic valve1.jpg|Aortic Stenosis, Bicuspid valve: Gross; excellent image of bicuspid and calcific valve showing a false raphe. | |||
Image:Bicuspid aortic valve2.jpg|Aortic Stenosis, Bicuspid valve: Gross; good example of bicuspid valve | |||
Image:Bicuspid aortic valve3.jpg|Aortic Stenosis, Bicuspid valve: Gross; image of bicuspid aortic valve, an excellent example | |||
Image:Bicuspid aortic valve4.jpg|Aortic Stenosis, Bicuspid valve: Gross; close-up image of bicuspid aortic valve. | |||
Image:Bicuspid aortic valve5.jpg|Aortic Stenosis, Bicuspid valve: Gross; close-up image of bicuspid aortic valve. | |||
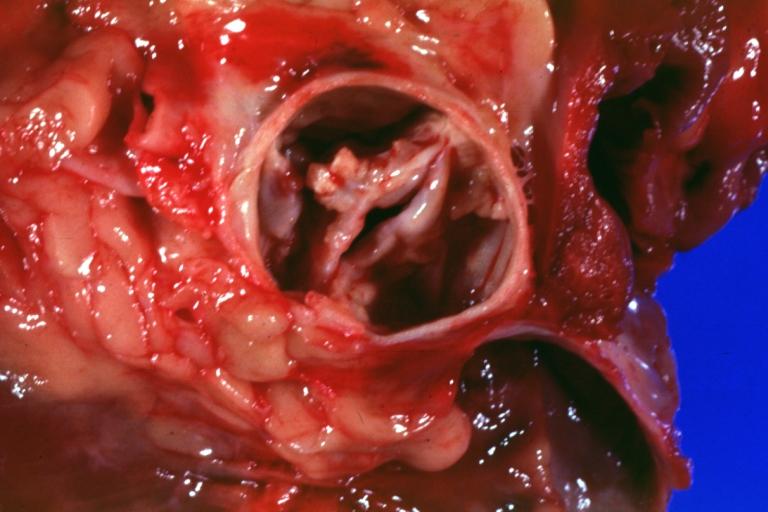
Image:Bicuspid aortic valve6.jpg|Bicuspid aortic valve | |||
Image:Bicuspid aortic valve7.jpg|Gross natural color opened first portion aortic arch with bicuspid aortic valve shows stenosis and aortic root is dilated | |||
Image:Bicuspid aortic valve8.jpg|Aortic Stenosis Bicuspid: Gross; natural color opened left ventricular outflow tract with calcific masses on valve as well as anterior leaflet mitral valve probably did not cause significant stenosis | |||
Image:Bicuspid aortic valve9.jpg|Bicuspid Aortic Valve with Repaired Aorta Coarctation: Gross natural color opened left ventricular outflow tract with uncomplicated bicuspid aortic valve repaired coarctation barely visible ruptured postoperative young female with ovaries Turner mosaic not ruled out | |||
Image:Bicuspid aortic valve10.jpg|Bicuspid Aortic Stenosis: Gross; fixed tissue | |||
Image:Bicuspid aortic valve11.jpg|Aortic Stenosis, Bicuspid: Gross; fixed tissue view of stenotic valve through ventricular outlet track | |||
Image:Bicuspid aortic valve12.jpg|Aortic Stenosis Bicuspid: Gross; fixed tissue. Bicuspid valve and false raphe classical | |||
Image:Bicuspid aortic valve13.jpg|Bicuspid aortic valve | |||
Image:Bicuspid aortic valve14.jpg|Bicuspid aortic valve | |||
Image:Bicuspid aortic valve15.jpg|Bicuspid aortic valve | |||
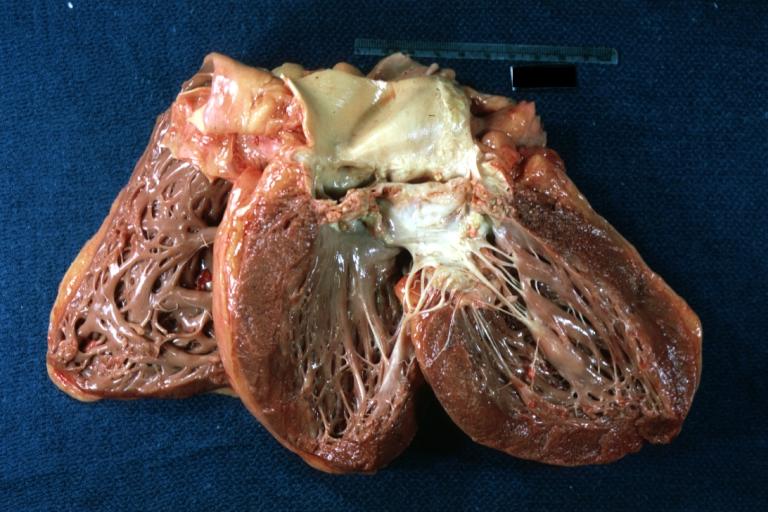
Image:Bicuspid aortic valve16.jpg|Left ventricular hypertrophy due to bicuspid aortic valve | |||
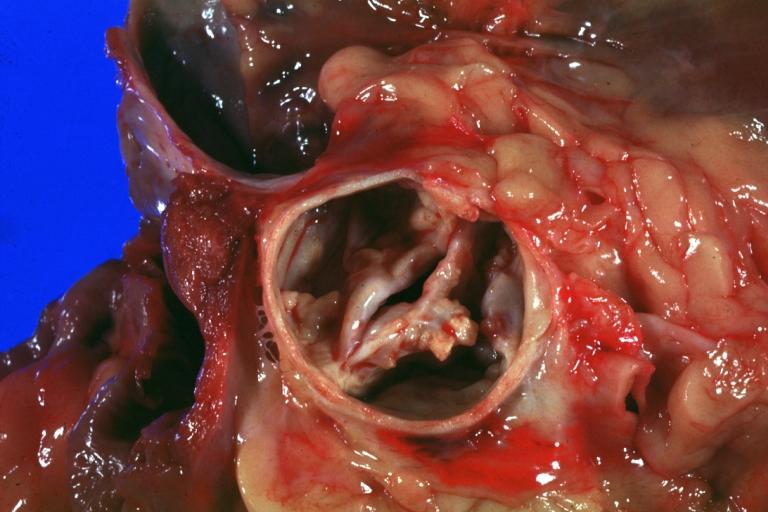
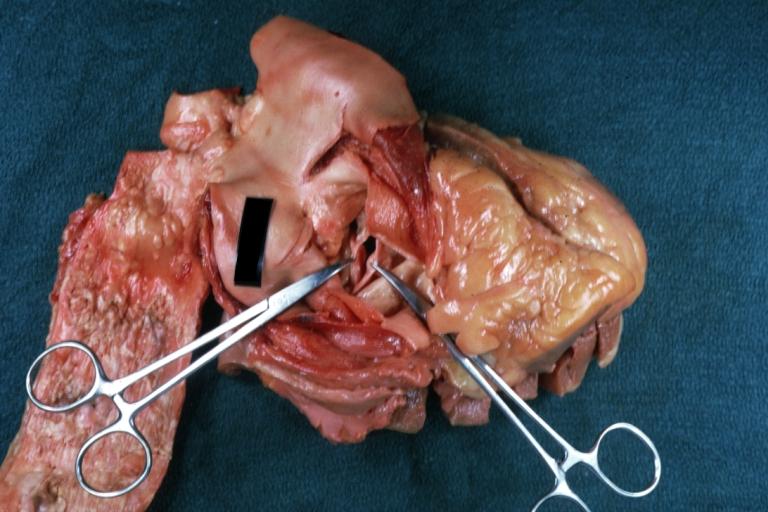
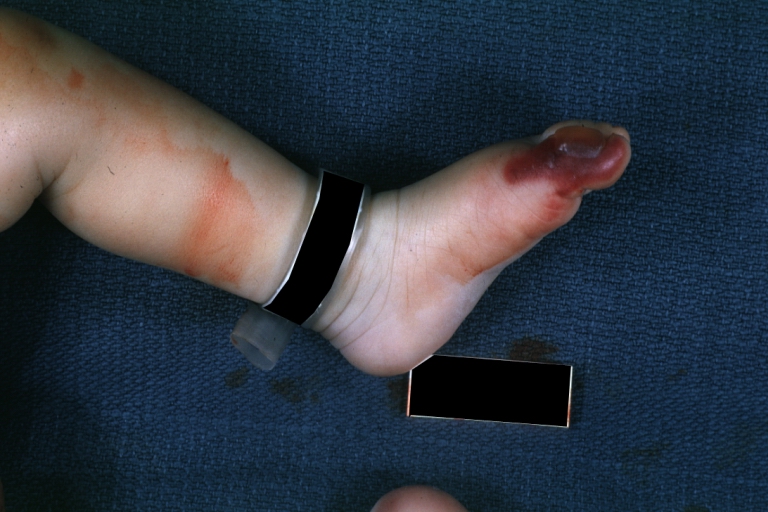
Image:Congenital aortic stenosis.jpg|Congenital aortic stenosis: Gangrene toe In Infant: Gross, natural color, 1-month-old child with congenital aortic stenosis | |||
Image:Unicuspid aortic stenosis.jpg|Unicuspid aortic stenosis | |||
</gallery> | |||
==Pathology | ==Microscopic Pathology== | ||
