Docetaxel: Difference between revisions
Gloria Picoy (talk | contribs) No edit summary |
Gloria Picoy (talk | contribs) No edit summary |
||
| Line 118: | Line 118: | ||
====Asthenia==== | ====Asthenia==== | ||
Severe asthenia has been reported in 14.9% (144/965) of metastatic breast cancer patients but has led to treatment discontinuation in only 1.8%. Symptoms of fatigue and weakness may last a few days up to several weeks and may be associated with deterioration of performance status in patients with progressive disease. | Severe asthenia has been reported in 14.9% (144/965) of metastatic breast cancer patients but has led to treatment discontinuation in only 1.8%. Symptoms of fatigue and weakness may last a few days up to several weeks and may be associated with deterioration of performance status in patients with progressive disease. | ||
|monitoring=Breast Cancer | |monitoring=====Breast Cancer==== | ||
* Patients who are dosed initially at 100 mg/m2 and who experience either febrile neutropenia, neutrophils <500 cells/mm3 for more than 1 week, or severe or cumulative cutaneous reactions during Docetaxel Injection therapy should have the dosage adjusted from 100 mg/m2 to 75 mg/m2. If the patient continues to experience these reactions, the dosage should either be decreased from 75 mg/m2 to 55 mg/m2 or the treatment should be discontinued. Conversely, patients who are dosed initially at 60 mg/m2 and who do not experience febrile neutropenia, neutrophils <500 cells/mm3 for more than 1 week, severe or cumulative cutaneous reactions, or severe peripheral neuropathy during Docetaxel Injection therapy may tolerate higher doses. Patients who develop ≥grade 3 peripheral neuropathy should have Docetaxel Injection treatment discontinued entirely. | |||
* Combination Therapy with Docetaxel Injection in the Adjuvant Treatment of Breast Cancer | |||
* Docetaxel Injection in combination with doxorubicin and cyclophosphamide should be administered when the neutrophil count is ≥1,500 cells/mm3. Patients who experience febrile neutropenia should receive G-CSF in all subsequent cycles. Patients who continue to experience this reaction should remain on G-CSF and have their Docetaxel Injection dose reduced to 60 mg/ m2. Patients who experience grade 3 or 4 stomatitis should have their Docetaxel Injection dose decreased to 60 mg/ m2. Patients who experience severe or cumulative cutaneous reactions or moderate neurosensory signs and/or symptoms during Docetaxel Injection therapy should have their dosage of Docetaxel Injection reduced from 75 to 60 mg/ m2. If the patient continues to experience these reactions at 60 mg/ m2, treatment should be discontinued. | |||
====Non-Small Cell Lung Cancer==== | |||
* Monotherapy with Docetaxel Injection for NSCLC treatment after failure of prior platinum-based chemotherapy | |||
* Patients who are dosed initially at 75 mg/m2 and who experience either febrile neutropenia, neutrophils <500 cells/mm3 for more than one week, severe or cumulative cutaneous reactions, or other grade 3/4 non-hematological toxicities during Docetaxel Injection treatment should have treatment withheld until resolution of the toxicity and then resumed at 55 mg/m2. Patients who develop ≥grade 3 peripheral neuropathy should have Docetaxel Injection treatment discontinued entirely. | |||
* Combination therapy with Docetaxel Injection for chemotherapy-naïve NSCLC | |||
* For patients who are dosed initially at Docetaxel Injection 75 mg/m2 in combination with cisplatin, and whose nadir of platelet count during the previous course of therapy is <25,000 cells/mm3, in patients who experience febrile neutropenia, and in patients with serious non-hematologic toxicities, the Docetaxel Injection dosage in subsequent cycles should be reduced to 65 mg/m2. In patients who require a further dose reduction, a dose of 50 mg/m2 is recommended. For cisplatin dosage adjustments, see manufacturers' prescribing information. | |||
====Prostate Cancer==== | |||
* Combination therapy with Docetaxel Injection for hormone-refractory metastatic prostate cancer | |||
* Docetaxel Injection should be administered when the neutrophil count is ≥1,500 cells/mm3. Patients who experience either febrile neutropenia, neutrophils <500 cells/mm3 for more than one week, severe or cumulative cutaneous reactions or moderate neurosensory signs and/or symptoms during Docetaxel Injection therapy should have the dosage of Docetaxel Injection reduced from 75 to 60 mg/ m2. If the patient continues to experience these reactions at 60 mg/m2, the treatment should be discontinued. | |||
