Prazosin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: João André Alves Silva, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Prazosin is a alpha-adrenergic blocker that is FDA approved for the {{{indicationType}}} of hypertension. Common adverse reactions include orthostatic hypotension, palpitations, nausea, asthenia, dizziness, headache, lethargy and somnolence.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Hypertension
- Initial dose:
- 1 mg two or three times a day
- Maintenance dose:
- Dosage may be slowly increased to a total daily dose of 20 mg given in divided doses. The therapeutic dosages most commonly employed have ranged from 6 mg to 15 mg daily given in divided doses. Doses higher than 20 mg usually do not increase efficacy, however a few patients may benefit from further increases up to a daily dose of 40 mg given in divided doses. After initial titration some patients can be maintained adequately on a twice daily dosage regimen.
- Use with other drugs:
- When adding a diuretic or other antihypertensive agent, the dose of prazosin should be reduced to 1 mg or 2 mg three times a day and retitration then carried out.
- Concomitant administration of prazosin with a PDE-5 inhibitor can result in additive blood pressure lowering effects and symptomatic hypotension; therefore, PDE-5 inhibitor therapy should be initiated at the lowest dose in patients taking prazosin.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Prazosin in adult patients.
Non–Guideline-Supported Use
Benign prostatic hyperplasia
- Prazosin has been used in the treatment benign prostatic hyperplasia.[1]
Congestive heart failure
- Prazosin has been successfully used in the hemodynamic management of patients with congestive heart failure.[2]
Dream disorder - posttraumatic stress disorder
- A 6-week open-label trial, with 5 patients, with history of exposure to civilian trauma, in a non-military environment, who subsequently developed posttraumatic stress disorder (PTSD):[3][4]
- 1 to 4 mg/day
- An 8-week open-label trial, with 4 patients, with history of combat trauma nightmares and chronic DSM-IV posttraumatic stress disorder (PTSD) with concomitant severe intractable combat trauma nightmares:[5]
- 2 to 10 mg/day
Erectile dysfunction
- The association of alprostadil and prazosin is an effective option for the treatment of erectile dysfunction.
- The therapeutic response to prazosin proved to be better than the placebo response for the following dosages of prazosin:
- 250, 500, 1000, and 2000 micrograms[6]
Poisoning due to scorpion venom
- A study shows that there is benefit in the use of prazosin in cases of envenomation by the Indian red scorpion, showed by the reduction of morbidity, mortality, and recovery time.
- 0.5 mg every 6 hours, until improvement of the clinical status.[7]
Raynaud's phenomenon
- According to a double-blind study, Prazosin shows therapeutic benefit when compared to the placebo, for the treatment of Raynaud phenomenon.
- 1 mg orally 3 times/day[8]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Label indications Use of Prazosin in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Prazosin in pediatric patients.
Non–Guideline-Supported Use
Poisoning due to scorpion venom
- A study shows that there is benefit in the use of prazosin in cases of envenomation by the Indian red scorpion, showed by the reduction of morbidity, mortality, and recovery time.
- 0.25 mg every 6 hours, until improvement of the clinical status.[7]
Contraindications
- Prazosin is contraindicated in patients with known sensitivity to:
- Quinazolines
- Prazosin
- Any of the inert ingredients
Warnings
- Prazosin, as other alpha-blockers, may cause:
- Syncope with sudden loss of consciousness:
- May be due to an excessive postural hypotensive effect
- Occasionally the syncopal episode has been preceded by a bout of severe tachycardia with heart rates of 120–160 beats per minute
- Syncopal episodes have usually occurred within 30 to 90 minutes of the initial dose of the drug
- These have often been reported in association with rapid dosage increases or the introduction of another antihypertensive drug into the regimen of a patient taking high doses of prazosin.
- The incidence of syncopal episodes is approximately 1% in patients given an initial dose of 2 mg or greater.
- Clinical trials conducted during the investigational phase of this drug suggest that syncopal episodes can be minimized by limiting the initial dose of the drug to 1 mg, by subsequently increasing the dosage slowly, and by introducing any additional antihypertensive drugs into the patient's regimen with caution.
