Vigabatrin: Difference between revisions
No edit summary |
No edit summary |
||
| Line 274: | Line 274: | ||
*SABRIL decreases [[alanine transaminase]] ([[ALT]]) and [[aspartate transaminase]] ([[AST]]) plasma activity in up to 90% of patients. In some patients, these enzymes become undetectable. The suppression of [[ALT]] and [[AST]] activity by SABRIL may preclude the use of these markers, especially [[ALT]], to detect early [[hepatic injury]]. | *SABRIL decreases [[alanine transaminase]] ([[ALT]]) and [[aspartate transaminase]] ([[AST]]) plasma activity in up to 90% of patients. In some patients, these enzymes become undetectable. The suppression of [[ALT]] and [[AST]] activity by SABRIL may preclude the use of these markers, especially [[ALT]], to detect early [[hepatic injury]]. | ||
*SABRIL may increase the amount of [[amino acids]] in the urine, possibly leading to a false positive test for certain rare genetic [[metabolic diseases]] (e.g., [[alpha aminoadipic aciduria]]). | *SABRIL may increase the amount of [[amino acids]] in the urine, possibly leading to a false positive test for certain rare genetic [[metabolic diseases]] (e.g., [[alpha aminoadipic aciduria]]). | ||
|FDAPregCat=C | |||
|useInPregnancyFDA=Vigabatrin produced developmental toxicity, including teratogenic and neurohistopathological effects, when administered to pregnant animals at clinically relevant doses. In addition, developmental neurotoxicity was observed in rats treated with vigabatrin during a period of postnatal development corresponding to the third trimester of human pregnancy. There are no adequate and well-controlled studies in pregnant women. SABRIL should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. | |||
Administration of vigabatrin (oral doses of 50 to 200 mg/kg) to pregnant rabbits throughout the period of organogenesis was associated with an increased incidence of malformations (cleft palate) and embryo-fetal death; these findings were observed in two separate studies. The no-effect dose for teratogenicity and embryolethality in rabbits (100 mg/kg) is approximately 1/2 the maximum recommended human dose (MRHD) of 3 g/day on a body surface area (mg/m2) basis. In rats, oral administration of vigabatrin (50, 100, or 150 mg/kg) throughout organogenesis resulted in decreased fetal body weights and increased incidences of fetal anatomic variations. The no-effect dose for embryo-fetal toxicity in rats (50 mg/kg) is approximately 1/5 the MRHD on a mg/m2 basis. Oral administration of vigabatrin (50, 100, 150 mg/kg) to rats from the latter part of pregnancy through weaning produced long-term neurohistopathological (hippocampal vacuolation) and neurobehavioral (convulsions) abnormalities in the offspring. A no-effect dose for developmental neurotoxicity in rats was not established; the low-effect dose (50 mg/kg) is approximately 1/5 the MRHD on a mg/m2 basis. | |||
In a published study, vigabatrin (300 or 450 mg/kg) was administered by intraperitoneal injection to a mutant mouse strain on a single day during organogenesis (day 7, 8, 9, 10, 11, or 12). An increase in malformations (including cleft palate) was observed at both doses. | |||
Oral administration of vigabatrin (5, 15, or 50 mg/kg) to young rats during the neonatal and juvenile periods of development (postnatal days 4-65) produced neurobehavioral (convulsions, neuromotor impairment, learning deficits) and neurohistopathological (brain vacuolation, decreased myelination, and retinal dysplasia) abnormalities in treated animals. The early postnatal period in rats is generally thought to correspond to late pregnancy in humans in terms of brain development. The no-effect dose for developmental neurotoxicity in juvenile rats (5 mg/kg) was associated with plasma vigabatrin exposures (AUC) less than 1/30 of those measured in pediatric patients receiving an oral dose of 50 mg/kg. | |||
|useInNursing=Vigabatrin is excreted in human milk. Because of the potential for serious adverse reactions from vigabatrin in nursing infants a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother | |||
|useInPed=The safety and effectiveness of SABRIL as adjunctive treatment of refractory complex partial seizures in pediatric patients aged 10 to 16 years of age have been established. The dosing recommendation in this population varies according to age group and is weight based. Adverse reactions in this pediatric population are similar to those observed in the adult population. | |||
*The safety and effectiveness of SABRIL have not been established in pediatric patients under 10 years of age with refractory complex partial seizures. | |||
*The safety and effectiveness of SABRIL as monotherapy for pediatric patients with infantile spasms (1 month to 2 years of age) have been established. | |||
*Duration of therapy for infantile spasms was evaluated in a post hoc analysis of a Canadian Pediatric Epilepsy Network (CPEN) study of developmental outcomes in infantile spasms patients. This analysis suggests that a total duration of 6 months of vigabatrin therapy is adequate for the treatment of infantile spasms. However, prescribers must use their clinical judgment as to the most appropriate duration of use. | |||
*Abnormal MRI signal changes were observed in infants. | |||
Oral administration of vigabatrin (5, 15, or 50 mg/kg) to young rats during the neonatal and juvenile periods of development (postnatal days 4-65) produced neurobehavioral (convulsions, neuromotor impairment, learning deficits) and neurohistopathological (brain vacuolation, decreased myelination, and retinal dysplasia) abnormalities in treated animals. The no-effect dose for developmental neurotoxicity in juvenile rats (5 mg/kg) was associated with plasma vigabatrin exposures (AUC) less than 1/30 of those measured in pediatric patients receiving an oral dose of 50 mg/kg | |||
|useInGeri=Clinical studies of vigabatrin did not include sufficient numbers of patients aged 65 and over to determine whether they responded differently from younger patients. Vigabatrin is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. | |||
Oral administration of a single dose of 1.5 g of vigabatrin to elderly (>65 years) patients with reduced creatinine clearance (<50 mL/min) was associated with moderate to severe sedation and confusion in 4 of 5 patients, lasting up to 5 days. The renal clearance of vigabatrin was 36% lower in healthy elderly subjects (>65 years) than in young healthy males. Adjustment of dose or frequency of administration should be considered. Such patients may respond to a lower maintenance dose. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. | |||
|useInRenalImpair=Dose adjustment, including initiating treatment with a lower dose, is necessary in pediatric patients 10 years of age and older and adults with mild (creatinine clearance >50-80 mL/min), moderate (creatinine clearance >30-50 mL/min) and severe (creatinine clearance >10-30 mL/min) renal impairment . | |||
SABRIL is primarily eliminated through the kidney. | |||
======Infants====== | |||
Information about how to adjust the dose in infants with renal impairment is unavailable. | |||
======Pediatric patients 10 years and older, and adult patients====== | |||
*Mild renal impairment (CLcr >50 - 80 mL/min): dose should be decreased by 25% | |||
*Moderate renal impairment (CLcr >30 - 50 mL/min): dose should be decreased by 50% | |||
*Severe renal impairment (CLcr >10 - 30 mL/min): dose should be decreased by 75%. | |||
CLcr in mL/min may be estimated from serum creatinine (mg/dL) using the following formulas: | |||
*Patients 10 to <12 years old: CLcr (mL/min/1.73 m2) = (K × Ht) / Scr****** | |||
**height (Ht) in cm; serum creatinine (Scr) in mg/dL | |||
**K (proportionality constant): Female Child (<12 years): K=0.55; | |||
*Male Child (<12 years): K=0.70 | |||
*Pediatric patients 12 years or older and adult patients: CLcr (mL/min) = [140-age (years)] × weight (kg) / [72 × serum creatinine (mg/dL)] (×0.85 for female patients) | |||
|alcohol=Alcohol-Vigabatrin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Vigabatrin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 17:08, 23 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
VISION LOSS
See full prescribing information for complete Boxed Warning.