====Gastric or Head and Neck Cancer==== | |||
* Docetaxel Injection in combination with cisplatin and fluorouracil in gastric cancer or head and neck cancer | |||
* Patients treated with Docetaxel Injection in combination with cisplatin and fluorouracil must receive antiemetics and appropriate hydration according to current institutional guidelines. In both studies, G-CSF was recommended during the second and/or subsequent cycles in case of febrile neutropenia, or documented infection with neutropenia, or neutropenia lasting more than 7 days. If an episode of febrile neutropenia, prolonged neutropenia or neutropenic infection occurs despite G-CSF use, the Docetaxel Injection dose should be reduced from 75 mg/m2 to 60 mg/m2. If subsequent episodes of complicated neutropenia occur the Docetaxel Injection dose should be reduced from 60 mg/m2 to 45 mg/m2. In case of grade 4 thrombocytopenia the Docetaxel Injection dose should be reduced from 75 mg/m2 to 60 mg/m2. Patients should not be retreated with subsequent cycles of Docetaxel Injection until neutrophils recover to a level >1,500 cells/mm3 and platelets recover to a level >100,000 cells/mm3. Discontinue treatment if these toxicities persist | |||
Patients treated with Docetaxel Injection in combination with cisplatin and fluorouracil must receive antiemetics and appropriate hydration according to current institutional guidelines. In both studies, G-CSF was recommended during the second and/or subsequent cycles in case of febrile neutropenia, or documented infection with neutropenia, or neutropenia lasting more than 7 days. If an episode of febrile neutropenia, prolonged neutropenia or neutropenic infection occurs despite G-CSF use, the Docetaxel Injection dose should be reduced from 75 mg/m2 to 60 mg/m2. If subsequent episodes of complicated neutropenia occur the Docetaxel Injection dose should be reduced from 60 mg/m2 to 45 mg/m2. In case of grade 4 thrombocytopenia the Docetaxel Injection dose should be reduced from 75 mg/m2 to 60 mg/m2. Patients should not be retreated with subsequent cycles of Docetaxel Injection until neutrophils recover to a level >1,500 cells/mm3 and platelets recover to a level >100,000 cells/mm3. Discontinue treatment if these toxicities persist | |||
[[File:Recommended Dose Modifications for Toxicities in Patients Treated with Docetaxel Injection in Combination with Cisplatin and Fluorouracil.png | [[File:Recommended Dose Modifications for Toxicities in Patients Treated with Docetaxel Injection in Combination with Cisplatin and Fluorouracil.png | ||
|thumb|None|600px]] | |thumb|None|600px]] | ||
Liver dysfunction | ====Liver dysfunction==== | ||
* In case of AST/ALT >2.5 to ≤5 × ULN and AP ≤2.5 × ULN, or AST/ALT >1.5 to ≤5 × ULN and AP >2.5 to ≤5 × ULN, Docetaxel Injection should be reduced by 20%. | |||
In case of AST/ALT >2.5 to ≤5 × ULN and AP ≤2.5 × ULN, or AST/ALT >1.5 to ≤5 × ULN and AP >2.5 to ≤5 × ULN, Docetaxel Injection should be reduced by 20%. | |||
In case of AST/ALT >5 × ULN and/or AP >5 × ULN Docetaxel Injection should be stopped. | * In case of AST/ALT >5 × ULN and/or AP >5 × ULN Docetaxel Injection should be stopped. | ||
The dose modifications for cisplatin and fluorouracil in the gastric cancer study are provided below: | * The dose modifications for cisplatin and fluorouracil in the gastric cancer study are provided below: | ||
=====Cisplatin dose modifications and delays===== | |||
Peripheral neuropathy: A neurological examination should be performed before entry into the study, and then at least every 2 cycles and at the end of treatment. In the case of neurological signs or symptoms, more frequent examinations should be performed and the following dose modifications can be made according to NCIC-CTC grade: | Peripheral neuropathy: A neurological examination should be performed before entry into the study, and then at least every 2 cycles and at the end of treatment. In the case of neurological signs or symptoms, more frequent examinations should be performed and the following dose modifications can be made according to NCIC-CTC grade: | ||
* Grade 2: Reduce cisplatin dose by 20%. | |||
* Grade 3: Discontinue treatment. | |||