- If syncope occurs, the patient should be placed in the recumbent position and treated supportively as necessary. This adverse effect is self-limiting and in most cases does not recur after the initial period of therapy or during subsequent dose titration.
- Hypotension may develop in patients given prazosin who are also receiving a beta-blocker such as propranolol.
- Patients should always be started on the 1 mg capsules of prazosin. The 2 and 5 mg capsules are not indicated for initial therapy.
- More common than loss of consciousness are the symptoms often associated with lowering of the blood pressure, namely:
- The patient should be cautioned about these possible adverse effects and advised what measures to take should they develop. The patient should also be cautioned to avoid situations where injury could result should syncope occur during the initiation of prazosin therapy.
Adverse Reactions
Clinical Trials Experience
- Clinical trials were conducted on more than 900 patients. During these trials and subsequent marketing experience, the most frequent reactions associated with prazosin therapy are:
- Dizziness 10.3%
- Headache 7.8%
- Drowsiness 7.6%
- Lack of energy 6.9%
- Weakness
- Palpitations 5.3%
- Nausea 4.9%.
- In most instances, side effects have disappeared with continued therapy or have been tolerated with no decrease in dose of drug.
- Less frequent adverse reactions which are reported to occur in 1–4% of patients are:
Central Nervous System
Cardiovascular
Gastrointestinal
Miscellaneous
- Rash
- Urinary frequency
- Blurred vision
- Reddened sclera
- Epistaxis
- Dry mouth
- Nasal congestion
- Fewer than 1% of patients have reported the following:
Gastrointestinal
- Abdominal discomfort
- Liver function abnormalities
- Pancreatitis
Cardiovascular
Central Nervous System
Dermatologic
Genitourinary
EENT
Miscellaneous
- Diaphoresis
- Fever
- Positive ANA titer
- Arthralgia
- Single reports of pigmentary mottling and serous retinopathy, and a few reports of cataract development or disappearance have been reported. In these instances, the exact causal relationship has not been established because the baseline observations were frequently inadequate.
- In more specific slit-lamp and funduscopic studies, which included adequate baseline examinations, no drug-related abnormal ophthalmological findings have been reported.
- Literature reports exist associating prazosin therapy with a worsening of pre-existing narcolepsy. A causal relationship is uncertain in these cases.
Postmarketing Experience
General
Autonomic Nervous System
Cardiovascular
Endocrine
Psychiatric
Skin/Appendages
Vascular
Vision
- Eye pain
Special Senses
- During cataract surgery, a variant of small pupil syndrome known as Intraoperative Floppy Iris Syndrome (IFIS) has been reported in association with alpha-1 blocker therapy
Drug Interactions
- Prazosin has been administered without any adverse drug interaction in limited clinical experience to date with the following:
- Tranquilizers and sedatives:
- Addition of a diuretic or other antihypertensive agent to prazosin has been shown to cause an additive hypotensive effect. This effect can be minimized by reducing the prazosin dose to 1 to 2 mg three times a day, by introducing additional antihypertensive drugs cautiously, and then by retitrating prazosin based on clinical response.
- Concomitant administration of prazosin with a phosphodiesterase-5 inhibitor can result in:
- Additive blood pressure lowering effects
- Symptomatic hypotension
Use in Specific Populations
Pregnancy
- Prazosin has been shown to be associated with decreased litter size at birth, 1, 4, and 21 days of age in rats when given doses more than 225 times the usual maximum recommended human dose. No evidence of drug-related external, visceral, or skeletal fetal abnormalities were observed. No drug-related external, visceral, or skeletal abnormalities were observed in fetuses of pregnant rabbits and pregnant monkeys at doses more than 225 times and 12 times the usual maximum recommended human dose, respectively.
- The use of prazosin and a beta-blocker for the control of severe hypertension in 44 pregnant women revealed no drug-related fetal abnormalities or adverse effects. Therapy with prazosin was continued for as long as 14 weeks. Prazosin has also been used alone or in combination with other hypotensive agents in severe hypertension of pregnancy by other investigators. No fetal or neonatal abnormalities have been reported with the use of prazosin.