Condition Name:
It is possible that vision loss can worsen despite discontinuation of SABRIL.
|
Overview
Vigabatrin is a anticonvulsant, gamma aminobutyric acid transaminase inhibitor that is FDA approved for the treatment of refractory complex partial seizures and infantile spams. There is a Black Box Warning for this drug as shown here. Common adverse reactions include weight increased, arthralgia, confusion, coordination problem, memory impairment, somnolence, tremor, blurred vision, diplopia, nystagmus, infection of ear, otitis media, aggressive behavior, dysmenorrhea, bronchitis, upper respiratory infection and fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Important Dosing Instructions
- SABRIL is given orally with or without food. The SABRIL dosing regimen depends on the indication, age group, weight, and dosage form (tablets or powder for oral solution). Patients with impaired renal function require dose adjustment.
- SABRIL tablets and powder for oral solution are bioequivalent. Either tablet or powder can be used for CPS. Powder for oral solution should be used for IS; tablets should not be used for IS because of difficulty in the administration of tablets to infants and young children.
- SABRIL powder for oral solution should be mixed with water prior to administration.
If using SABRIL powder for oral solution, physicians should review and discuss the Medication Guide and instructions for mixing and giving SABRIL with the patient or caregiver(s). Physicians should confirm that patients or caregiver(s) understand how to mix SABRIL powder with water and administer the correct daily dose. Empty the entire contents of each 500 mg packet into a clean cup, and dissolve in 10 mL of cold or room temperature water per packet (see Table 2). Administer the resulting solution using the 10 mL oral syringe supplied with the medication. The concentration of the final solution is 50 mg/mL. Discard the resulting solution if it is not clear (or free of particles) and colorless. Each individual dose should be prepared and used immediately. Discard any unused portion of the solution after administering the correct dose. Monitoring of SABRIL plasma concentrations to optimize therapy is not helpful. If a decision is made to discontinue SABRIL, the dose should be gradually reduced
Refractory Complex Partial Seizures
Adults (Patients >16 Years of Age)
Treatment should be initiated at 1000 mg/day (500 mg twice daily). Total daily dose may be increased in 500 mg increments at weekly intervals depending on response. The recommended dose of SABRIL in adults is 3000 mg/day (1500 mg twice daily). A 6000 mg/day dose has not been shown to confer additional benefit compared to the 3000 mg/day dose and is associated with an increased incidence of adverse events. In controlled clinical studies in adults with complex partial seizures, SABRIL was tapered by decreasing the daily dose 1000 mg/day on a weekly basis until discontinued.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Vigabatrin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Vigabatrin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Refractory Complex Partial Seizures
Adults (Patients >16 Years of Age)
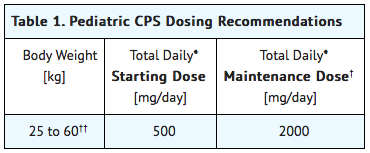
Treatment is based on body weight as shown in Table 1. Treatment should be initiated at a total daily dose of 500 mg/day (250 mg twice daily) and may be increased weekly to a total maintenance dose of 2000 mg/day (1000 mg twice daily). Patients weighing more than 60 kg should be dosed according to adult recommendations.
Infantile Spasms
The initial daily dosing is 50 mg/kg/day given in two divided doses; subsequent dosing can be titrated by 25 mg/kg/day to 50 mg/kg/day increments every 3 days up to a maximum of 150 mg/kg/day given in 2 divided doses [see USE IN SPECIFIC POPULATIONS (8.4)].
Table 2 below describes how many packets and how many milliliters (mL) of water will be needed to prepare each individual dose. The concentration after reconstitution is 50 mg/mL.

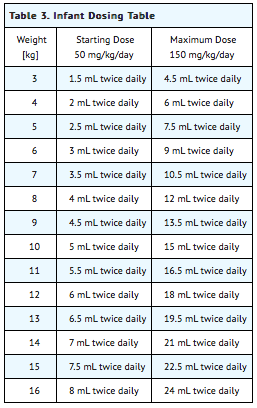
Table 3 provides the volume of the 50 mg/mL dosing solution that should be administered as individual doses in infants of various weights.
In a controlled clinical study in patients with infantile spasms, SABRIL was tapered by decreasing the daily dose at a rate of 25 mg/kg to 50 mg/kg every 3 to 4 days
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Vigabatrin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Vigabatrin in pediatric patients.
Contraindications
None
Warnings
|
VISION LOSS
See full prescribing information for complete Boxed Warning.
Condition Name:
It is possible that vision loss can worsen despite discontinuation of SABRIL.
|
Vision Loss
Because of the risk of vision loss, and because, when it is effective, SABRIL provides an observable symptomatic benefit, patient response and continued need for treatment should be periodically assessed.
In patients with refractory complex partial seizures, SABRIL should be withdrawn if a substantial clinical benefit is not observed within 3 months of initiating treatment. If, in the clinical judgment of the prescriber, evidence of treatment failure becomes obvious earlier than 3 months, treatment should be discontinued at that time.
