Alfaxalone: Difference between revisions
m (Bot: Automated text replacement (-{{SIB}} + & -{{EH}} + & -{{EJ}} + & -{{Editor Help}} + & -{{Editor Join}} +)) |
m (Protected "Alfaxalone": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (2 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{Drugbox | ||
| IUPAC_name | | Verifiedfields = changed | ||
| image | | Watchedfields = changed | ||
| verifiedrevid = 477317167 | |||
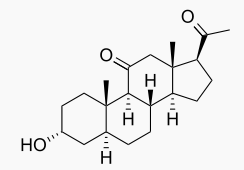
| | | IUPAC_name = 3α-hydroxy-5α-pregnane-11,20-dione | ||
| | | image = Alfaxalone Wiki Str.png | ||
<!--Clinical data--> | |||
| tradename = | |||
| Drugs.com = {{drugs.com|international|alfaxalone}} | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| | | pregnancy_US = <!-- A / B / C / D / X --> | ||
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> | |||
| legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| | | legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> | ||
| pregnancy_AU | | legal_US = Schedule IV | ||
| pregnancy_US | |||
<!--Pharmacokinetic data--> | |||
| legal_AU | | bioavailability = The alfaxalone molecule is solubilised using SBECD. Cyclodextrins are complex polysaccharides derived from starch that supply a hydrophobic centre for lipophilic drugs like alfaxalone. | ||
| legal_CA | |||
| legal_UK | <!--Identifiers--> | ||
| legal_US | | CAS_number_Ref = {{cascite|changed|??}} | ||
| | | CAS_number = 23930-19-0 | ||
| | | ATC_prefix = N01 | ||
| ATC_suffix = AX05 | |||
| PubChem = 104845 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 94637 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = BD07M97B2A | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D07282 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 190279 | |||
<!--Chemical data--> | |||
| C=21 | H=32 | O=3 | |||
| molecular_weight = 332.477 g/mol | |||
| smiles = O=C2[C@H]3[C@H]([C@@H]1CC[C@H](C(=O)C)[C@@]1(C)C2)CC[C@H]4C[C@H](O)CC[C@]34C | |||
| InChI = 1/C21H32O3/c1-12(22)16-6-7-17-15-5-4-13-10-14(23)8-9-20(13,2)19(15)18(24)11-21(16,17)3/h13-17,19,23H,4-11H2,1-3H3/t13-,14+,15-,16+,17-,19+,20-,21+/m0/s1 | |||
| InChIKey = DUHUCHOQIDJXAT-OLVMNOGEBO | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C21H32O3/c1-12(22)16-6-7-17-15-5-4-13-10-14(23)8-9-20(13,2)19(15)18(24)11-21(16,17)3/h13-17,19,23H,4-11H2,1-3H3/t13-,14+,15-,16+,17-,19+,20-,21+/m0/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = DUHUCHOQIDJXAT-OLVMNOGESA-N | |||
}} | }} | ||
__NOTOC__ | |||
{{SI}} | |||
{{CMG}} | |||
== Overview == | |||
'''Alfaxalone''' ([[International Nonproprietary Name|INN]], [[Japanese Accepted Name|JAN]]), also known as '''alphaxalone''' or '''alphaxolone''' ([[British Approved Name|BAN]]), is a [[neuroactive steroid]] and [[general anaesthetic]].<ref name="GanellinTriggle1996">{{cite book|author1=C.R. Ganellin|author2=David J. Triggle|title=Dictionary of Pharmacological Agents|url=http://books.google.com/books?id=A0THacd46ZsC&pg=PA1094|date=21 November 1996|publisher=CRC Press|isbn=978-0-412-46630-4|pages=1094–}}</ref> It is used in veterinary practice under the trade name '''Alfaxan''',<ref>{{ cite web| url = http://www.drugs.com/international/alfaxalone.html | title = Alfaxalone| publisher = Drugs.com}}</ref> and is licensed for use in both dogs and cats.{{citation needed|date=November 2011}} Along with [[alfadolone]], it is also one of the constituents of anesthetic [[combination drug|drug mixture]] [[althesin]]. | |||
{{ | Unlike some of its predecessors alfaxalone is not associated with histamine release and anaphylaxis.{{citation needed|date=November 2011}} | ||
A study 1987 found the primary mechanism for the anaesthetic action of alfaxalone to be modulation of neuronal cell membrane chloride ion transport, induced by binding of alfaxalone to [[GABAA|GABA<sub>A</sub>]] cell surface receptors. | |||
<ref>{{ cite pmid | 3819824 }}</ref> | |||
A 1994 study found that alfaxalone binds to a different region of this receptor than the [[benzodiazepine]]s. | |||
.<ref>{{ cite pmid | 7806498 }}</ref> These benzodiazepine-insensitive [[GABAA|GABA<sub>A</sub>]] receptors are located extrasynaptically and are responsible for tonic inhibition. The occurrence of tonic [[GABAA|GABA<sub>A</sub>]] inhibition coincides with the expression of relatively rare receptor subunits, particularly the α4, α6, and δ subunits, and as a general rule-of-thumb, δ subunit-containing receptors are extrasynaptic.<ref>Wahab A, Heinemann U, Albus K (October 2009). "Effects of gamma-aminobutyric acid (GABA) agonists and a GABA uptake inhibitor on pharmacoresistant seizure like events in organotypic hippocampal slice cultures". Epilepsy Research 86 (2-3): 113–23. doi:10.1016/j.eplepsyres.2009.05.008. PMID 19535226.</ref> | |||