- There are no adequate and well controlled studies which establish the safety of prazosin in pregnant women. Prazosin should be used during pregnancy only if the potential benefit justifies the potential risk to the mother and fetus.
Pregnancy Category (AUS): B2
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of prazosin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Prazosin during labor and delivery.
Nursing Mothers
- Prazosin has been shown to be excreted in small amounts in human milk. Caution should be exercised when prazosin is administered to a nursing woman.
Pediatric Use
- Safety and effectiveness in children have not been established.
Geriatic Use
There is no FDA guidance on the use of Prazosin in geriatric settings.
Gender
There is no FDA guidance on the use of Prazosin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Prazosin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Prazosin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Prazosin in patients with hepatic impairment.
Females of Reproductive Potential and Males
- No carcinogenic potential was demonstrated in an 18 month study in rats with prazosin at dose levels more than 225 times the usual maximum recommended human dose of 20 mg per day.
- Prazosin was not mutagenic in in vivo genetic toxicology studies.
- In a fertility and general reproductive performance study in rats, both males and females, treated with 75 mg/kg (225 times the usual maximum recommended human dose), demonstrated decreased fertility, while those treated with 25 mg/kg (75 times the usual maximum recommended human dose) did not.
- In chronic studies (one year or more) of prazosin in rats and dogs, testicular changes consisting of atrophy and necrosis occurred at 25 mg/kg/day (75 times the usual maximum recommended human dose). No testicular changes were seen in rats or dogs at 10 mg/kg/day (30 times the usual maximum recommended human dose). In view of the testicular changes observed in animals, 105 patients on long term prazosin therapy were monitored for 17-ketosteroid excretion and no changes indicating a drug effect were observed. In addition, 27 males on prazosin for up to 51 months did not have changes in sperm morphology suggestive of drug effect.
Immunocompromised Patients
There is no FDA guidance one the use of Prazosin in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
Hypotension
- Hypotension may develop in patients given prazosin who are also receiving a beta-blocker such as propranolol.
IV Compatibility
There is limited information regarding the compatibility of Prazosin and IV administrations.
Overdosage
- Accidental ingestion of at least 50 mg of prazosin in a two year old child resulted in:
- Profound drowsiness
- Depressed reflexes
- No decrease in blood pressure was noted.
- Recovery was uneventful.
Acute Overdose
Hypotension
- Should overdosage lead to hypotension, support of the cardiovascular system is of first importance.
Management
- Restoration of blood pressure and normalization of heart rate may be accomplished by keeping the patient in the supine position.
- If this measure is inadequate:
- Shock should first be treated with volume expanders.
- If necessary, vasopressors should then be used.
- Renal function should be monitored and supported as needed.
Pharmacology
| |
Prazosin
| |
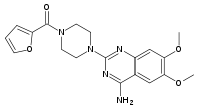
| Systematic (IUPAC) name | |
| 2-[4-(2-Furoyl)piperazin-1-yl]-6,7-dimethoxyquinazolin-4-amine | |
| Identifiers | |
| CAS number | |
| ATC code | C02 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 383.401 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ~60% |
| Protein binding | 97% |
| Metabolism | ? |
| Half life | 2–3 hours |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | Oral |
Mechanism of Action
- The exact mechanism of the hypotensive action of prazosin is unknown:
- Prazosin causes a decrease in total peripheral resistance and was originally thought to have a direct relaxant action on vascular smooth muscle.
- Recent animal studies, however, have suggested that the vasodilator effect of prazosin is also related to blockade of postsynaptic alpha-adrenoceptors. The results of dog forelimb experiments demonstrate that the peripheral vasodilator effect of prazosin is confined mainly to the level of the resistance vessels (arterioles).
- Unlike conventional alpha-blockers, the antihypertensive action of prazosin is usually not accompanied by a reflex tachycardia. Tolerance has not been observed to develop in long term therapy.