In patients with infantile spasms, SABRIL should be withdrawn if a substantial clinical benefit is not observed within 2 to 4 weeks. If, in the clinical judgment of the prescriber, evidence of treatment failure becomes obvious earlier than 2 to 4 weeks, treatment should be discontinued at that time [see BOXED WARNING].
Monitoring of Vision
Monitoring of vision by an ophthalmic professional with expertise in visual field interpretation and the ability to perform dilated indirect ophthalmoscopy of the retina is required, unless a patient is formally exempted from periodic ophthalmologic assessment as documented in the Support, Help And Resources for Epilepsy (SHARE) program [see WARNINGS AND PRECAUTIONS (5.2)]. Because vision testing in infants is difficult, vision loss may not be detected until it is severe. For patients receiving SABRIL who are not exempted, vision assessment is required at baseline (no later than 4 weeks after starting SABRIL) and at least every 3 months while on therapy and about 3-6 months after the discontinuation of therapy.
The diagnostic approach should be individualized for the patient and clinical situation. For all patients, attempts to monitor vision periodically and/or formal exemptions must be documented under the SHARE program. In adults and cooperative pediatric patients, perimetry is recommended, preferably by automated threshold visual field testing. Additional testing may also include electrophysiology (e.g., electroretinography [ERG]), retinal imaging (e.g., optical coherence tomography [OCT]), and/or other methods appropriate for the patient, but this additional testing is not required. In patients exempted from vision testing, treatment may continue according to clinical judgment, with appropriate patient counseling and with documentation in the SHARE program of the exemption. Because of variability, results from ophthalmic monitoring must be interpreted with caution, and repeat assessment is recommended if results are abnormal or uninterpretable. Repeat assessment in the first few weeks of treatment is recommended to establish if, and to what degree, reproducible results can be obtained, and to guide selection of appropriate ongoing monitoring for the patient.
The onset and progression of vision loss from SABRIL is unpredictable, and it may occur or worsen precipitously between assessments. Once detected, vision loss due to SABRIL is not reversible. It is expected that even with frequent monitoring, some SABRIL patients will develop severe vision loss. Drug discontinuation should be considered, balancing benefit and risk, if visual loss is
SABRIL is available only through a restricted distribution program called the SHARE program, because of the risk of vision loss.
Notable requirements components of the SHARE Program include the following:
- Prescribers must be certified with the program by enrolling and reviewing educational materials and comply with the following:
- Assess vision prior to initiating therapy and then every 3 months during therapy.
- Remove patients from SABRIL therapy if the patients do not experience a meaningful reduction in seizures.
- The prescriber may, with appropriate documentation and caregiver counseling, exempt certain patients from vision assessment, using the Ophthalmologic Assessment Form, if:
- The patient is blind (subsequent Ophthalmologic Assessment Forms do not need to be submitted to the REMS coordinating center)
- The patient’s general neurological and/or mental condition permanently precludes the need for visual assessment (subsequent Ophthalmologic Assessment Forms do not need to be submitted to the REMS coordinating center)
- The patient’s general neurological condition temporarily precludes the ability to assess visual function. The evaluation, however, may be performed at a later time as clinically appropriate.
- The patient’s medical condition prevents visual assessment being performed safely
- For other reasons specified by the prescriber
- Patient/parent/legal guardian must understand the risks and benefits and sign a Patient-Prescriber Agreement.
- Pharmacies that dispense SABRIL must be certified and agree to comply with the REMS requirements. Certified pharmacies must only dispense SABRIL to patients who are enrolled in the program.
Magnetic Resonance Imaging (MRI) Abnormalities in Infants
Abnormal MRI signal changes characterized by increased T2 signal and restricted diffusion in a symmetric pattern involving the thalamus, basal ganglia, brain stem, and cerebellum have been observed in some infants treated with vigabatrin for infantile spasms. In a retrospective epidemiologic study in infants with IS (N=205), the prevalence of these changes was 22% in vigabatrin treated patients versus 4% in patients treated with other therapies.
In the study above, in post marketing experience, and in published literature reports, these changes generally resolved with discontinuation of treatment. In a few patients, the lesion resolved despite continued use. It has been reported that some infants exhibited coincident motor abnormalities, but no causal relationship has been established and the potential for long-term clinical sequelae has not been adequately studied.
Neurotoxicity (brain histopathology and neurobehavioral abnormalities) was observed in rats exposed to vigabatrin during late gestation and the neonatal and juvenile periods of development. The relationship between these findings and the abnormal MRI findings in infants treated with vigabatrin for infantile spasms is unknown [see WARNINGS AND PRECAUTIONS (5.4) and USE IN SPECIFIC POPULATIONS (8.1)].
The specific pattern of signal changes observed in IS patients was not observed in older pediatric and adult patients treated with vigabatrin for refractory CPS. In a blinded review of MRI images obtained in prospective clinical trials in patients with refractory CPS 3 years and older (N=656), no difference was observed in anatomic distribution or prevalence of MRI signal changes between vigabatrin treated and placebo treated patients.
For adults treated with SABRIL, routine MRI surveillance is unnecessary as there is no evidence that vigabatrin causes MRI changes in this population.
Neurotoxicity
Vacuolation, characterized by fluid accumulation and separation of the outer layers of myelin, has been observed in brain white matter tracts in adult and juvenile rats and adult mice, dogs, and possibly monkeys following administration of vigabatrin. This lesion, referred to as intramyelinic edema (IME), was seen in animals at doses within the human therapeutic range. A no-effect dose was not established in rodents or dogs. In the rat and dog, vacuolation was reversible following discontinuation of vigabatrin treatment, but, in the rat, pathologic changes consisting of swollen or degenerating axons, mineralization, and gliosis were seen in brain areas in which vacuolation had been previously observed. Vacuolation in adult animals was correlated with alterations in MRI and changes in visual and somatosensory evoked potentials (EP).