Ototoxicity: In the case of grade 3 toxicity, discontinue treatment. | Ototoxicity: In the case of grade 3 toxicity, discontinue treatment. | ||
| Line 171: | Line 166: | ||
[[File:Dose Reductions for Evaluation of Creatinine Clearance.jpeg|thumb|None|600px]] | [[File:Dose Reductions for Evaluation of Creatinine Clearance.jpeg|thumb|None|600px]] | ||
Fluorouracil dose modifications and treatment delays | =====Fluorouracil dose modifications and treatment delays===== | ||
In the event of grade 2 or greater plantar-palmar toxicity, fluorouracil should be stopped until recovery. The fluorouracil dosage should be reduced by 20%. | In the event of grade 2 or greater plantar-palmar toxicity, fluorouracil should be stopped until recovery. The fluorouracil dosage should be reduced by 20%. | ||
Revision as of 15:21, 29 December 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warning: Toxic deaths, hepatotoxicity, neutropenia, hipersensitivity reactions and fluid retention
See full prescribing information for complete Boxed Warning.
The incidence of treatment-related mortality associated with docetaxel therapy is increased in patients with abnormal liver function, in patients receiving higher doses, and in patients with non-small cell lung carcinoma and a history of prior treatment with platinum-based chemotherapy who receive docetaxel as a single agent at a dose of 100 mg/m2.
Docetaxel Injection should not be given to patients with bilirubin > upper limit of normal (ULN), or to patients with AST and/or ALT >1.5 x ULN concomitant with alkaline phosphatase >2.5 x ULN. Patients with elevations of bilirubin or abnormalities of transaminase concurrent with alkaline phosphatase are at increased risk for the development of grade 4 neutropenia, febrile neutropenia, infections, severe thrombocytopenia, severe stomatitis, severe skin toxicity, and toxic death. Patients with isolated elevations of transaminase >1.5 x ULN also had a higher rate of febrile neutropenia grade 4 but did not have an increased incidence of toxic death. Bilirubin, AST or ALT, and alkaline phosphatase values should be obtained prior to each cycle of Docetaxel Injection therapy. Docetaxel Injection therapy should not be given to patients with neutrophil counts of <1500 cells/mm3. In order to monitor the occurrence of neutropenia, which may be severe and result in infection, frequent blood cell counts should be performed on all patients receiving Docetaxel Injection. Severe hypersensitivity reactions characterized by generalized rash/erythema, hypotension and/or bronchospasm, or very rarely fatal anaphylaxis, have been reported in patients who received a 3-day dexamethasone premedication. Hypersensitivity reactions require immediate discontinuation of the Docetaxel Injection infusion and administration of appropriate therapy. Docetaxel Injection must not be given to patients who have a history of severe hypersensitivity reactions to docetaxel or to other drugs formulated with polysorbate 80. Severe fluid retention occurred in 6.5% (6/92) of patients despite use of a 3-day dexamethasone premedication regimen. It was characterized by one or more of the following events: poorly tolerated peripheral edema, generalized edema, pleural effusion requiring urgent drainage, dyspnea at rest, cardiac tamponade, or pronounced abdominal distention (due to ascites) |
Overview
Docetaxel is an antineoplastic agent that is FDA approved for the treatment of breast cancer, non-small cell lung cancer, prostate cancer, gastric adenocarcinoma, head and Neck Cancer. There is a Black Box Warning for this drug as shown here. Common adverse reactions include body fluid retention, vasodilatation, alopecia, disorder of skin and/or subcutaneous tissue, nail changes, pruritus, rash, diarrhea, inflammatory disease of mucous membrane, nausea, stomatitis, vomiting, any grade of anemia, leukopenia, neutropenia, asthenia, neuropathy, amenorrhea, fever of unknown origin..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Premedication Regimen
- All patients should be premedicated with oral corticosteroids (see below for prostate cancer) such as dexamethasone 16 mg per day (e.g., 8 mg twice daily) for 3 days starting 1 day prior to Docetaxel Injection administration in order to reduce the incidence and severity of fluid retention as well as the severity of hypersensitivity reactions.