Structure
Prazosin, a quinazoline derivative, is the first of a new chemical class of antihypertensives. It is the hydrochloride salt of 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furoyl) piperazine and its structural formula is:

Molecular formula C19H21N5O4•HCl It is a white, crystalline substance, slightly soluble in water and isotonic saline, and has a molecular weight of 419.87. Each 1 mg capsule of prazosin for oral use contains drug equivalent to 1 mg free base. Inert ingredients in the formulations are: hard gelatin capsules (which may contain Blue 1, Red 3, Red 28, Red 40, and other inert ingredients); magnesium stearate; sodium lauryl sulfate; starch; sucrose.
Pharmacodynamics
- Hemodynamic studies have been carried out in man following acute single dose administration and during the course of long term maintenance therapy.
- The results confirm that the therapeutic effect is a fall in blood pressure unaccompanied by a clinically significant change in cardiac output, heart rate, renal blood flow and glomerular filtration rate.
- There is no measurable negative chronotropic effect.
- In clinical studies to date, prazosin hydrochloride has not increased plasma renin activity.
- In man, blood pressure is lowered in both the supine and standing positions. This effect is most pronounced on the diastolic blood pressure.
- In clinical studies in which lipid profiles were followed, there were generally no adverse changes noted between pre- and post-treatment lipid levels.
Pharmacokinetics
- Following oral administration, human plasma concentrations reach a peak at about three hours with a plasma half-life of two to three hours. The drug is highly bound to plasma protein. Bioavailability studies have demonstrated that the total absorption relative to the drug in a 20% alcoholic solution is 90%, resulting in peak levels approximately 65% of that of the drug in solution.
- Animal studies indicate that prazosin hydrochloride is extensively metabolized, primarily by demethylation and conjugation, and excreted mainly via bile and feces. Less extensive human studies suggest similar metabolism and excretion in man.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of prazosin in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of prazosin in the drug label.
How Supplied

Storage
There is limited information regarding storage conditions of doxazosin in the drug label.
Images
Drug Images
{{#ask: Page Name::Prazosin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Prazosin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Dizziness or drowsiness may occur after the first dose of this medicine. Avoid driving or performing hazardous tasks for the first 24 hours after taking this medicine or when the dose is increased. Dizziness, lightheadedness, or fainting may occur, especially when rising from a lying or sitting position. Getting up slowly may help lessen the problem. These effects may also occur if you drink alcohol, stand for long periods of time, exercise, or if the weather is hot. While taking MINIPRESS, be careful in the amount of alcohol you drink. Also, use extra care during exercise or hot weather, or if standing for long periods. Check with your physician if you have any questions.
Precautions with Alcohol
- Effects such as such as dizziness, lightheadedness, or fainting may occur when taking prazosin, especially when rising from a lying or sitting position:
Brand Names
- Minipress
Look-Alike Drug Names
- N/A
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Lowe F (1999). "Alpha-1-adrenoceptor blockade in the treatment of benign prostatic hyperplasia". Prostate Cancer Prostatic Dis. 2 (3): 110–119. doi:10.1038/sj.pcan.4500302. PMID 12496820.
- ↑ Westheim A, Koss A, Sivertssen E (1986). "Hemodynamic effects at rest and during exercise in long-term treatment with prazosin in chronic congestive heart failure". Acta Med Scand. 219 (5): 449–53. PMID 3526819.
- ↑ Raskind MA, Thompson C, Petrie EC, Dobie DJ, Rein RJ, Hoff DJ; et al. (2002). "Prazosin reduces nightmares in combat veterans with posttraumatic stress disorder". J Clin Psychiatry. 63 (7): 565–8. PMID 12143911.
- ↑ Taylor F, Raskind MA (2002). "The alpha1-adrenergic antagonist prazosin improves sleep and nightmares in civilian trauma posttraumatic stress disorder". J Clin Psychopharmacol. 22 (1): 82–5. PMID 11799347.
- ↑ Raskind MA, Dobie DJ, Kanter ED, Petrie EC, Thompson CE, Peskind ER (2000). "The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: a report of 4 cases". J Clin Psychiatry. 61 (2): 129–33. PMID 10732660.