Administration of vigabatrin to rats during the neonatal and juvenile periods of development produced vacuolar changes in the brain gray matter (areas including the thalamus, midbrain, deep cerebellar nuclei, substantia nigra, hippocampus, and forebrain) which are considered distinct from the IME observed in vigabatrin treated adult animals. Decreased myelination and evidence of oligodendrocyte injury were additional findings in the brains of vigabatrin-treated rats. An increase in apoptosis was seen in some brain regions following vigabatrin exposure during the early postnatal period. Long-term neurobehavioral abnormalities (convulsions, neuromotor impairment, learning deficits) were also observed following vigabatrin treatment of young rats. These effects in young animals occurred at doses lower than those producing neurotoxicity in adult animals and were associated with plasma vigabatrin levels substantially lower than those achieved clinically in infants and children [see USE IN SPECIFIC POPULATIONS (8.1)].
In a published study, vigabatrin (200, 400 mg/kg/day) induced apoptotic neurodegeneration in the brain of young rats when administered by intraperitoneal injection on postnatal days 5-7.
Administration of vigabatrin to female rats during pregnancy and lactation at doses below those used clinically resulted in hippocampal vacuolation and convulsions in the mature offspring.
Abnormal MRI signal changes characterized by increased T2 signal and restricted diffusion in a symmetric pattern involving the thalamus, basal ganglia, brain stem, and cerebellum have been observed in some infants treated for IS with vigabatrin. Studies of the effects of vigabatrin on MRI and EP in adult epilepsy patients have demonstrated no clear-cut abnormalities [see WARNINGS AND PRECAUTIONS (5.3)].
Suicidal Behavior and Ideation
Antiepileptic drugs (AEDs), including SABRIL, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI: 1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED treated patients was 0.43%, compared to 0.24% among 16,029 placebo treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug treated patients in the trials and none in placebo treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5-100 years) in the clinical trials analyzed. Table 4 shows absolute and relative risk by indication for all evaluated AEDs.
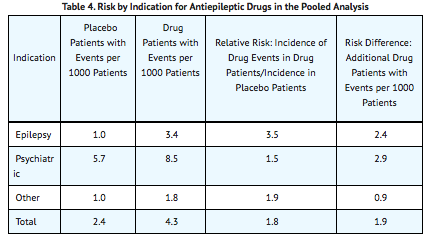
The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
Anyone considering prescribing SABRIL or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
Withdrawal of Antiepileptic Drugs (AEDs)
- As with all AEDs, SABRIL should be withdrawn gradually. Patients and caregivers should be told not to suddenly discontinue SABRIL therapy.
In controlled clinical studies in adults with complex partial seizures, SABRIL was tapered by decreasing the daily dose 1000 mg/day on a weekly basis until discontinued.
- In a controlled study in pediatric patients with complex partial seizures, SABRIL was tapered by decreasing the daily dose by one third every week for three weeks.
- In a controlled clinical study in patients with infantile spasms, SABRIL was tapered by decreasing the daily dose at a rate of 25-50 mg/kg every 3-4 days.
Anemia
In North American controlled trials in adults, 6% of patients (16/280) receiving SABRIL and 2% of patients (3/188) receiving placebo had adverse events of anemia and/or met criteria for potentially clinically important hematology changes involving hemoglobin, hematocrit, and/or RBC indices. Across U.S. controlled trials, there were mean decreases in hemoglobin of about 3% and 0% in SABRIL and placebo treated patients, respectively, and a mean decrease in hematocrit of about 1% in SABRIL treated patients compared to a mean gain of about 1% in patients treated with placebo.
In controlled and open label epilepsy trials in adults and pediatric patients, 3 SABRIL patients (0.06%, 3/4855) discontinued for anemia and 2 SABRIL patients experienced unexplained declines in hemoglobin to below 8 g/dL and/or hematocrit below 24%.
Somnolence and Fatigue
SABRIL causes somnolence and fatigue. Patients should be advised not to drive a car or operate other complex machinery until they are familiar with the effects of SABRIL on their ability to perform such activities.
Pooled data from two SABRIL controlled trials in adults demonstrated that 24% (54/222) of SABRIL patients experienced somnolence compared to 10% (14/135) of placebo patients. In those same studies, 28% of SABRIL patients experienced fatigue compared to 15% (20/135) of placebo patients. Almost 1% of SABRIL patients discontinued from clinical trials for somnolence and almost 1% discontinued for fatigue.
Pooled data from three SABRIL controlled trials in pediatric patients demonstrated that 6% (10/165) of SABRIL patients experienced somnolence compared to 5% (5/104) of placebo patients. In those same studies, 10% (17/165) of SABRIL patients experienced fatigue compared to 7% (7/104) of placebo patients. No SABRIL patients discontinued from clinical trials due to somnolence or fatigue.
Peripheral Neuropathy
SABRIL causes symptoms of peripheral neuropathy in adults. Pediatric clinical trials were not designed to assess symptoms of peripheral neuropathy, but observed incidence of symptoms based on pooled data from controlled pediatric studies appeared similar for pediatric patients on vigabatrin and placebo. In a pool of North American controlled and uncontrolled epilepsy studies, 4.2% (19/457) of SABRIL patients developed signs and/or symptoms of peripheral neuropathy. In the subset of North American placebo-controlled epilepsy trials, 1.4% (4/280) of SABRIL treated patients and no (0/188) placebo patients developed signs and/or symptoms of peripheral neuropathy. Initial manifestations of peripheral neuropathy in these trials included, in some combination, symptoms of numbness or tingling in the toes or feet, signs of reduced distal lower limb vibration or position sensation, or progressive loss of reflexes, starting at the ankles. Clinical studies in the development program were not designed to investigate peripheral neuropathy systematically and did not include nerve conduction studies, quantitative sensory testing, or skin or nerve biopsy. There is insufficient evidence to determine if development of these signs and symptoms were related to duration of SABRIL treatment, cumulative dose, or if the findings of peripheral neuropathy were completely reversible upon discontinuation of SABRIL.
Weight Gain
- SABRIL causes weight gain in adult and pediatric patients.
- Data pooled from randomized controlled trials in adults found that 17% (77/443) of SABRIL patients versus 8% (22/275) of placebo patients gained ≥7% of baseline body weight. In these same trials, the mean weight change among SABRIL patients was 3.5 kg compared to 1.6 kg for placebo patients.
- Data pooled from randomized controlled trials in pediatric patients with refractory complex partial seizures found that 47% (77/163) of SABRIL patients versus 19% (19/102) of placebo patients gained ≥7% of baseline body weight.
- In all epilepsy trials, 0.6% (31/4855) of SABRIL patients discontinued for weight gain. The long term effects of SABRIL related weight gain are not known. Weight gain was not related to the occurrence of edema.
