Sacrococcygeal teratoma pathophysiology: Difference between revisions
(Mahshid) |
No edit summary |
||
| Line 4: | Line 4: | ||
==Overview== | ==Overview== | ||
Sacrococcygeal teratoma originates from the pluripotent cells in primitive knot or | Sacrococcygeal teratoma originates from the [[Pluripotency|pluripotent]] [[Cell (biology)|cells]] in primitive knot or Hensen's node, which is the primary organizer of [[Embryo|embryonic]] development, located on the [[anterior]] surface of the [[sacrum]] or [[coccyx]] by the 2nd or 3rd [[Gestation|gestational]] week. Development of sacrococcygeal teratoma is associated with gain of [[Chromosome|chromosomes]] ''1q32-qter'' regions and loss of the ''6q24-qter'' and ''18q21-qter'' regions. The [[pathophysiology]] of sacrococcygeal teratoma depends on the [[Histology|histological]] subtype. | ||
==Pathogenesis== | ==Pathogenesis== | ||
Sacrococcygeal teratoma originates from the pluripotent cells in primitive knot or Hensen's node, which is the primary organizer of embryonic development, located on the anterior surface of the sacrum or coccyx by | * Sacrococcygeal teratoma originates from the [[Pluripotency|pluripotent]] [[Cell (biology)|cells]] in primitive knot or Hensen's node, which is the primary organizer of [[Embryo|embryonic]] development, located on the [[Anatomical terms of location|anterior]] surface of the [[sacrum]] or [[coccyx]] by the 2nd or 3rd [[Gestation|gestational]] week.<ref name="pat">Sacrococcygeal teratoma. Hindawi (2015)http://www.hindawi.com/journals/criog/2012/131369/ Accessed on December 15th, 2015</ref> | ||
==Genetics== | ==Genetics== | ||
Development of Sacrococcygeal teratoma is associated with gain of chromosomes ''1q32-qter'' regions and | * Development of Sacrococcygeal teratoma is associated with gain of [[Chromosome|chromosomes]] ''1q32-qter'' regions and loss of the ''6q24-qter'' and ''18q21-qter'' regions.<ref name="aa">{{cite journal |vauthors=Harms D, Zahn S, Göbel U, Schneider DT |title=Pathology and molecular biology of teratomas in childhood and adolescence |journal=Klin Padiatr |volume=218 |issue=6 |pages=296–302 |year=2006 |pmid=17080330 |doi=10.1055/s-2006-942271 |url=}}</ref><ref name="aaa">{{cite journal |vauthors=Veltman I, Veltman J, Janssen I, Hulsbergen-van de Kaa C, Oosterhuis W, Schneider D, Stoop H, Gillis A, Zahn S, Looijenga L, Göbel U, van Kessel AG |title=Identification of recurrent chromosomal aberrations in germ cell tumors of neonates and infants using genomewide array-based comparative genomic hybridization |journal=Genes Chromosomes Cancer |volume=43 |issue=4 |pages=367–76 |year=2005 |pmid=15880464 |doi=10.1002/gcc.20208 |url=}}</ref> | ||
==Associated Conditions== | ==Associated Conditions== | ||
Following conditions are associated with sacrococcygeal teratoma: | Following conditions are associated with sacrococcygeal teratoma: | ||
*[[Myelomeningocoele]]<ref name = path>Sacrococcygel Teratoma. Radiopedia (2015) http://radiopaedia.org/articles/sacrococcygeal-teratoma Accessed on December 15, 2015</ref> | *[[Spina bifida|Myelomeningocoele]]<ref name="path">Sacrococcygel Teratoma. Radiopedia (2015) http://radiopaedia.org/articles/sacrococcygeal-teratoma Accessed on December 15, 2015</ref> | ||
*[[Vertebral anomalies]] | *[[Vertebral anomalies]] | ||
*[[Bladder outlet obstruction]] | *[[Bladder outlet obstruction]] | ||