- For hormone-refractory metastatic prostate cancer, given the concurrent use of prednisone, the recommended premedication regimen is oral dexamethasone 8 mg, at 12 hours, 3 hours and 1 hour before the Docetaxel Injection infusion.
Breast Cancer
- Locally advanced or metastatic breast cancer after failure of prior chemotherapy
- Dose of Docetaxel Injection is 60 mg/m2 to 100 mg/m2 administered intravenously over 1 hour every 3 weeks.
- Adjuvant treatment of operable node-positive breast cancer
- Docetaxel Injection dose is 75 mg/m2 administered 1 hour after doxorubicin 50 mg/m2
- Cyclophosphamide 500 mg/m2 every 3 weeks for 6 courses.
Non-Small Cell Lung Cancer
- Treatment after failure of prior platinum-based chemotherapy
- Dose is 75 mg/m 2 administered intravenously over 1 hour every 3 weeks.
- For chemotherapy-naïve patients, docetaxel was evaluated in combination with cisplatin
- Dosage: Docetaxel Injection is 75 mg/m2 administered intravenously over 1 hour immediately followed by cisplatin 75 mg/m2 over 30-60 minutes every 3 weeks.
Prostate Cancer
- For hormone-refractory metastatic prostate cancer
- Docetaxel Injection 75 mg/m2 every 3 weeks as a 1 hour intravenous infusion.
- Prednisone 5 mg orally twice daily is administered continuously
Gastric Adenocarcinoma
- Dosage: Docetaxel Injection is 75 mg/m2 as a 1 hour intravenous infusion, followed by cisplatin 75 mg/m2, as a 1 to 3 hour intravenous infusion (both on day 1 only), followed by fluorouracil 750 mg/m 2 per day given as a 24-hour continuous intravenous infusion for 5 days, starting at the end of the cisplatin infusion.
- Treatment is repeated every three weeks.
- Patients must receive premedication with antiemetics and appropriate hydration for cisplatin administration.
Head and Neck Cancer
- Docetaxel Injection in combination with cisplatin and fluorouracil is indicated for the induction treatment of patients with locally advanced squamous cell carcinoma of the head and neck (SCCHN).
- Patients must receive premedication with antiemetics, and appropriate hydration (prior to and after cisplatin administration). Prophylaxis for neutropenic infections should be administered. All patients treated on the docetaxel containing arms of the TAX323 and TAX324 studies received prophylactic antibiotics.
- Induction chemotherapy followed by radiotherapy (TAX323)
For the induction treatment of locally advanced inoperable SCCHN, the recommended dose of Docetaxel Injection is 75 mg/m2 as a 1 hour intravenous infusion followed by cisplatin 75 mg/m2 intravenously over 1 hour, on day one, followed by fluorouracil as a continuous intravenous infusion at 750 mg/m2 per day for five days. This regimen is administered every 3 weeks for 4 cycles. Following chemotherapy, patients should receive radiotherapy.
- Induction chemotherapy followed by chemoradiotherapy (TAX324)
For the induction treatment of patients with locally advanced (unresectable, low surgical cure, or organ preservation) SCCHN, the recommended dose of Docetaxel Injection is 75 mg/m2 as a 1 hour intravenous infusion on day 1, followed by cisplatin 100 mg/m2 administered as a 30-minute to 3 hour infusion, followed by fluorouracil 1000 mg/m2/day as a continuous infusion from day 1 to day 4. This regimen is administered every 3 weeks for 3 cycles. Following chemotherapy, patients should receive chemoradiotherapy.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Docetaxel in adult patients.