Edema
SABRIL causes edema in adults. Pediatric clinical trials were not designed to assess edema, but observed incidence of edema based pooled data from controlled pediatric studies appeared similar for pediatric patients on vigabatrin and placebo.
Pooled data from controlled trials demonstrated increased risk among SABRIL patients compared to placebo patients for peripheral edema (SABRIL 2%, placebo 1%), and edema (SABRIL 1%, placebo 0%). In these studies, one SABRIL and no placebo patients discontinued for an edema related AE. In adults, there was no apparent association between edema and cardiovascular adverse events such as hypertension or congestive heart failure. Edema was not associated with laboratory changes suggestive of deterioration in renal or hepatic function.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions in U.S. and Primary Non-U.S. Clinical Studies
In U.S. and primary non-U.S. clinical studies of 4,079 SABRIL treated patients, the most commonly observed (≥5%) adverse reactions associated with the use of SABRIL in combination with other AEDs were headache, somnolence, fatigue, dizziness, convulsion, nasopharyngitis, weight increased, upper respiratory tract infection, visual field defect, depression, tremor, nystagmus, nausea, diarrhea, memory impairment, insomnia, irritability, coordination abnormal, vision blurred, diplopia, vomiting, influenza, pyrexia, and rash.
The adverse reactions most commonly associated with SABRIL treatment discontinuation in ≥1% of patients were convulsion and depression.
In patients with infantile spasms, the adverse reactions most commonly associated with SABRIL treatment discontinuation in ≥1% of patients were infections, status epilepticus, developmental coordination disorder, dystonia, hypotonia, hypertonia, weight increased, and insomnia.
Most Common Adverse Reactions in Controlled Clinical Trials
Refractory Complex Partial Seizures
Adults
Table 5 lists the treatment emergent adverse reactions that occurred in ≥2% and more than one patient per SABRIL treated group and that occurred more frequently than in placebo patients from 2 U.S. add-on clinical studies of refractory CPS in adults.

Pediatrics 10 to 16 years of age
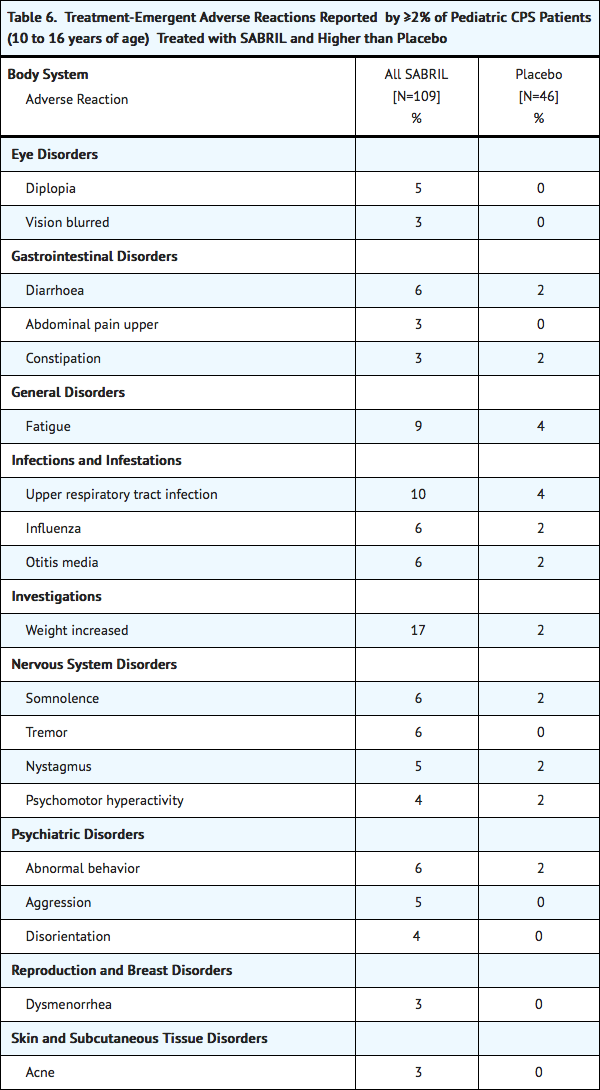
Table 6 lists adverse reactions from controlled clinical studies of pediatric patients receiving SABRIL or placebo as add-on therapy for refractory complex partial seizures. Adverse reactions that are listed occurred in at least 2% of SABRIL treated patients and more frequently than placebo. The median SABRIL dose was 49.4 mg/kg, (range of 8.0 – 105.9 mg/kg).
Infantile Spasms
In a randomized, placebo-controlled IS study with a 5 day double-blind treatment phase (n=40), the adverse events reported by >5% of patients receiving SABRIL and that occurred more frequently than in placebo patients, were somnolence (SABRIL 45%, placebo 30%), bronchitis (SABRIL 30%, placebo 15%), ear infection (SABRIL 10%, placebo 5%), and otitis media acute SABRIL 10%, placebo 0%).
In a dose response study of low-dose (18-36 mg/kg/day) versus high-dose (100-148 mg/kg/day) vigabatrin, no clear correlation between dose and incidence of adverse events was observed. The treatment emergent adverse reactions (≥5% in either dose group) are summarized in Table 7.

Postmarketing Experience
The following adverse reactions have been reported during post approval use of SABRIL worldwide. All adverse reactions that are not listed above as adverse reactions reported in clinical trials, that are not relatively common in the population and are not too vague to be useful are listed in this section. These reactions are reported voluntarily from a population of uncertain size; therefore, it is not possible to estimate their frequency or establish a causal relationship to drug exposure. Adverse reactions are categorized by system organ class.
Birth Defects
- Congenital cardiac defects
- Congenital external ear anomaly
- Congenital hemangioma
- Congenital hydronephrosis
- Congenital male genital malformation
- Congenital oral malformation
- Congenital vesicoureteric reflux
- Dentofacial anomaly dysmorphism
- Fetal anticonvulsant syndrome
- Hamartomas
- Hip dysplasia
- Limb malformation
- Limb reduction defect
- Low set ears
- Renal aplasia
- Retinitis pigmentosa
- Supernumerary nipple
- Talipes
Ear Disorders
Endocrine Disorders
- Delayed puberty
Gastrointestinal Disorders
General Disorders
Hepatobiliary Disorders
Nervous System Disorders
Psychiatric Disorders
Respiratory Disorders
Skin and Subcutaneous Tissue Disorders
- Angioedema
- Maculo-papular rash
- Pruritus
- Stevens-Johnson syndrome (SJS)
- Toxic epidermal necrolysis (TEN)
Drug Interactions
Antiepileptic Drugs
Phenytoin
Although phenytoin dose adjustments are not routinely required, dose adjustment of phenytoin should be considered if clinically indicated, since SABRIL may cause a moderate reduction in total phenytoin plasma levels.