==== | On microscopic histopathological analysis, the calcific aortic stenosis shows pink amorphous material around the calcific foci indicating deposition of calcium. Congenital and calcific aortic stenosis show the areas of fibrosis, thickening, fat cell infiltration and elastosis.<ref name="pmid23833294">{{cite journal| author=Towler DA| title=Molecular and cellular aspects of calcific aortic valve disease. | journal=Circ Res | year= 2013 | volume= 113 | issue= 2 | pages= 198-208 | pmid=23833294 | doi=10.1161/CIRCRESAHA.113.300155 | pmc=4057916 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23833294 }} </ref> | ||
==References== | ==References== | ||
{{reflist|2}} | {{reflist|2}} | ||
{{WH}} | |||
{{WS}} | |||
[[CME Category::Cardiology]] | |||
[[Category:Disease]] | [[Category:Disease]] | ||
[[Category:Valvular heart disease]] | [[Category:Valvular heart disease]] | ||
| Line 208: | Line 255: | ||
[[Category:Surgery]] | [[Category:Surgery]] | ||
[[Category:Needs CMG review]] | [[Category:Needs CMG review]] | ||
Latest revision as of 16:39, 3 March 2020
| Resident Survival Guide |
|
Aortic Stenosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Percutaneous Aortic Balloon Valvotomy (PABV) or Aortic Valvuloplasty |
|
Transcatheter Aortic Valve Replacement (TAVR) |
|
Case Studies |
|
Aortic stenosis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Aortic stenosis pathophysiology |
|
Directions to Hospitals Treating Aortic stenosis pathophysiology |
|
Risk calculators and risk factors for Aortic stenosis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-In-Chief: Mohammed A. Sbeih, M.D. [2], Lakshmi Gopalakrishnan, M.B.B.S. [3]; Usama Talib, BSc, MD [4] , Claudia P. Hochberg, M.D. [5], Abdul-Rahman Arabi, M.D. [6], Keri Shafer, M.D. [7], Priyamvada Singh, MBBS [8], Aysha Anwar, M.B.B.S[9] Cafer Zorkun, M.D., Aysha Anwar, M.B.B.S[10] Mandana Chitsazan, M.D. [11] Assistant Editor-In-Chief: Kristin Feeney, B.S. [12]; Rim Halaby
Overview
Aortic stenosis is the progressive narrowing of the aortic valve. Calcific aortic stenosis, in particular, is an active atherosclerotic pathology where inflammation, fibrosis, and calcification are involved in the progressive narrowing of the effective aortic valve area in the absence of any commissural fusion. In contrast, rheumatic aortic stenosis is due to the fusion of the commissures with valvular scarring and calcification. Aortic stenosis causes an impedance to the antegrade blood flow not only at the level of the aortic valve itself but also at the subvalvular (below the aortic valve) or supravalvular (above the aortic valve) levels. As a result, chronic pressure overload develops in the left ventricle. The left ventricle undergoes hypertrophy as an initial adaptive mechanism to overcome the increased afterload. This compensatory mechanism ends up being maladaptive by causing apoptosis of the hypertrophied myocytes and subsequent heart failure. Hence, aortic stenosis is a progressive valvular disease which progression depends mainly on the degree of the narrowing of the aortic valve as well as on the maladaptive ventricular wall response. Gross anatomy dissection may be used as a diagnostic tool in the evaluation of aortic stenosis. Common findings associated with aortic stenosis include left ventricular hypertrophy and heart block.