Non–Guideline-Supported Use
- As first-line chemotherapy for breast cancer locally advanced/metastatic disease
- Neoadjuvant treatment in combination with an anthracycline-containing regimen for breast cancer
- Carcinoma of bladder
- Carcinoma of esophagus
- Mobilization of harvesting of peripheral blood stem cells
- Head and neck cancer
- Previously treated advanced ovarian cancer
- First line in combination with carboplatin ovarian cancer
- Small cell carcinoma of lung
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Docetaxel FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Docetaxel in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Docetaxel in pediatric patients.
Contraindications
Docetaxel Injection is contraindicated in patients who have a history of severe hypersensitivity reactions to docetaxel or to other drugs formulated with polysorbate 80. Severe reactions, including anaphylaxis, have occurred.
Docetaxel Injection should not be used in patients with neutrophil counts of <1500 cells/mm3.
Warnings
|
Warning: Toxic deaths, hepatotoxicity, neutropenia, hipersensitivity reactions and fluid retention
See full prescribing information for complete Boxed Warning.
The incidence of treatment-related mortality associated with docetaxel therapy is increased in patients with abnormal liver function, in patients receiving higher doses, and in patients with non-small cell lung carcinoma and a history of prior treatment with platinum-based chemotherapy who receive docetaxel as a single agent at a dose of 100 mg/m2.
Docetaxel Injection should not be given to patients with bilirubin > upper limit of normal (ULN), or to patients with AST and/or ALT >1.5 x ULN concomitant with alkaline phosphatase >2.5 x ULN. Patients with elevations of bilirubin or abnormalities of transaminase concurrent with alkaline phosphatase are at increased risk for the development of grade 4 neutropenia, febrile neutropenia, infections, severe thrombocytopenia, severe stomatitis, severe skin toxicity, and toxic death. Patients with isolated elevations of transaminase >1.5 x ULN also had a higher rate of febrile neutropenia grade 4 but did not have an increased incidence of toxic death. Bilirubin, AST or ALT, and alkaline phosphatase values should be obtained prior to each cycle of Docetaxel Injection therapy. Docetaxel Injection therapy should not be given to patients with neutrophil counts of <1500 cells/mm3. In order to monitor the occurrence of neutropenia, which may be severe and result in infection, frequent blood cell counts should be performed on all patients receiving Docetaxel Injection. Severe hypersensitivity reactions characterized by generalized rash/erythema, hypotension and/or bronchospasm, or very rarely fatal anaphylaxis, have been reported in patients who received a 3-day dexamethasone premedication. Hypersensitivity reactions require immediate discontinuation of the Docetaxel Injection infusion and administration of appropriate therapy. Docetaxel Injection must not be given to patients who have a history of severe hypersensitivity reactions to docetaxel or to other drugs formulated with polysorbate 80. Severe fluid retention occurred in 6.5% (6/92) of patients despite use of a 3-day dexamethasone premedication regimen. It was characterized by one or more of the following events: poorly tolerated peripheral edema, generalized edema, pleural effusion requiring urgent drainage, dyspnea at rest, cardiac tamponade, or pronounced abdominal distention (due to ascites) |
Toxic Deaths
Breast Cancer
Docetaxel administered at 100 mg/m2 was associated with deaths considered possibly or probably related to treatment in 2.0% (19/965) of metastatic breast cancer patients, both previously treated and untreated, with normal baseline liver function and in 11.5% (7/61) of patients with various tumor types who had abnormal baseline liver function (AST and/or ALT >1.5 times ULN together with AP >2.5 times ULN). Among patients dosed at 60 mg/m2, mortality related to treatment occurred in 0.6% (3/481) of patients with normal liver function, and in 3 of 7 patients with abnormal liver function. Approximately half of these deaths occurred during the first cycle. Sepsis accounted for the majority of the deaths.
