Vinflunine: Difference between revisions
No edit summary |
m (Protected "Vinflunine": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (One intermediate revision by one other user not shown) | |||
| Line 3: | Line 3: | ||
| verifiedrevid = 449351504 | | verifiedrevid = 449351504 | ||
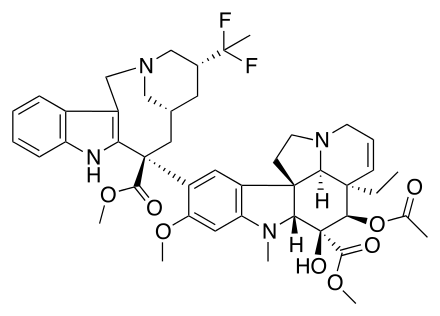
| IUPAC_name = methyl (2β,3β,4β,5α,12β,19α)- 4-(acetyloxy)- 15-[(4''R'',6''R'',8''S'')- 4-(1,1-difluoroethyl)- 8-(methoxycarbonyl)- 1,3,4,5,6,7,8,9-octahydro- 2,6-methanoazecino[4,3-''b'']indol- 8-yl]- 3-hydroxy- 16-methoxy- 1-methyl- 6,7-didehydroaspidospermidine- 3-carboxylate | | IUPAC_name = methyl (2β,3β,4β,5α,12β,19α)- 4-(acetyloxy)- 15-[(4''R'',6''R'',8''S'')- 4-(1,1-difluoroethyl)- 8-(methoxycarbonyl)- 1,3,4,5,6,7,8,9-octahydro- 2,6-methanoazecino[4,3-''b'']indol- 8-yl]- 3-hydroxy- 16-methoxy- 1-methyl- 6,7-didehydroaspidospermidine- 3-carboxylate | ||
| image = Vinflunine. | | image = Vinflunine.png | ||
<!--Clinical data--> | <!--Clinical data--> | ||
| Line 46: | Line 46: | ||
| smiles = CC[C@@]12C=CCN3[C@@H]1[C@]4(CC3)[C@H]([C@]([C@@H]2OC(=O)C)(C(=O)OC)O)N(C5=CC(=C(C=C45)[C@]6(C[C@H]7C[C@H](CN(C7)CC8=C6NC9=CC=CC=C89)C(C)(F)F)C(=O)OC)OC)C | | smiles = CC[C@@]12C=CCN3[C@@H]1[C@]4(CC3)[C@H]([C@]([C@@H]2OC(=O)C)(C(=O)OC)O)N(C5=CC(=C(C=C45)[C@]6(C[C@H]7C[C@H](CN(C7)CC8=C6NC9=CC=CC=C89)C(C)(F)F)C(=O)OC)OC)C | ||
}} | }} | ||
__NOTOC__ | |||
{{SI}} | |||
{{CMG}} | |||
==Overview== | |||
'''Vinflunine''' ([[International Nonproprietary Name|INN]], trade name '''Javlor''') is a novel fluorinated [[Vinca alkaloid]]<ref name="pmid9515574">{{cite journal |author=Kruczynski A, Barret JM, Etiévant C, Colpaert F, Fahy J, Hill BT |title=Antimitotic and tubulin-interacting properties of vinflunine, a novel fluorinated Vinca alkaloid |journal=Biochem. Pharmacol. |volume=55 |issue=5 |pages=635–48 |date=March 1998 |pmid=9515574 |doi= 10.1016/S0006-2952(97)00505-4|url=http://linkinghub.elsevier.com/retrieve/pii/S0006-2952(97)00505-4}}</ref> undergoing research for the treatment of bladder cancer. It was originally discovered by the team of the Professor Jean-Claude Jacquesy (UMR CNRS 6514 - Poitiers University),<ref>Vinca Alkaloids in Superacidic Media: A Method for Creating a New Family of Antitumor Derivatives Fahy, J.; Duflos, A.; Ribet, J.-P.; Jacquesy, J.-C.; Berrier, C.; Jouannetaud, M.-P.; Zunino, F. | '''Vinflunine''' ([[International Nonproprietary Name|INN]], trade name '''Javlor''') is a novel fluorinated [[Vinca alkaloid]]<ref name="pmid9515574">{{cite journal |author=Kruczynski A, Barret JM, Etiévant C, Colpaert F, Fahy J, Hill BT |title=Antimitotic and tubulin-interacting properties of vinflunine, a novel fluorinated Vinca alkaloid |journal=Biochem. Pharmacol. |volume=55 |issue=5 |pages=635–48 |date=March 1998 |pmid=9515574 |doi= 10.1016/S0006-2952(97)00505-4|url=http://linkinghub.elsevier.com/retrieve/pii/S0006-2952(97)00505-4}}</ref> undergoing research for the treatment of bladder cancer. It was originally discovered by the team of the Professor Jean-Claude Jacquesy (UMR CNRS 6514 - Poitiers University),<ref>Vinca Alkaloids in Superacidic Media: A Method for Creating a New Family of Antitumor Derivatives Fahy, J.; Duflos, A.; Ribet, J.-P.; Jacquesy, J.-C.; Berrier, C.; Jouannetaud, M.-P.; Zunino, F. | ||