Pathophysiology
- Aortic stenosis in the progressive narrowing of the aortic valve.[1]
- The decrease in aortic valve area does not cause a change in the antegrade velocity unless the area is decreased by at least half. Aortic stenosis causes an impedance to the antegrade blood flow not only at the level of the aortic valve itself but also at the subvalvular (below the aortic valve) or supravalvular (above the aortic valve) levels. As a result, chronic pressure overload develops in the left ventricle. As a result, chronic pressure overload develops in the left ventricle.[2]
- Slow compensatory mechanisms occur in the heart to adapt to the pressure changes caused by aortic stenosis. The most prominent adaptive mechanism is ventricular hypertrophy which leads early on to diastolic dysfunction and later on to systolic dysfunction.[3][4]
Shown below is an image summarizing the pathophysiology of aortic stenosis:[4]
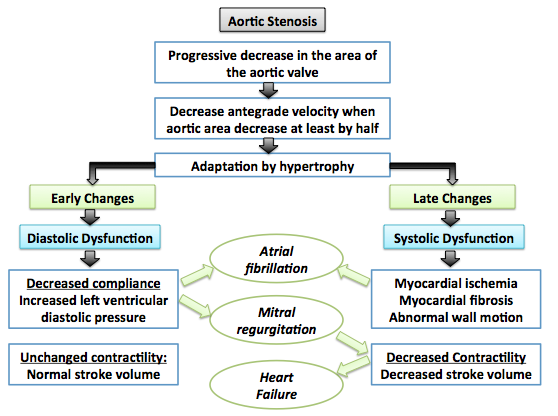
Associated Conditions
Heyde's Syndrome
- In Heyde's syndrome, aortic stenosis is associated with angiodysplasia of the colon. Presenting symptoms include:[5]
Von Willebrand Disease
- Aortic stenosis may result in a form of von Willebrand disease due to an increased turbulence around the stenosed aortic valve which subsequently triggers the break down of coagulation factor VIII-associated antigen, (also called von Willebrand factor) and thus results in a variant of von Willebrand disease.[6][7]
Narrowing of the Aortic Valve
Calcific Aortic Stenosis
Calcific aortic stenosis has long been thought to be the result of prolonged wear and tear mechanism and age-related degeneration of the valvular cusps as opposed to cusp fusion of the aortic valve in rheumatic heart disease. However, recent studies have revealed that the underlying pathophysiology of calcific aortic stenosis is active inflammation, fibrosis, and calcification. Genetic predisposition plays a role in the rate of progression of aortic stenosis. The stages of the formation of the calcific aortic stenosis are the following:[4]
- Endothelial Damage:
- Endothelial damage is the initial event starting the cascade of events in calcific aortic stenosis.
- It results from increased mechanical stress and decreased shear stress.
- Endothelial damage happens at a faster rate in bicuspid aortic valves than in tricuspid aortic valves as a result of the difference in distribution of stress forces on the valvular cusps.[4]
- Inflammation:
- Endothelial damage triggers accumulation of lipids (LDL and lipoprotens) that subsequently undergo oxidative changes.