Clonazepam
SABRIL may moderately increase the Cmax of clonazepam resulting in an increase of clonazepam-associated adverse reactions.
Other AEDs
There are no clinically significant pharmacokinetic interactions between SABRIL and either phenobarbital or sodium valproate. Based on population pharmacokinetics, carbamazepine, clorazepate, primidone, and sodium valproate appear to have no effect on plasma concentrations of vigabatrin.
Oral Contraceptives
SABRIL is unlikely to affect the efficacy of steroid oral contraceptives.
Drug-Laboratory Test Interactions
- SABRIL decreases alanine transaminase (ALT) and aspartate transaminase (AST) plasma activity in up to 90% of patients. In some patients, these enzymes become undetectable. The suppression of ALT and AST activity by SABRIL may preclude the use of these markers, especially ALT, to detect early hepatic injury.
- SABRIL may increase the amount of amino acids in the urine, possibly leading to a false positive test for certain rare genetic metabolic diseases (e.g., alpha aminoadipic aciduria).
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C Vigabatrin produced developmental toxicity, including teratogenic and neurohistopathological effects, when administered to pregnant animals at clinically relevant doses. In addition, developmental neurotoxicity was observed in rats treated with vigabatrin during a period of postnatal development corresponding to the third trimester of human pregnancy. There are no adequate and well-controlled studies in pregnant women. SABRIL should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Administration of vigabatrin (oral doses of 50 to 200 mg/kg) to pregnant rabbits throughout the period of organogenesis was associated with an increased incidence of malformations (cleft palate) and embryo-fetal death; these findings were observed in two separate studies. The no-effect dose for teratogenicity and embryolethality in rabbits (100 mg/kg) is approximately 1/2 the maximum recommended human dose (MRHD) of 3 g/day on a body surface area (mg/m2) basis. In rats, oral administration of vigabatrin (50, 100, or 150 mg/kg) throughout organogenesis resulted in decreased fetal body weights and increased incidences of fetal anatomic variations. The no-effect dose for embryo-fetal toxicity in rats (50 mg/kg) is approximately 1/5 the MRHD on a mg/m2 basis. Oral administration of vigabatrin (50, 100, 150 mg/kg) to rats from the latter part of pregnancy through weaning produced long-term neurohistopathological (hippocampal vacuolation) and neurobehavioral (convulsions) abnormalities in the offspring. A no-effect dose for developmental neurotoxicity in rats was not established; the low-effect dose (50 mg/kg) is approximately 1/5 the MRHD on a mg/m2 basis.
In a published study, vigabatrin (300 or 450 mg/kg) was administered by intraperitoneal injection to a mutant mouse strain on a single day during organogenesis (day 7, 8, 9, 10, 11, or 12). An increase in malformations (including cleft palate) was observed at both doses.
Oral administration of vigabatrin (5, 15, or 50 mg/kg) to young rats during the neonatal and juvenile periods of development (postnatal days 4-65) produced neurobehavioral (convulsions, neuromotor impairment, learning deficits) and neurohistopathological (brain vacuolation, decreased myelination, and retinal dysplasia) abnormalities in treated animals. The early postnatal period in rats is generally thought to correspond to late pregnancy in humans in terms of brain development. The no-effect dose for developmental neurotoxicity in juvenile rats (5 mg/kg) was associated with plasma vigabatrin exposures (AUC) less than 1/30 of those measured in pediatric patients receiving an oral dose of 50 mg/kg.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Vigabatrin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Vigabatrin during labor and delivery.
Nursing Mothers
Vigabatrin is excreted in human milk. Because of the potential for serious adverse reactions from vigabatrin in nursing infants a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother
Pediatric Use
The safety and effectiveness of SABRIL as adjunctive treatment of refractory complex partial seizures in pediatric patients aged 10 to 16 years of age have been established. The dosing recommendation in this population varies according to age group and is weight based. Adverse reactions in this pediatric population are similar to those observed in the adult population.
- The safety and effectiveness of SABRIL have not been established in pediatric patients under 10 years of age with refractory complex partial seizures.
- The safety and effectiveness of SABRIL as monotherapy for pediatric patients with infantile spasms (1 month to 2 years of age) have been established.
- Duration of therapy for infantile spasms was evaluated in a post hoc analysis of a Canadian Pediatric Epilepsy Network (CPEN) study of developmental outcomes in infantile spasms patients. This analysis suggests that a total duration of 6 months of vigabatrin therapy is adequate for the treatment of infantile spasms. However, prescribers must use their clinical judgment as to the most appropriate duration of use.
- Abnormal MRI signal changes were observed in infants.
Oral administration of vigabatrin (5, 15, or 50 mg/kg) to young rats during the neonatal and juvenile periods of development (postnatal days 4-65) produced neurobehavioral (convulsions, neuromotor impairment, learning deficits) and neurohistopathological (brain vacuolation, decreased myelination, and retinal dysplasia) abnormalities in treated animals. The no-effect dose for developmental neurotoxicity in juvenile rats (5 mg/kg) was associated with plasma vigabatrin exposures (AUC) less than 1/30 of those measured in pediatric patients receiving an oral dose of 50 mg/kg
Geriatic Use
Clinical studies of vigabatrin did not include sufficient numbers of patients aged 65 and over to determine whether they responded differently from younger patients. Vigabatrin is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Oral administration of a single dose of 1.5 g of vigabatrin to elderly (>65 years) patients with reduced creatinine clearance (<50 mL/min) was associated with moderate to severe sedation and confusion in 4 of 5 patients, lasting up to 5 days. The renal clearance of vigabatrin was 36% lower in healthy elderly subjects (>65 years) than in young healthy males. Adjustment of dose or frequency of administration should be considered. Such patients may respond to a lower maintenance dose. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
Gender
There is no FDA guidance on the use of Vigabatrin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Vigabatrin with respect to specific racial populations.