J. Am. Chem. Soc.; (Communication); 1997; 119(36); 8576-8577.</ref> developed by [[Laboratoires Pierre Fabre]] and was licensed to [[Bristol-Myers Squibb]] for development in certain countries, including the [[United States]]. | J. Am. Chem. Soc.; (Communication); 1997; 119(36); 8576-8577.</ref> developed by [[Laboratoires Pierre Fabre]] and was licensed to [[Bristol-Myers Squibb]] for development in certain countries, including the [[United States]]. | ||
| Line 53: | Line 57: | ||
==References== | ==References== | ||
{{Reflist}} | {{Reflist|2}} | ||
{{Chemotherapeutic agents}} | {{Chemotherapeutic agents}} | ||
[[Category:Alkaloids]] | [[Category:Alkaloids]] | ||
[[Category:Bristol-Myers Squibb]] | [[Category:Bristol-Myers Squibb]] | ||
[[Category: | [[Category:Drug]] | ||
Latest revision as of 17:24, 20 August 2015
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| [[Regulation of therapeutic goods |Template:Engvar data]] | |
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C45H54F2N4O8 |
| Molar mass | 816.92 g/mol |
| 3D model (JSmol) | |
| |
| | |
|
WikiDoc Resources for Vinflunine |
|
Articles |
|---|
|
Most recent articles on Vinflunine |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Vinflunine at Clinical Trials.gov Clinical Trials on Vinflunine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Vinflunine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Vinflunine Discussion groups on Vinflunine Patient Handouts on Vinflunine Directions to Hospitals Treating Vinflunine Risk calculators and risk factors for Vinflunine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Vinflunine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Vinflunine (INN, trade name Javlor) is a novel fluorinated Vinca alkaloid[1] undergoing research for the treatment of bladder cancer. It was originally discovered by the team of the Professor Jean-Claude Jacquesy (UMR CNRS 6514 - Poitiers University),[2] developed by Laboratoires Pierre Fabre and was licensed to Bristol-Myers Squibb for development in certain countries, including the United States.
On November 23, 2007, Pierre Fabre and BMS announced that they are terminating their license agreement for the development of vinflunine.[3]
References
- ↑ Kruczynski A, Barret JM, Etiévant C, Colpaert F, Fahy J, Hill BT (March 1998). "Antimitotic and tubulin-interacting properties of vinflunine, a novel fluorinated Vinca alkaloid". Biochem. Pharmacol. 55 (5): 635–48. doi:10.1016/S0006-2952(97)00505-4. PMID 9515574.
- ↑ Vinca Alkaloids in Superacidic Media: A Method for Creating a New Family of Antitumor Derivatives Fahy, J.; Duflos, A.; Ribet, J.-P.; Jacquesy, J.-C.; Berrier, C.; Jouannetaud, M.-P.; Zunino, F. J. Am. Chem. Soc.; (Communication); 1997; 119(36); 8576-8577.
- ↑ "Bristol -Myers Squibb press release - Bristol-Myers Squibb and Pierre Fabre Provide Update On Vinflunine Development Status". Retrieved 2008-06-27.
- Pages with script errors
- CS1 maint: Multiple names: authors list
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- Alkaloids
- Bristol-Myers Squibb
- Drug