Non-Small Cell Lung Cancer
Docetaxel administered at a dose of 100 mg/m2 in patients with locally advanced or metastatic non-small cell lung cancer who had a history of prior platinum-based chemotherapy was associated with increased treatment-related mortality (14% and 5% in two randomized, controlled studies). There were 2.8% treatment-related deaths among the 176 patients treated at the 75 mg/m2 dose in the randomized trials. Among patients who experienced treatment-related mortality at the 75 mg/m2 dose level, 3 of 5 patients had an ECOG PS of 2 at study entry.
Hepatic Impairment
Patients with combined abnormalities of transaminases and alkaline phosphatase should not be treated with Docetaxel Injection.
Hematologic Effects
- Perform frequent peripheral blood cell counts on all patients receiving Docetaxel Injection. Patients should not be retreated with subsequent cycles of Docetaxel Injection until neutrophils recover to a level >1500 cells/mm3 and platelets recover to a level > 100,000 cells/mm3.
- A 25% reduction in the dose of Docetaxel Injection is recommended during subsequent cycles following severe neutropenia (<500 cells/mm3) lasting 7 days or more, febrile neutropenia, or a grade 4 infection in a Docetaxel Injection cycle.
- Neutropenia (<2000 neutrophils/mm3) occurs in virtually all patients given 60 mg/m2 to 100 mg/m2 of docetaxel and grade 4 neutropenia (<500 cells/mm3) occurs in 85% of patients given 100 mg/m2 and 75% of patients given 60 mg/m2. Frequent monitoring of blood counts is, therefore, essential so that dose can be adjusted. Docetaxel Injection should not be administered to patients with neutrophils <1500 cells/mm3.
- Febrile neutropenia occurred in about 12% of patients given 100 mg/m2 but was very uncommon in patients given 60 mg/m2. Hematologic responses, febrile reactions and infections, and rates of septic death for different regimens are dose related.
- Three breast cancer patients with severe liver impairment (bilirubin >1.7 times ULN) developed fatal gastrointestinal bleeding associated with severe drug-induced thrombocytopenia. In gastric cancer patients treated with docetaxel in combination with cisplatin and fluorouracil (TCF), febrile neutropenia and/or neutropenic infection occurred in 12% of patients receiving G-CSF compared to 28% who did not. Patients receiving TCF should be closely monitored during the first and subsequent cycles for febrile neutropenia and neutropenic infection.
Hypersensitivity Reactions
Patients should be observed closely for hypersensitivity reactions, especially during the first and second infusions. Severe hypersensitivity reactions characterized by generalized rash/erythema, hypotension and/or bronchospasm, or very rarely fatal anaphylaxis, have been reported in patients premedicated with 3 days of corticosteroids. Severe hypersensitivity reactions require immediate discontinuation of the Docetaxel Injection infusion and aggressive therapy. Patients with a history of severe hypersensitivity reactions should not be rechallenged with Docetaxel Injection.
Hypersensitivity reactions may occur within a few minutes following initiation of a Docetaxel Injection infusion. If minor reactions such as flushing or localized skin reactions occur, interruption of therapy is not required. All patients should be premedicated with an oral corticosteroid prior to the initiation of the infusion of Docetaxel Injection.
Fluid Retention
Severe fluid retention has been reported following docetaxel therapy. Patients should be premedicated with oral corticosteroids prior to each Docetaxel Injection administration to reduce the incidence and severity of fluid retention. Patients with pre-existing effusions should be closely monitored from the first dose for the possible exacerbation of the effusions.
When fluid retention occurs, peripheral edema usually starts in the lower extremities and may become generalized with a median weight gain of 2 kg.