Renal Impairment
Dose adjustment, including initiating treatment with a lower dose, is necessary in pediatric patients 10 years of age and older and adults with mild (creatinine clearance >50-80 mL/min), moderate (creatinine clearance >30-50 mL/min) and severe (creatinine clearance >10-30 mL/min) renal impairment .
SABRIL is primarily eliminated through the kidney.
Infants
Information about how to adjust the dose in infants with renal impairment is unavailable.
Pediatric patients 10 years and older, and adult patients
- Mild renal impairment (CLcr >50 - 80 mL/min): dose should be decreased by 25%
- Moderate renal impairment (CLcr >30 - 50 mL/min): dose should be decreased by 50%
- Severe renal impairment (CLcr >10 - 30 mL/min): dose should be decreased by 75%.
CLcr in mL/min may be estimated from serum creatinine (mg/dL) using the following formulas:
- Patients 10 to <12 years old: CLcr (mL/min/1.73 m2) = (K × Ht) / Scr******
**height (Ht) in cm; serum creatinine (Scr) in mg/dL
**K (proportionality constant): Female Child (<12 years): K=0.55;
*Male Child (<12 years): K=0.70
- Pediatric patients 12 years or older and adult patients: CLcr (mL/min) = [140-age (years)] × weight (kg) / [72 × serum creatinine (mg/dL)] (×0.85 for female patients)
Hepatic Impairment
There is no FDA guidance on the use of Vigabatrin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Vigabatrin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Vigabatrin in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Vigabatrin Administration in the drug label.
Monitoring
There is limited information regarding Vigabatrin Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Vigabatrin and IV administrations.
Overdosage
There is limited information regarding Vigabatrin overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Vigabatrin Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Vigabatrin Mechanism of Action in the drug label.
Structure
There is limited information regarding Vigabatrin Structure in the drug label.
Pharmacodynamics
There is limited information regarding Vigabatrin Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Vigabatrin Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Vigabatrin Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Vigabatrin Clinical Studies in the drug label.
How Supplied
There is limited information regarding Vigabatrin How Supplied in the drug label.
Storage
There is limited information regarding Vigabatrin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Vigabatrin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Vigabatrin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Vigabatrin Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Vigabatrin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Vigabatrin Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Vigabatrin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
| File:Vigabatrin.png | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 80-90% |
| Protein binding | 0 |
| Metabolism | Almost no metabolic transformation occurs |
| Elimination half-life | 5-8 hours in young adults, 12-13 hours in the elderly. |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C6H11NO2 |
| Molar mass | 129.157 g/mol |
Vigabatrin is an anticonvulsant that inhibits the catabolism of GABA. It is an analog of GABA, but it is not a receptor agonist.[1]
Mechanism of action
Vigabatrin is an irreversible inhibitor of gamma-aminobutyric acid transaminase (GABA-T), the enzyme responsible for the catabolism of GABA, which increases the level of GABA in the synapses.[1]
Vigabatrin is a racemic compound, and its [S]-enantiomer is pharmacologically active.[2],[3]
Pharmacokinetics
With most drugs, elimination half-life is a useful predictor of dosing schedules and the time needed to reach steady state concentrations. In the case of vigabatrin, however, it has been found that the half-life of biologic activity is far longer than the elimination half-life.[4]
For vigabatrin, there is no range of target concentrations because researchers found no difference between the serum concentration levels of responders and those of non-responders.[5] Instead, the duration of action is believed to be more a function of the GABA-T resynthesis rate; levels of GABA-T do not usually return to their normal state until six days after stopping the medication.[3]
Uses
Approved/clinically proven
Canada
In Canada, vigabatrin is approved for use as an adjunctive treatment (with other drugs) in treatment resistant epilepsy, complex partial seizures, secondary generalized seizures, and for monotherapy use in infantile spasms in West syndrome.[1]
Mexico
As of 2003, vigabatrin is approved in Mexico for the treatment of epilepsy that is not satisfactorily controlled by conventional therapy (adjunctive or monotherapy) or in recently diagnosed patients who have not tried other agents (monotherapy).[6]
Vigabatrin is also indicated for monotherapy use in secondarily generalized tonic-clonic seizures, partial seizures, and in infantile spasms due to West syndrome.[6]
Unapproved/Investigational
In November of 2001, a team of scientists lead by Peter Zwanzger of the University of Munich reported that vigabatrin reduced cholecystokinin tetrapeptide-induced symptoms of panic disorder, in addition to elevated cortisol and ACTH levels, in healthy volunteers.[7]
In 1994, Feucht and Brantner-Inthaler reported that vigabatrin reduced seizures by 50-100% in 85% of children with Lennox-Gastaut syndrome who had poor results with a valproate.[8]
In 1984, a double-blind crossover-study of six Huntington's disease patients—five of them on antipsychotics—reported that vigabatrin did little, if anything, to improve hyperkinetic movements, the ability to carry out daily activities, or normalize motor function.[9]
Adverse effects
Central nervous system
Common
Out of 2,081 subjects, somnolence (12.5%), headache (3.8%), dizziness (3.8%), nervousness (2.7%), depression (2.5%), memory disturbances (2.3%), diplopia (2.2%), aggression (2.0%), ataxia (1.9%), vertigo (1.9%), hyperactivity (1.8%), vision abnormalities (1.6%), confusion (1.4%), insomnia (1.3%), impaired concentration (1.2%), personality disorder (1.1%).[1] Out of 299 children, 33 (11%) became hyperactive.[1]
Rare
Some patients develop psychosis during the course of vigabatrin therapy,[10] which is more common in adults than in children.[11] This can happen even in patients with no prior history of psychosis.[12] Other rare CNS side effects include anxiety, emotional lability, irritability, tremor, abnormal gait, and speech disorder.[1]
Gastrointestinal
Common
Abdominal pain (1.6%), constipation (1.4%), vomiting (1.4%), and nausea (1.4%).[1]
Rare
Dyspepsia and increased appetite occurred in less than 1% of subjects in clinical trials.[1]
Body as a Whole
Common
Fatigue (9.2%), weight gain (5.0%), asthenia (1.1%).[1]
Teratogenicity
A teratology study conducted in rabbits found that a dose of 150mg/kg/day caused cleft palate in 2% of pups and a dose of 200 mg/kg/day caused it in 9%.[1] This may be due to a decrease in methionine levels, according to a study published in March of 2001.[13] In 2005, a study conducted at the University of Catania was published stating that rats whose mothers had consumed 250-1000 mg/kg/day had poorer performance in the water maze and open-field tasks, rats in the 750-mg group were underweight at birth and did not catch up to the control group, and rats in the 1000 mg group did not survive pregnancy.[14]
There is no controlled teratology data in humans to date.