| Line 30: | Line 30: | ||
==Microscopic Pathology== | ==Microscopic Pathology== | ||
Sacrococcygeal teratoma can be divided into following three types depending on the microscopic pathology: <ref name = "micro">{{cite journal |vauthors=Calaminus G, Schneider DT, Bökkerink JP, Gadner H, Harms D, Willers R, Göbel U |title=Prognostic value of tumor size, metastases, extension into bone, and increased tumor marker in children with malignant sacrococcygeal germ cell tumors: a prospective evaluation of 71 patients treated in the German cooperative protocols Maligne Keimzelltumoren (MAKEI) 83/86 and MAKEI 89 |journal=J. Clin. Oncol. |volume=21 |issue=5 |pages=781–6 |year=2003 |pmid=12610174 |doi= |url=}}</ref> | Sacrococcygeal teratoma can be divided into following three types depending on the microscopic pathology: <ref name="micro">{{cite journal |vauthors=Calaminus G, Schneider DT, Bökkerink JP, Gadner H, Harms D, Willers R, Göbel U |title=Prognostic value of tumor size, metastases, extension into bone, and increased tumor marker in children with malignant sacrococcygeal germ cell tumors: a prospective evaluation of 71 patients treated in the German cooperative protocols Maligne Keimzelltumoren (MAKEI) 83/86 and MAKEI 89 |journal=J. Clin. Oncol. |volume=21 |issue=5 |pages=781–6 |year=2003 |pmid=12610174 |doi= |url=}}</ref> | ||
===Mature Teratoma=== | ===Mature Teratoma=== | ||
*[[Benign]] | *[[Benign]] | ||
| Line 40: | Line 40: | ||
==Grading Based Upon Microscopic Features== | ==Grading Based Upon Microscopic Features== | ||
According to Gonzalez-Crussi System, sacrococcygeal teratoma is graded on a scale from 0-3, based on the histology:<ref name = "aa">{{cite journal | authors=Harms D, Zahn S, Göbel U, Schneider DT |title=Pathology and molecular biology of teratomas in childhood and adolescence |journal=Klin Padiatr |volume=218 |issue=6 |pages=296–302 |year=2006 |pmid=17080330 |doi=10.1055/s-2006-942271 |url=}}</ref> | According to Gonzalez-Crussi System, sacrococcygeal teratoma is graded on a scale from 0-3, based on the histology:<ref name="aa">{{cite journal | authors=Harms D, Zahn S, Göbel U, Schneider DT |title=Pathology and molecular biology of teratomas in childhood and adolescence |journal=Klin Padiatr |volume=218 |issue=6 |pages=296–302 |year=2006 |pmid=17080330 |doi=10.1055/s-2006-942271 |url=}}</ref> | ||
{| style="border: 0px; font-size: 90%; margin: 3px; width: 800px" | {| style="border: 0px; font-size: 90%; margin: 3px; width: 800px" | ||
|valign=top| | | valign="top" | | ||
|+ | |+ | ||
! style="background: #4479BA; width: 100px;" | {{fontcolor|#FFF|Grade}} | ! style="background: #4479BA; width: 100px;" | {{fontcolor|#FFF|Grade}} | ||
Revision as of 19:04, 2 May 2019
|
Sacrococcygeal teratoma Microchapters |
|
Diagnosis |
|---|
| Echocardiography and Ultrasound |
|
Treatment |
|
Case Studies |
|
Sacrococcygeal teratoma pathophysiology On the Web |
|
American Roentgen Ray Society Images of Sacrococcygeal teratoma pathophysiology |
|
Risk calculators and risk factors for Sacrococcygeal teratoma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]Associate Editor(s)-in-Chief: Mirdula Sharma, MBBS [2]
Overview