Among 92 breast cancer patients premedicated with 3-day corticosteroids, moderate fluid retention occurred in 27.2% and severe fluid retention in 6.5%. The median cumulative dose to onset of moderate or severe fluid retention was 819 mg/m2. Nine of 92 patients (9.8%) of patients discontinued treatment due to fluid retention: 4 patients discontinued with severe fluid retention; the remaining 5 had mild or moderate fluid retention. The median cumulative dose to treatment discontinuation due to fluid retention was 1021 mg/m2. Fluid retention was completely, but sometimes slowly, reversible with a median of 16 weeks from the last infusion of docetaxel to resolution (range: 0 to 42+ weeks). Patients developing peripheral edema may be treated with standard measures, e.g., salt restriction, oral diuretic(s).
Acute Myeloid Leukemia
Treatment-related acute myeloid leukemia (AML) or myelodysplasia has occurred in patients given anthracyclines and/or cyclophosphamide, including use in adjuvant therapy for breast cancer. In the adjuvant breast cancer trial (TAX316) AML occurred in 3 of 744 patients who received docetaxel, doxorubicin and cyclophosphamide (TAC) and in 1 of 736 patients who received fluorouracil, doxorubicin and cyclophosphamide [see Clinical Studies (14.2)]. In TAC-treated patients, the risk of delayed myelodysplasia or myeloid leukemia requires hematological follow-up.
Cutaneous Reactions
Localized erythema of the extremities with edema followed by desquamation has been observed. In case of severe skin toxicity, an adjustment in dosage is recommended [see Dosage and Administration (2.7)]. The discontinuation rate due to skin toxicity was 1.6% (15/965) for metastatic breast cancer patients. Among 92 breast cancer patients premedicated with 3-day corticosteroids, there were no cases of severe skin toxicity reported and no patient discontinued docetaxel due to skin toxicity.
Neurologic Reactions
Severe neurosensory symptoms (e.g. paresthesia, dysesthesia, pain) were observed in 5.5% (53/965) of metastatic breast cancer patients, and resulted in treatment discontinuation in 6.1%. When these symptoms occur, dosage must be adjusted. If symptoms persist, treatment should be discontinued. Patients who experienced neurotoxicity in clinical trials and for whom follow-up information on the complete resolution of the event was available had spontaneous reversal of symptoms with a median of 9 weeks from onset (range: 0 to 106 weeks). Severe peripheral motor neuropathy mainly manifested as distal extremity weakness occurred in 4.4% (42/965).
Asthenia
Severe asthenia has been reported in 14.9% (144/965) of metastatic breast cancer patients but has led to treatment discontinuation in only 1.8%. Symptoms of fatigue and weakness may last a few days up to several weeks and may be associated with deterioration of performance status in patients with progressive disease.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Docetaxel Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Docetaxel Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Docetaxel Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Docetaxel in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Docetaxel in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Docetaxel during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Docetaxel in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Docetaxel in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Docetaxel in geriatric settings.
Gender
There is no FDA guidance on the use of Docetaxel with respect to specific gender populations.
Race
There is no FDA guidance on the use of Docetaxel with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Docetaxel in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Docetaxel in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Docetaxel in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Docetaxel in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Docetaxel Administration in the drug label.
Monitoring
Breast Cancer
- Patients who are dosed initially at 100 mg/m2 and who experience either febrile neutropenia, neutrophils <500 cells/mm3 for more than 1 week, or severe or cumulative cutaneous reactions during Docetaxel Injection therapy should have the dosage adjusted from 100 mg/m2 to 75 mg/m2. If the patient continues to experience these reactions, the dosage should either be decreased from 75 mg/m2 to 55 mg/m2 or the treatment should be discontinued. Conversely, patients who are dosed initially at 60 mg/m2 and who do not experience febrile neutropenia, neutrophils <500 cells/mm3 for more than 1 week, severe or cumulative cutaneous reactions, or severe peripheral neuropathy during Docetaxel Injection therapy may tolerate higher doses. Patients who develop ≥grade 3 peripheral neuropathy should have Docetaxel Injection treatment discontinued entirely.