More on "abnormal vision"
In 2003, vigabatrin was shown by Frisén and Malmgren to cause irreversible diffuse atrophy of the retinal nerve fiber layer in a retrospective study of 25 patients.[15] This has the most effect on the outer area (as opposed to the macular, or central area) of the retina.[16]
Drug interactions
A study published in 2002 found that vigabatrin causes a statistically significant increase in plasma clearance of carbamazepine.[17]
In 1984, Drs Rimmer and Richens at the University of Wales reported that administering vigabatrin with phenytoin lowered the serum phenytoin concentration in patients with treatment-resistant epilepsy.[18] The concentration of phenytoin falls to 23% within five weeks, according to an experiment published in 1989 by the same two scientists that tried and failed to elucidate the mechanism behind this interaction.[19]
Brand names
Vigabatrin is sold as Sabril® in Canada,[20] Mexico,[6] and the United Kingdom.[21] The brand name in Denmark is Sabrilex®.
References and end notes
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 Long, Phillip W. "Vigabatrin." Internet Mental Health. 1995-2003.
- ↑ Sheean, G. (1992). "Vigabatrin--plasma enantiomer concentrations and clinical effects". Clinical and Experimental Neurology. 29: 107–16. PMID 1343855. Unknown parameter
|coauthors=ignored (help) - ↑ 3.0 3.1 Gram L, Larsson OM, Johnsen A, Schousboe A (1989). "Experimental studies of the influence of vigabatrin on the GABA system". British Journal of Clinical Pharmacology. 27 (Suppl 1): 13S–17S. PMID 2757904.
- ↑ Browne TR (1998). "Pharmacokinetics of antiepileptic drugs". Neurology. 51 (5 suppl 4): S2–7. PMID 9818917.
- ↑ Lindberger M, Luhr O, Johannessen SI, Larsson S, Tomson T (2003). "Serum concentrations and effects of gabapentin and vigabatrin: observations from a dose titration study". Therapeutic Drug Monitoring. 25 (4): 457–62. PMID 12883229.
- ↑ 6.0 6.1 6.2 DEF MEXICO: SABRIL Diccionario de Especialdades Farmaceuticas. Edicion 49, 2003.
- ↑ Zwanzger P, Baghai TC, Schuele C, Strohle A, Padberg F, Kathmann N, Schwarz M, Moller HJ, Rupprecht R (2001). "Vigabatrin decreases cholecystokinin-tetrapeptide (CCK-4) induced panic in healthy volunteers". Neuropsychopharmacology. 25 (5): 699–703. PMID 11682253.
- ↑ Feucht M, Brantner-Inthaler S (1994). "Gamma-vinyl-GABA (vigabatrin) in the therapy of Lennox-Gastaut syndrome: an open study" (PDF). Epilepsia. 35 (5): 993–8. PMID 7925171. Retrieved 2006-05-25.
- ↑ Scigliano G, Giovannini P, Girotti F, Grassi MP, Caraceni T, Schechter PJ (1984). "Gamma-vinyl GABA treatment of Huntington's disease". Neurology. 34 (1): 94–6. PMID 6228746.
- ↑ Sander JW, Hart YM (1990). "Vigabatrin and behaviour disturbance". Lancet. 335 (8680): 57. PMID 1967367.
- ↑ Chiaretti A, Castorina M, Tortorolo L, Piastra M, Polidori G (1994). "[Acute psychosis and vigabatrin in childhood]". La Pediatria Medica e Chirurgica : Medical and surgical pediatrics. 16 (5): 489–90. [Article in Italian] PMID 7885961
- ↑ Sander JW, Hart YM, Trimble MR, Shorvon SD (1991). "Vigabatrin and psychosis". Journal of Neurology, Neurosurgery, and Psychiatry. 54 (5): 435–9. PMID 1865207.
- ↑ Abdulrazzaq YM, Padmanabhan R, Bastaki SM, Ibrahim A, Bener A (2001). "Placental transfer of vigabatrin (gamma-vinyl GABA) and its effect on concentration of amino acids in the embryo of TO mice". Teratology. 63 (3): 127–33. PMID 11283969.
- ↑ Lombardo SA, Leanza G, Meli C, Lombardo ME, Mazzone L, Vincenti I, Cioni M (2005). "Maternal exposure to the antiepileptic drug vigabatrin affects postnatal development in the rat". Neurological Sciences. 26 (2): 89–94. PMID 15995825.
- ↑ Frisén L, Malmgren K (2003). "Characterization of vigabatrin-associated optic atrophy". Acta Ophthalmologica Scandinavica. 81 (5): 466–73. PMID 14510793.
- ↑ Buncic JR, Westall CA, Panton CM, Munn JR, MacKeen LD, Logan WJ (2004). "Characteristic retinal atrophy with secondary "inverse" optic atrophy identifies vigabatrin toxicity in children". Ophthalmology. 111 (10): 1935–42. PMID 15465561.
- ↑ Sanchez-Alcaraz, Agustín (2002). "Effect of vigabatrin on the pharmacokinetics of carbamazepine". Journal of Clinical Pharmacology and Therapeutics. 27 (6): 427–30. PMID 12472982. Unknown parameter
|coauthors=ignored (help) - ↑ Rimmer EM, Richens A (1984). "Double-blind study of gamma-vinyl GABA in patients with refractory epilepsy". Lancet. 1 (8370): 189–90. PMID 6141335.
- ↑ Rimmer EM, Richens A (1989). "Interaction between vigabatrin and phenytoin". British Journal of Clinical Pharmacology. 27 (Suppl 1): 27S–33S. PMID 2757906.
- ↑ drugs.com Vigabatrin Drug Information
- ↑ Treatments for Epilepsy - Vigabatrin Norfolk and Waveney Mental Health Partnership NHS Trust
- Pages with script errors
- Pages with citations using unsupported parameters
- CS1 maint: Multiple names: authors list
- Pages with broken file links
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Anticonvulsants