Sacrococcygeal teratoma originates from the pluripotent cells in primitive knot or Hensen's node, which is the primary organizer of embryonic development, located on the anterior surface of the sacrum or coccyx by the 2nd or 3rd gestational week. Development of sacrococcygeal teratoma is associated with gain of chromosomes 1q32-qter regions and loss of the 6q24-qter and 18q21-qter regions. The pathophysiology of sacrococcygeal teratoma depends on the histological subtype.
Pathogenesis
- Sacrococcygeal teratoma originates from the pluripotent cells in primitive knot or Hensen's node, which is the primary organizer of embryonic development, located on the anterior surface of the sacrum or coccyx by the 2nd or 3rd gestational week.[1]
Genetics
- Development of Sacrococcygeal teratoma is associated with gain of chromosomes 1q32-qter regions and loss of the 6q24-qter and 18q21-qter regions.[2][3]
Associated Conditions
Following conditions are associated with sacrococcygeal teratoma:
- Myelomeningocoele[4]
- Vertebral anomalies
- Bladder outlet obstruction
- Hydronephrosis
- Rectal stenosis or atresia
- Hydrops fetalis
- Cardiomegaly due to vascular shunting and high output cardiac failure
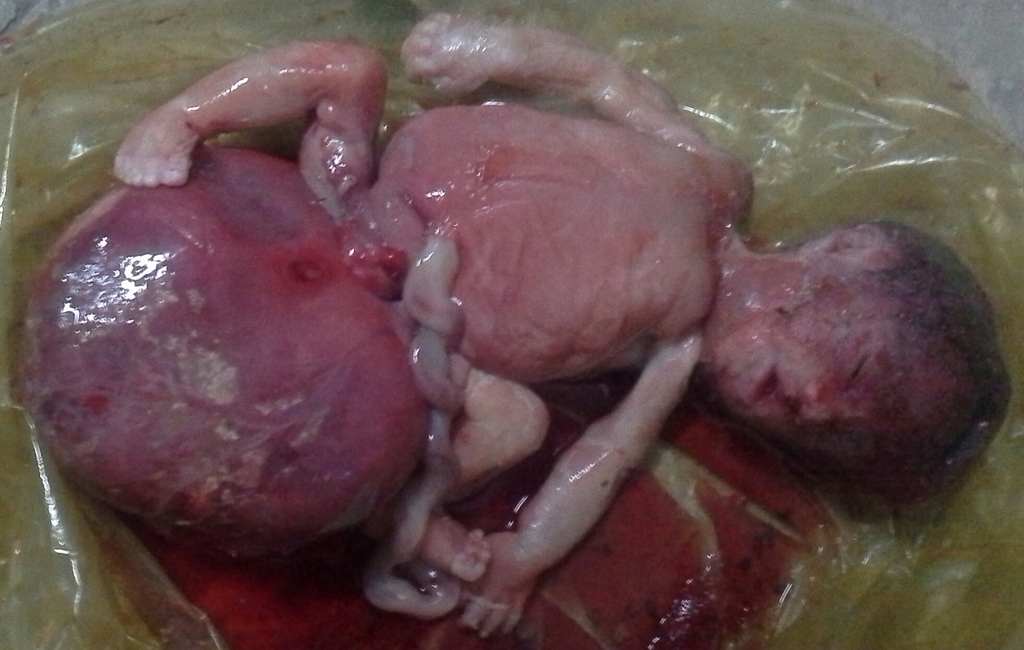
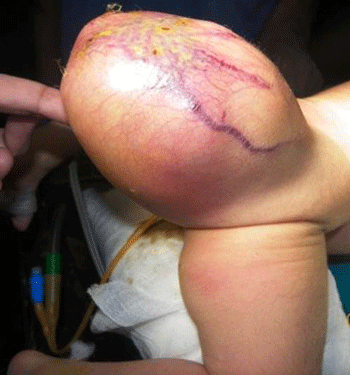
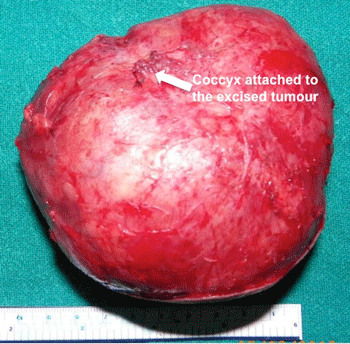
Gross Pathology
-
Gross Image of Sacrococcygeal teratoma
-
Gross Image of Sacrococcygeal teratoma
-
Gross Image of Excised Sacrococcygeal teratoma
Microscopic Pathology
Sacrococcygeal teratoma can be divided into following three types depending on the microscopic pathology: [5]
Mature Teratoma
- Benign
- Consist of fully differentiated somatic tissue
Immature Teratoma
- Malignant
- Consist of small fraction of incompletely differentiated tissue
- They have elevated tumor markers including yolk sac component secreting alpha fetoprotein or primitive neuroectodermal tumor (PNET).
Grading Based Upon Microscopic Features
According to Gonzalez-Crussi System, sacrococcygeal teratoma is graded on a scale from 0-3, based on the histology:[2]
| Grade | Microscopic Features |
|---|---|
|
Grade 0 |
|
|
Grade I |
|
|
Grade II |
|
|
Grade III |
|
References
- ↑ Sacrococcygeal teratoma. Hindawi (2015)http://www.hindawi.com/journals/criog/2012/131369/ Accessed on December 15th, 2015
- ↑ 2.0 2.1 Harms D, Zahn S, Göbel U, Schneider DT (2006). "Pathology and molecular biology of teratomas in childhood and adolescence". Klin Padiatr. 218 (6): 296–302. doi:10.1055/s-2006-942271. PMID 17080330.
- ↑ Veltman I, Veltman J, Janssen I, Hulsbergen-van de Kaa C, Oosterhuis W, Schneider D, Stoop H, Gillis A, Zahn S, Looijenga L, Göbel U, van Kessel AG (2005). "Identification of recurrent chromosomal aberrations in germ cell tumors of neonates and infants using genomewide array-based comparative genomic hybridization". Genes Chromosomes Cancer. 43 (4): 367–76. doi:10.1002/gcc.20208. PMID 15880464.
- ↑ Sacrococcygel Teratoma. Radiopedia (2015) http://radiopaedia.org/articles/sacrococcygeal-teratoma Accessed on December 15, 2015
- ↑ Calaminus G, Schneider DT, Bökkerink JP, Gadner H, Harms D, Willers R, Göbel U (2003). "Prognostic value of tumor size, metastases, extension into bone, and increased tumor marker in children with malignant sacrococcygeal germ cell tumors: a prospective evaluation of 71 patients treated in the German cooperative protocols Maligne Keimzelltumoren (MAKEI) 83/86 and MAKEI 89". J. Clin. Oncol. 21 (5): 781–6. PMID 12610174.