Glutamate dehydrogenase
| glutamate dehydrogenase 1 | |
|---|---|
| Identifiers | |
| Symbol | GLUD1 |
| Alt. symbols | GLUD |
| Entrez | 2746 |
| HUGO | 4335 |
| OMIM | 138130 |
| RefSeq | NM_005271 |
| UniProt | P00367 |
| Other data | |
| EC number | 1.4.1.3 |
| Locus | Chr. 10 q21.1-24.3 |
| glutamate dehydrogenase 2 | |
|---|---|
| Identifiers | |
| Symbol | GLUD2 |
| Alt. symbols | GLUDP1 |
| Entrez | 2747 |
| HUGO | 4336 |
| OMIM | 300144 |
| RefSeq | NM_012084 |
| UniProt | P49448 |
| Other data | |
| Locus | Chr. X q25 |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
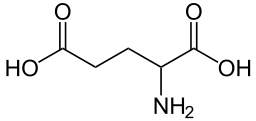
Glutamate dehydrogenase is an enzyme, present in mitochondria of eukaryotes, as are some of the other enzymes required for urea synthesis, that converts glutamate to α-Ketoglutarate, and vice versa.
The enzyme represents a key link between catabolic and metabolic pathways, and is therefore ubiquitous in both higher and lower organisms.
Cofactors
Its cofactor for the glutamate to α-Ketoglutarate reaction, which produces ammonium as a bi-product, is NAD+.
Its cofactor for the reverse reaction, α-Ketoglutarate to glutamate, is NADP+. This reverse reaction uses ammonium to incorporate nitrogen and α-Ketoglutarate into glutamate.
Role in flow of nitrogen
Ammonia incorporation in animals occurs through the actions of glutamate dehydrogenase and glutamine synthetase. Glutamate plays the central role in mammalian nitrogen flow, serving as both a nitrogen donor and nitrogen acceptor.
Regulation of glutamate dehydrogenase
In Humans the activity of glutamate dehydrogenase is controlled through ADP-ribosilation, a covalent modification carried out by the gene sirt4. This regulation is relaxed in response to caloric restriction and low blood glucose. Under these curcumstances glutamate dehydrogenase activity is raised to increase the amount of α-Ketoglutarate that is produced. The product α-Ketoglutarate can be used to provide energy by being used in the citric acid cycle to ultimately produce ATP.
The control of GDH through ADP-ribosilation is particularly important in insulin producing β cells. Beta cells secrete insulin in response to an increase in the ATP:ADP ratio, and as amino acids are broken down by GDH into α-ketoglutarate, this ratio rises and more insulin is secreted. SIRT4 is necessary to regulate the metabolism of amino acids as a method of controlling insulin secretion and to regulate blood glucose levels.
Regulation
- Adenosine triphosphate (ATP)
- Guanosine triphosphate (GTP)
Activators:
- Adenosine diphosphate (ADP)
- Guanosine diphosphate (GDP)
See also
External links
- Glutamate+dehydrogenase at the US National Library of Medicine Medical Subject Headings (MeSH)
| This EC 1.4 enzyme-related article is a stub. You can help Wikipedia by expanding it. |