- ↑ Peterson CA, Bennett AH, Hellstrom WJ, Kaiser FE, Morley JE, Nemo KJ; et al. (1998). "Erectile response to transurethral alprostadil, prazosin and alprostadil-prazosin combinations". J Urol. 159 (5): 1523–7, discussion 1527-8. doi:10.1097/00005392-199805000-00030. PMID 9554347.
- ↑ 7.0 7.1 Bawaskar HS, Bawaskar PH (1996). "Severe envenoming by the Indian red scorpion Mesobuthus tamulus: the use of prazosin therapy". QJM. 89 (9): 701–4. PMID 8917746.
- ↑ Wollersheim H, Thien T, Fennis J, van Elteren P, van 't Laar A (1986). "Double-blind, placebo-controlled study of prazosin in Raynaud's phenomenon". Clin Pharmacol Ther. 40 (2): 219–25. PMID 3731684.
{{#subobject:
|Page Name=Prazosin |Pill Name=000694310.jpg |Drug Name=Prazosin |Pill Ingred=* fd&c blue no. 1, fd&c red no. 3, d&c red no. 28, fd&c red no. 40, magnesium stearate, sodium laurel sulfate and sucrose|+sep=; |Pill Imprint=Pfizer;431;Minipress |Pill Dosage=1 mg |Pill Color=White|+sep=; |Pill Shape=Capsule |Pill Size (mm)=18.00 |Pill Scoring=1 |Pill Image= |Drug Author=Pfizer Laboratories Div Pfizer Inc |NDC=
}}
{{#subobject:
|Page Name=Prazosin |Pill Name=Minipress 2mg.jpg |Drug Name=Prazosin |Pill Ingred=fd&c blue no. 1, fd&c red no. 3, d&c red no. 28, fd&c red no. 40, magnesium stearate, sodium lauryl sulfate and sucrose|+sep=; |Pill Imprint=Pfizer;437;Minipress |Pill Dosage=2 mg |Pill Color=Red|+sep=; |Pill Shape=Capsule |Pill Size (mm)=18.00 |Pill Scoring=1 |Pill Image= |Drug Author=Pfizer Laboratories Div Pfizer Inc |NDC=
}}
{{#subobject:
|Page Name=Prazosin |Pill Name=Minipress 5mg.jpg |Drug Name=Prazosin |Pill Ingred=fd&c blue no. 1, fd&c red no. 3, d&c red no. 28, fd&c red no. 40, magnesium stearate, sodium laurel sulfate and sucrose|+sep=; |Pill Imprint=Pfizer;438;Minipress |Pill Dosage=5 mg |Pill Color=Blue|+sep=; |Pill Shape=Capsule |Pill Size (mm)=22.00 |Pill Scoring=1 |Pill Image= |Drug Author=Pfizer Laboratories Div Pfizer Inc |NDC=
}}
{{#subobject:
|Label Page=Prazosin |Label Name=Prazosin 1.jpg
}}
{{#subobject:
|Label Page=Prazosin |Label Name=MINIPRESS (PRAZOSIN HYDROCHLORIDE) CAPSULE copy 10.jpg
}}
{{#subobject:
|Label Page=Prazosin |Label Name=MINIPRESS (PRAZOSIN HYDROCHLORIDE) CAPSULE copy 12.jpg
}}
{{#subobject:
|Label Page=Prazosin |Label Name=MINIPRESS (PRAZOSIN HYDROCHLORIDE) CAPSULE copy 9.jpg
}}
{{#subobject:
|Label Page=Prazosin |Label Name=MINIPRESS (PRAZOSIN HYDROCHLORIDE) CAPSULE copy 11.jpg
}}
{{#subobject:
|Label Page=Prazosin |Label Name=MINIPRESS (PRAZOSIN HYDROCHLORIDE) CAPSULE copy 8.jpg
}}
{{#subobject:
|Label Page=Prazosin |Label Name=MINIPRESS (PRAZOSIN HYDROCHLORIDE) CAPSULE copy 7.jpg
}}