- As a result, inflammation is accentuated by:
- Elevated CRP in patients with aortic stenosis is an evidence of inflammation[8]
- Fibrosis:
- Inflammation eventually leads to activation of fibroblasts and remodeling of the extracellular matrix[9]
- Calcification:
- Calcification is induced by long standing inflammation and fibrosis.
- The following disorders of mineral metabolism accelerates calcification:[4][10][11][12][13]
- Hemodialysis
- Osteoporosis
- Paget's disease
- Vitamin D receptor polymorphism
- Calcification starts at the base of the cusps and then reaches the leaflets. Calcification results in a decrease in the leflet motion and hence decrease in the effective valve area in the absence of any fusion of the commissures.[14]
- Calcification is coordinated by osteoblasts in the context of a highly regulated mechanism similar to bone formation.[4]
Rheumatic Aortic Stenosis
- Rheumatic aortic stenosis is due to the fusion of the valve commissures and subsequent scarring and calcification. Rheumatic aortic stenosis is always accompanied by mitral regurgitation.[14]
- Rheumatic fever which depends on a prior autoimmunological stimulation, is a systemic disease affecting the peri-arteriolar connective tissue and can occur after an untreated Group A streptococcal pharyngeal infection. It is believed to be caused by antibody cross-reactivity which is a type II hypersensitivity reaction and is termed 'molecular mimicry'.
- Chronic rheumatic heart disease is characterized by repeated inflammation with fibrinous resolution. The cardinal anatomic changes of the valve include leaflet thickening, commissural fusion and shortening and thickening of the tendinous cords.[15]Rheumatic heart disease may lead to up to 99% stenosis of the aortic valve often resulting in a “fish mouth” gross appearance.
Congenital Aortic Stenosis
Congenital malformation of the aortic valve is one of the less common causes of aortic stenosis.[16]Congenital aortic stenosis is detected in young adults.
Left Ventricular Hypertrophy
- Long-standing aortic stenosis exposes the left ventricle to prolonged pressure overload which leads to concentric hypertrophy as a compensatory mechanism to preserve left ventricular function.[17][18][19] The left ventricular wall increases in thickness (i.e. concentric hypertrophy occurs) as a result of the parallel replication of the sarcomeres
- However, left ventricle hypertrophy ends up being a maladpative mechanism and a marker of a bad prognosis. Hypertrophied myocardial cells undergo apoptosis at a rate larger to that of regeneration.[4] In fact, angiotensin II promotes the apoptosis of the hypertrophied vessels, thus angiotensin receptor blockers (ARB) paly a role in the treatment of heart failure secondary to aortic stenosis even without having a great role in decreasing the blood pressure.[20]
- Left ventricular hypertrophy weakly relates to the degree of stenosis itself but rather to advanced age, male sex and obesity. Other factors that participate in the hypertrophy of the left ventricle are hypertension and increased arterial stiffness.
- Fibrosis is an integral component of the myocardial hypertrophy.[4]
Early Diastolic Dysfunction
During the initial period of concentric hypertrophy, the left ventricle is not dilated and there is preservation of the left ventricular systolic function. Diastolic function, however, may be reduced due to a reduction in diastolic compliance.[21][22][23]
This diastolic dysfunction may in turn lead to a rise in pulmonary capillary wedge pressure and consequently lead to dyspnea. Cardiac output may also be reduced as a result of diastolic dysfunction and impaired filling of the left ventricle. Early in the course of aortic stenosis, there may be a failure to augment cardiac output during exercise resulting in dyspnea on exertion.
Late Systolic Dysfunction
Later in the course of aortic stenosis, left ventricular dysfunction may develop due to a variety of pathophysiological processes. Systolic dysfunction is associated with a poor prognosis and it often does not partially or fully reverse following operative repair.[22]
Excess Hypertrophy Causes Systolic Dysfunction
The massive concentric hypertrophy, characterized by a reduced diastolic radius-to-wall thickness ratio, has shown to initially counter balance the increased systolic left ventricular pressure; nevertheless, if this process continues, an inverse relationship has been observed such that the ejection fraction eventually goes down as the left ventricular mass increases beyond a certain point.[24][22][25][26]
Myocardial Ischemia
The hypertrophied left ventricle and the prolonged ejection time (the time for the heart to eject blood) result in an increase in the myocardial oxygen requirements. In addition, the elevated diastolic filling pressure reduces the gradient between the aorta and the right atrium ("the height of the waterfall") which normally drives coronary blood flow. There may be a relative reduction in the density of the capillary network. The hypertrophied ventricle may also compress the capillaries. All of the above lead to a reduction in coronary blood flow even in the absence of obstructive epicardial stenosis. This may lead to subendocardial ischemia during stress or exercise.[27][28]
Myocardial Fibrosis
Myocardial scarring or fibrosis may develop with prolonged aortic stenosis, probably due to chronic subendocardial ischemia or increased wall stress. [29]
Dyssynchronous Contraction
Another factor that may contribute to the reduced left ventricular systolic function is the dyssynchronous contraction subsequent to regional wall motion abnormalities, fibrosis or ischemia.[30]
Atrial Fibrillation
The stiff non-compliant left ventricle can become increasingly dependent on the left atrium for filling, which predisposes to atrial fibrillation. The presence of atrial fibrillation and the associated loss of atrial contractility can result in reduced left ventricular filling and reduced cardiac output.[31]
Hemodynamics of Aortic Stenosis
Pressure Gradient and Valve Area
When the aortic valve becomes stenosed, it can result in the formation of a pressure gradient between the left ventricle (LV) and the aorta.[32] The more constricted the valve is, the bigger the gradient between the LV and the aorta is.