Alfaxalone is metabolised rapidly in the liver. It has a very short plasma elimination half-life in dogs and cats.{{citation needed|date=November 2011}} | |||
==See also== | |||
* [[Ganaxolone]] | |||
* [[Hydroxydione]] | |||
* [[Minaxolone]] | |||
* [[Pregnanolone]] | |||
* [[Renanolone]] | |||
==References== | |||
{{Reflist|2}} | |||
{{General anesthetics}} | {{General anesthetics}} | ||
{{GABAAR PAMs}} | |||
{{Glycinergics}} | |||
[[Category:Drug]] | |||
[[Category:General anesthetics]] | |||
[[Category:Neurosteroids]] | |||
[[Category:Alcohols]] | |||
[[Category:Ketones]] | |||
[[Category:GABAA receptor positive allosteric modulators]] | |||
[[Category:Glycine receptor agonists]] | |||
{{ | {{nervous-system-drug-stub}} | ||
Latest revision as of 17:19, 18 August 2015
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | The alfaxalone molecule is solubilised using SBECD. Cyclodextrins are complex polysaccharides derived from starch that supply a hydrophobic centre for lipophilic drugs like alfaxalone. |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C21H32O3 |
| Molar mass | 332.477 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Alfaxalone |
|
Articles |
|---|
|
Most recent articles on Alfaxalone |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Alfaxalone at Clinical Trials.gov Clinical Trials on Alfaxalone at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Alfaxalone
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Alfaxalone Discussion groups on Alfaxalone Patient Handouts on Alfaxalone Directions to Hospitals Treating Alfaxalone Risk calculators and risk factors for Alfaxalone
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Alfaxalone |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Alfaxalone (INN, JAN), also known as alphaxalone or alphaxolone (BAN), is a neuroactive steroid and general anaesthetic.[1] It is used in veterinary practice under the trade name Alfaxan,[2] and is licensed for use in both dogs and cats.[citation needed] Along with alfadolone, it is also one of the constituents of anesthetic drug mixture althesin.
Unlike some of its predecessors alfaxalone is not associated with histamine release and anaphylaxis.[citation needed]
A study 1987 found the primary mechanism for the anaesthetic action of alfaxalone to be modulation of neuronal cell membrane chloride ion transport, induced by binding of alfaxalone to GABAA cell surface receptors. [3]
A 1994 study found that alfaxalone binds to a different region of this receptor than the benzodiazepines. .[4] These benzodiazepine-insensitive GABAA receptors are located extrasynaptically and are responsible for tonic inhibition. The occurrence of tonic GABAA inhibition coincides with the expression of relatively rare receptor subunits, particularly the α4, α6, and δ subunits, and as a general rule-of-thumb, δ subunit-containing receptors are extrasynaptic.[5]
Alfaxalone is metabolised rapidly in the liver. It has a very short plasma elimination half-life in dogs and cats.[citation needed]
See also
References
- ↑ C.R. Ganellin; David J. Triggle (21 November 1996). Dictionary of Pharmacological Agents. CRC Press. pp. 1094–. ISBN 978-0-412-46630-4.
- ↑ "Alfaxalone". Drugs.com.
- ↑ PMID 3819824 (PMID 3819824)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 7806498 (PMID 7806498)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ Wahab A, Heinemann U, Albus K (October 2009). "Effects of gamma-aminobutyric acid (GABA) agonists and a GABA uptake inhibitor on pharmacoresistant seizure like events in organotypic hippocampal slice cultures". Epilepsy Research 86 (2-3): 113–23. doi:10.1016/j.eplepsyres.2009.05.008. PMID 19535226.
- Pages with script errors
- Pages with incomplete PMID references
- Template:drugs.com link with non-standard subpage
- Articles with changed CASNo identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- All articles with unsourced statements
- Articles with unsourced statements from November 2011
- Articles with invalid date parameter in template
- Drug
- General anesthetics
- Neurosteroids
- Alcohols
- Ketones
- GABAA receptor positive allosteric modulators
- Glycine receptor agonists