For instance, the pressure gradient in patients with mild AS might be 20 mmHg. This means that, at peak systole, while the LV may generate a pressure of 140 mmHg, the pressure that is transmitted into the aorta will only be 120 mmHg. Therefore, while a blood pressure cuff may measure a normal systolic blood pressure the actual pressure generated by and inside the LV would be considerably higher. As the left ventricle fails, it may no longer be able to mount the contractility necessary to generate a large gradient across the aortic valve.
Therefore, the absence of a large gradient across the aortic valve does not exclude the presence of critical aortic stenosis. The presence of a low gradient and a low ejection results in a low flow aortic stenosis. It is for this reason that the best measure of the severity of aortic stenosis is the aortic valve area and not the aortic valve gradient.
Read in detail by clicking on the topics:
Subvalvular Gradients in AS in the Absence of Anatomic Obstruction
Subvalvular pressure gradients are often present in patients with severe aortic stenosis in the absence of an anatomic subvalvular obstruction. In fact, the subvalvular pressure gradients constitute around 50% of the total measured transvalvular gradient. The extent of increase in cardiac output during exercise is inversely related to the magnitude of subvalvular gradient.[33]
Flow Velocity
If the left ventricular function and contractility are preserved, a flow velocity across the stenosed valve of at least 2.6 m/sec is deemed consistent with aortic stenosis. This is based on the echocardiographic estimation of the aortic jet velocity, the aortic valve area and the mean transvalvular gradient. The aortic valve becomes calcified in aortic valve sclerosis (not stenosis); however, the aortic jet velocity is ≤ 2.5 m/sec (without a significant gradient). Aortic valve sclerosis is commonly characterized by a focal thickening of the aortic cusps with calcific nodules generally at the base of leaflets and a transvalvular velocity within the normal range (Vmax <2 m/s). Until a few years ago, aortic valve sclerosis was considered to be a physiologic process related to aging without any clinical relevance. However, aortic valve sclerosis is not observed in about 50% of people over 80 years old. Furthermore, several experimental and clinical studies have demonstrated that aortic valve sclerosis could represent an active phenomenon significantly related to the risk factors of atherosclerosis.[34][35]
Relationship of Hemodynamic Severity to Symptoms of Aortic Stenosis
- Patients with aortic stenosis become symptomatic when:[36][37]
- The valve area is less than 1.0 cm2.
- The jet velocity is over 4.0 m/sec.
- Mean transvalvular pressure gradient exceeds 40 mm Hg.
- However, many patients develop symptoms only when more severe valve obstruction is present, other patients become symptomatic at a less severe degree of stenosis, particularly if there is coexisting aortic regurgitation.
ACC/AHA Guidelines - Severity Classification [36]
Indicator Mild Moderate Severe Jet velocity (m per s) Less than 3.0 3.0–4.0 Greater than 4.0 Mean gradient (mm Hg)† Less than 25 25–40 Greater than 40 Valve area (cm2) Greater than 1.5 1.0–1.5 Less than 1.0 Valve area index (cm2 per m2) Less than 0.6
† Valve gradients are flow-dependent. They should be assessed with knowledge of cardiac output or antegrade flow across the valve when used to estimate of the severity of valve stenosis.
Low Flow, Low Gradient Aortic Stenosis
- In aortic stenosis, as the aortic orifice area decreases the transvalvular gradient increases. In fact, when the aortic valve effective orifice area decreases below 1.0 cm2 the mean transvalvular gradient is expected to be larger than 40 mm Hg. The transvalvular gradient is highly dependent on the flow of blood through the valve.
- However, when severe systolic or/and diastolic myocardial dysfunction coexist with the aortic stenosis, there is a decrease in the flow through the valve leading to a prominent decrease in the transvalvular gradient. This is called low flow, low gradient aortic stenosis (LFLG AS).
- LFLG AS is a challenging diagnosis that must be done in order to tailor the management plan. It is important to recognize these entities because they might cause either underestimation or overestimation of the degree of severity of the aortic stenosis.
- Two various scenarios occur in the setting of LFLG AS depending on the status of the left ventricular ejection fraction:
- LFLG AS with low LVEF
- LFLG AS with normal LVEF[38]
Shown below is a table depicting the differences between LFLG AS with low LVEF and LFLG AS with normal LVEF:
| LFLG AS | Percentage of the Cases of Severe AS Caused by LFLG AS | Pathophysiology | Diagnostic Challenges |
|---|---|---|---|
| LFLG AS with low LVEF | 5-10% of severe aortic stenosis | Decreased systolic function:
Dilated left ventricle Decreased contractility |
Differentiation between severe aortic stenosis and pseudo-severe aortic stenosis |
| LFLG AS with normal LVEF | 10-25% of severe aortic stenosis | Decreased diastolic function:
Small left ventricle Decreased compliance |
Underestimation of the severity of aortic stenosis
Differentiation from confounding measurement errors and small ventricle body size |
Low Flow, Low Gradient Aortic Stenosis with Low Ejection Fraction
- When ventricular systolic dysfunction is present, the myocardium can not contract strongly enough to pump blood with a lot of pressure. In that case, low flow and subsequent low transvalvular gradient are present and this entity is called LFLG AS with low LVEF.
- LFLG AS with low LVEF is defined as:[36][37][38]
- An aortic valve areas < 1.0 cm2
- A left ventricular ejection fraction < 40%
- A mean pressure difference or gradient across the aortic valve of < 30 mm Hg
- When low flow, low gradient aortic stenosis is present, the challenge is to differentiate whether the LFLG AS with low LVEF is a true severe aortic stenosis or a pseudo-severe aortic stenosis. It is very important to differentiate between these two entities as they have different outcomes following aortic valve replacement.[38]
True Severe Aortic Stenosis
- The aortic stenosis is so severe that it caused secondary left ventricular dysfunction. This systolic dysfunction causes decreased contractility and hence causes decreased ejection force and low transvalvular flow and gradient.[38]
Pseudo-Severe Aortic Stenosis
The aortic stenosis in Pseudo-Severe Aortic Stenosis is mild or moderate but it co-exists with another myocardial disease that is independent from the aortic stenosis. Overestimation of the severity of the aortic stenosis happens in this context.
- The presence of fibrosis in the left ventricle may cause an incomplete recovery after aortic valve replacement[22].
- This scenario can also occur among patients in whom there is a history of myocardial infarction due to the absence of sufficient contractility to mount an aortic gradient.
- It may also occur when myocardial fibrosis develops due to longstanding aortic stenosis.[38]
Low Flow, Low Gradient Aortic Stenosis with Normal Ejection Fraction
LFLG AS with normal ejection fraction has been recently described and it is usually an advanced stage of valvular and myocardial diseases.
- LFLG AS with normal ejection fraction has a lot of similarities with normal ejection fraction diastolic heart failure. In fact, it is usually present in older females in the context of systemic hypertension. The underlying pathophysiology is a restrictive myocardium.
- The characteristics of LFLG AS with normal ejection fraction is the presence of extensive remodeling due to predominant diastolic dysfunction as well as systolic dysfunction. However, the decrease in the systolic performance of the left ventricle does not show a decrease in the ejection fraction.[38]
Gross Pathology
Pathological findings of congenital or acquired aortic stenosis in adults result in thickening and calcification of aortic valve. The following patterns may be seen:[39]
- Calcified bicuspid valve involving anterior or posterior cusps
- Calcified aortic valve cusps with the fusion of commissures seen in post rheumatic cases
- Degenerative calcific aortic stenosis which shows sinuses of Valsalva filled with calcium deposits seen in age >70
Images shown below are courtesy of Professor Peter Anderson DVM Ph.D. and published with permission. © PEIR, the University of Alabama at Birmingham, Department of Pathology
-
Aortic Stenosis, Bicuspid valve: Gross; excellent image of bicuspid and calcific valve showing a false raphe.
-
Aortic Stenosis, Bicuspid valve: Gross; good example of bicuspid valve
-
Aortic Stenosis, Bicuspid valve: Gross; image of bicuspid aortic valve, an excellent example
-
Aortic Stenosis, Bicuspid valve: Gross; close-up image of bicuspid aortic valve.
-
Aortic Stenosis, Bicuspid valve: Gross; close-up image of bicuspid aortic valve.
-
Bicuspid aortic valve
-
Gross natural color opened first portion aortic arch with bicuspid aortic valve shows stenosis and aortic root is dilated
-
Aortic Stenosis Bicuspid: Gross; natural color opened left ventricular outflow tract with calcific masses on valve as well as anterior leaflet mitral valve probably did not cause significant stenosis
-
Bicuspid Aortic Valve with Repaired Aorta Coarctation: Gross natural color opened left ventricular outflow tract with uncomplicated bicuspid aortic valve repaired coarctation barely visible ruptured postoperative young female with ovaries Turner mosaic not ruled out
-
Bicuspid Aortic Stenosis: Gross; fixed tissue
-
Aortic Stenosis, Bicuspid: Gross; fixed tissue view of stenotic valve through ventricular outlet track
-
Aortic Stenosis Bicuspid: Gross; fixed tissue. Bicuspid valve and false raphe classical
-
Bicuspid aortic valve
-
Bicuspid aortic valve
-
Bicuspid aortic valve
-
Left ventricular hypertrophy due to bicuspid aortic valve
-
Congenital aortic stenosis: Gangrene toe In Infant: Gross, natural color, 1-month-old child with congenital aortic stenosis
-
Unicuspid aortic stenosis
Microscopic Pathology
On microscopic histopathological analysis, the calcific aortic stenosis shows pink amorphous material around the calcific foci indicating deposition of calcium. Congenital and calcific aortic stenosis show the areas of fibrosis, thickening, fat cell infiltration and elastosis.[40]
References
- ↑ Galli D, Manuguerra R, Monaco R, Manotti L, Goldoni M, Becchi G; et al. (2016). "Understanding the structural features of symptomatic calcific aortic valve stenosis: A broad-spectrum clinicopathologic study in 236 consecutive surgical cases". Int J Cardiol. 228: 364–374. doi:10.1016/j.ijcard.2016.11.180. PMID 27866029.
- ↑ Joseph J, Naqvi SY, Giri J, Goldberg S (2016). "Aortic stenosis: pathophysiology, diagnosis and therapy". Am J Med. doi:10.1016/j.amjmed.2016.10.005. PMID 27810479.
- ↑ Otto CM, Prendergast B (2014). "Aortic-valve stenosis--from patients at risk to severe valve obstruction". N Engl J Med. 371 (8): 744–56. doi:10.1056/NEJMra1313875. PMID 25140960.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 Dweck MR, Boon NA, Newby DE (2012). "Calcific aortic stenosis: a disease of the valve and the myocardium". J Am Coll Cardiol. 60 (19): 1854–63. doi:10.1016/j.jacc.2012.02.093. PMID 23062541.
- ↑ Ledingham D (2013). "Heyde's syndrome: exploring the link between aortic stenosis and an acquired bleeding disorder". BMJ Case Rep. 2013. doi:10.1136/bcr-2013-009306. PMC 3645074. PMID 23605838.
- ↑ Vincentelli A, Susen S, Le Tourneau T, Six I, Fabre O, Juthier F; et al. (2003). "Acquired von Willebrand syndrome in aortic stenosis". N Engl J Med. 349 (4): 343–9. doi:10.1056/NEJMoa022831. PMID 12878741.
- ↑ Tamura T, Horiuchi H, Imai M, Tada T, Shiomi H, Kuroda M; et al. (2015). "Unexpectedly High Prevalence of Acquired von Willebrand Syndrome in Patients with Severe Aortic Stenosis as Evaluated with a Novel Large Multimer Index". J Atheroscler Thromb. 22 (11): 1115–23. doi:10.5551/jat.30809. PMID 26269004.
- ↑ Galante A, Pietroiusti A, Vellini M, Piccolo P, Possati G, De Bonis M; et al. (2001). "C-reactive protein is increased in patients with degenerative aortic valvular stenosis". J Am Coll Cardiol. 38 (4): 1078–82. PMID 11583885.
- ↑ Frantz C, Stewart KM, Weaver VM (2010). "The extracellular matrix at a glance". J Cell Sci. 123 (Pt 24): 4195–200. doi:10.1242/jcs.023820. PMC 2995612. PMID 21123617.
- ↑ Ureña P, Malergue MC, Goldfarb B, Prieur P, Guédon-Rapoud C, Pétrover M (1999). "Evolutive aortic stenosis in hemodialysis patients: analysis of risk factors". Nephrologie. 20 (4): 217–25. PMID 10480155.
- ↑ Aksoy Y, Yagmur C, Tekin GO, Yagmur J, Topal E, Kekilli E; et al. (2005). "Aortic valve calcification: association with bone mineral density and cardiovascular risk factors". Coron Artery Dis. 16 (6): 379–83. PMID 16118543.
- ↑ Strickberger SA, Schulman SP, Hutchins GM (1987). "Association of Paget's disease of bone with calcific aortic valve disease". Am J Med. 82 (5): 953–6. PMID 3578364.
- ↑ Ortlepp JR, Hoffmann R, Ohme F, Lauscher J, Bleckmann F, Hanrath P (2001). "The vitamin D receptor genotype predisposes to the development of calcific aortic valve stenosis". Heart. 85 (6): 635–8. PMC 1729782. PMID 11359741.
- ↑ 14.0 14.1 Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD; et al. (2008). "2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons". Circulation. 118 (15): e523–661. doi:10.1161/CIRCULATIONAHA.108.190748. PMID 18820172.
- ↑ Kumar RK, Tandon R (2013). "Rheumatic fever & rheumatic heart disease: the last 50 years". Indian J Med Res. 137 (4): 643–58. PMC 3724245. PMID 23703332.
- ↑ Mordi I, Tzemos N (2012). "Bicuspid aortic valve disease: a comprehensive review". Cardiol Res Pract. 2012: 196037. doi:10.1155/2012/196037. PMC 3368178. PMID 22685681.
- ↑ Sasayama S, Ross J, Franklin D, Bloor CM, Bishop S, Dilley RB (1976). "Adaptations of the left ventricle to chronic pressure overload". Circulation Research. 38 (3): 172–8. PMID 129304. Retrieved 2012-04-10. Unknown parameter
|month=ignored (help) - ↑ Gaasch WH (1979). "Left ventricular radius to wall thickness ratio". The American Journal of Cardiology. 43 (6): 1189–94. PMID 155986. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Spann JF, Bove AA, Natarajan G, Kreulen T (1980). "Ventricular performance, pump function and compensatory mechanisms in patients with aortic stenosis". Circulation. 62 (3): 576–82. PMID 6446989. Retrieved 2012-04-10. Unknown parameter
|month=ignored (help) - ↑ González A, López B, Ravassa S, Querejeta R, Larman M, Díez J; et al. (2002). "Stimulation of cardiac apoptosis in essential hypertension: potential role of angiotensin II". Hypertension. 39 (1): 75–80. PMID 11799082.
- ↑ Gaasch WH, Levine HJ, Quinones MA, Alexander JK (1976). "Left ventricular compliance: mechanisms and clinical implications". The American Journal of Cardiology. 38 (5): 645–53. PMID 136186. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ 22.0 22.1 22.2 22.3 Hess OM, Ritter M, Schneider J, Grimm J, Turina M, Krayenbuehl HP (1984). "Diastolic stiffness and myocardial structure in aortic valve disease before and after valve replacement". [[]]: 855–65. Retrieved 2012-04-10. Unknown parameter
|month=ignored (help) - ↑ Gaasch WH (1994). "Diagnosis and treatment of heart failure based on left ventricular systolic or diastolic dysfunction". JAMA : the Journal of the American Medical Association. 271 (16): 1276–80. PMID 8151903. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Krayenbuehl HP, Hess OM, Ritter M, Monrad ES, Hoppeler H (1988). "Left ventricular systolic function in aortic stenosis". European Heart Journal. 9 Suppl E: 19–23. PMID 2969811. Retrieved 2012-04-10. Unknown parameter
|month=ignored (help) - ↑ Gunther S, Grossman W (1979). "Determinants of ventricular function in pressure-overload hypertrophy in man". Circulation. 59 (4): 679–88. PMID 154367. Retrieved 2012-04-10. Unknown parameter
|month=ignored (help) - ↑ Huber D, Grimm J, Koch R, Krayenbuehl HP (1981). "Determinants of ejection performance in aortic stenosis". Circulation. 64 (1): 126–34. PMID 7237709. Retrieved 2012-04-10. Unknown parameter
|month=ignored (help) - ↑ Marcus ML, Doty DB, Hiratzka LF, Wright CB, Eastham CL (1982). "Decreased coronary reserve: a mechanism for angina pectoris in patients with aortic stenosis and normal coronary arteries". N Engl J Med. 307 (22): 1362–6. doi:10.1056/NEJM198211253072202. PMID 6215582.
- ↑ Carabello BA (2002). "Clinical practice. Aortic stenosis". N Engl J Med. 346 (9): 677–82. doi:10.1056/NEJMcp010846. PMID 11870246.
- ↑ Yarbrough WM, Mukherjee R, Ikonomidis JS, Zile MR, Spinale FG (2012). "Myocardial remodeling with aortic stenosis and after aortic valve replacement: mechanisms and future prognostic implications". J Thorac Cardiovasc Surg. 143 (3): 656–64. doi:10.1016/j.jtcvs.2011.04.044. PMC 3210937. PMID 21762938.
- ↑ Jin XY, Pepper JR, Gibson DG (1996). "Effects of incoordination on left ventricular force-velocity relation in aortic stenosis". Heart (British Cardiac Society). 76 (6): 495–501. PMC 484601. PMID 9014797. Retrieved 2012-04-10. Unknown parameter
|month=ignored (help) - ↑ Burup Kristensen C, Jensen JS, Sogaard P, Carstensen HG, Mogelvang R (2012). "Atrial fibrillation in aortic stenosis--echocardiographic assessment and prognostic importance". Cardiovasc Ultrasound. 10: 38. doi:10.1186/1476-7120-10-38. PMC 3517318. PMID 23006976.
- ↑ Lilly LS (editor) (2003). Pathophysiology of Heart Disease (3rd ed. ed.). Lippincott Williams & Wilkins. ISBN 0-7817-4027-4.
- ↑ Laskey WK, Kussmaul WG (2001). "Subvalvular gradients in patients with valvular aortic stenosis: prevalence, magnitude, and physiological importance". Circulation. 104 (9): 1019–22. PMID 11524395. Retrieved 2012-04-12. Unknown parameter
|month=ignored (help) - ↑ Branch KR, O'Brien KD, Otto CM (2002). "Aortic valve sclerosis as a marker of active atherosclerosis". Curr Cardiol Rep. 4 (2): 111–7. PMID 11827633.
- ↑ {{Faggiano P, D'Aloia A, Antonini-Canterin F, Pinamonti B, DiLenarda A, Brentana L, Metra M, Nodari S, Dei Cas L. Usefulness of cardiac calcification on two-dimensional echocardiography for distinguishing ischemic from nonischemic dilated cardiomyopathy: a preliminary report. J Cardiovasc Med. 2006.}}
- ↑ 36.0 36.1 36.2 Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD; et al. (2008). "2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons". J Am Coll Cardiol. 52 (13): e1–142. doi:10.1016/j.jacc.2008.05.007. PMID 18848134.
- ↑ 37.0 37.1 Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H; et al. (2013). "[Guidelines on the management of valvular heart disease (version 2012). The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)]". G Ital Cardiol (Rome). 14 (3): 167–214. doi:10.1714/1234.13659. PMID 23474606.
- ↑ 38.0 38.1 38.2 38.3 38.4 38.5 Pibarot P, Dumesnil JG (2012). "Low-flow, low-gradient aortic stenosis with normal and depressed left ventricular ejection fraction". J Am Coll Cardiol. 60 (19): 1845–53. doi:10.1016/j.jacc.2012.06.051. PMID 23062546.
- ↑ Normand J, Loire R, Zambartas C (1988). "The anatomical aspects of adult aortic stenosis". Eur Heart J. 9 Suppl E: 31–6. PMID 3402479.
- ↑ Towler DA (2013). "Molecular and cellular aspects of calcific aortic valve disease". Circ Res. 113 (2): 198–208. doi:10.1161/CIRCRESAHA.113.300155. PMC 4057916. PMID 23833294.
- Pages with reference errors
- CS1 maint: Explicit use of et al.
- CS1 maint: Multiple names: authors list
- CS1 maint: PMC format
- Pages with citations using unsupported parameters
- Pages using citations with accessdate and no URL
- CS1 maint: Extra text: authors list
- CS1 maint: Extra text
- Disease
- Valvular heart disease
- Cardiology
- Congenital heart disease
- Cardiac surgery
- Surgery
- Needs CMG review