- Combination Therapy with Docetaxel Injection in the Adjuvant Treatment of Breast Cancer
- Docetaxel Injection in combination with doxorubicin and cyclophosphamide should be administered when the neutrophil count is ≥1,500 cells/mm3. Patients who experience febrile neutropenia should receive G-CSF in all subsequent cycles. Patients who continue to experience this reaction should remain on G-CSF and have their Docetaxel Injection dose reduced to 60 mg/ m2. Patients who experience grade 3 or 4 stomatitis should have their Docetaxel Injection dose decreased to 60 mg/ m2. Patients who experience severe or cumulative cutaneous reactions or moderate neurosensory signs and/or symptoms during Docetaxel Injection therapy should have their dosage of Docetaxel Injection reduced from 75 to 60 mg/ m2. If the patient continues to experience these reactions at 60 mg/ m2, treatment should be discontinued.
Non-Small Cell Lung Cancer
- Monotherapy with Docetaxel Injection for NSCLC treatment after failure of prior platinum-based chemotherapy
- Patients who are dosed initially at 75 mg/m2 and who experience either febrile neutropenia, neutrophils <500 cells/mm3 for more than one week, severe or cumulative cutaneous reactions, or other grade 3/4 non-hematological toxicities during Docetaxel Injection treatment should have treatment withheld until resolution of the toxicity and then resumed at 55 mg/m2. Patients who develop ≥grade 3 peripheral neuropathy should have Docetaxel Injection treatment discontinued entirely.
- Combination therapy with Docetaxel Injection for chemotherapy-naïve NSCLC
- For patients who are dosed initially at Docetaxel Injection 75 mg/m2 in combination with cisplatin, and whose nadir of platelet count during the previous course of therapy is <25,000 cells/mm3, in patients who experience febrile neutropenia, and in patients with serious non-hematologic toxicities, the Docetaxel Injection dosage in subsequent cycles should be reduced to 65 mg/m2. In patients who require a further dose reduction, a dose of 50 mg/m2 is recommended. For cisplatin dosage adjustments, see manufacturers' prescribing information.
Prostate Cancer
- Combination therapy with Docetaxel Injection for hormone-refractory metastatic prostate cancer
- Docetaxel Injection should be administered when the neutrophil count is ≥1,500 cells/mm3. Patients who experience either febrile neutropenia, neutrophils <500 cells/mm3 for more than one week, severe or cumulative cutaneous reactions or moderate neurosensory signs and/or symptoms during Docetaxel Injection therapy should have the dosage of Docetaxel Injection reduced from 75 to 60 mg/ m2. If the patient continues to experience these reactions at 60 mg/m2, the treatment should be discontinued.
Gastric or Head and Neck Cancer
- Docetaxel Injection in combination with cisplatin and fluorouracil in gastric cancer or head and neck cancer
- Patients treated with Docetaxel Injection in combination with cisplatin and fluorouracil must receive antiemetics and appropriate hydration according to current institutional guidelines. In both studies, G-CSF was recommended during the second and/or subsequent cycles in case of febrile neutropenia, or documented infection with neutropenia, or neutropenia lasting more than 7 days. If an episode of febrile neutropenia, prolonged neutropenia or neutropenic infection occurs despite G-CSF use, the Docetaxel Injection dose should be reduced from 75 mg/m2 to 60 mg/m2. If subsequent episodes of complicated neutropenia occur the Docetaxel Injection dose should be reduced from 60 mg/m2 to 45 mg/m2. In case of grade 4 thrombocytopenia the Docetaxel Injection dose should be reduced from 75 mg/m2 to 60 mg/m2. Patients should not be retreated with subsequent cycles of Docetaxel Injection until neutrophils recover to a level >1,500 cells/mm3 and platelets recover to a level >100,000 cells/mm3. Discontinue treatment if these toxicities persist
[[File:Recommended Dose Modifications for Toxicities in Patients Treated with Docetaxel Injection in Combination with Cisplatin and Fluorouracil.png |thumb|None|600px]]
Liver dysfunction
- In case of AST/ALT >2.5 to ≤5 × ULN and AP ≤2.5 × ULN, or AST/ALT >1.5 to ≤5 × ULN and AP >2.5 to ≤5 × ULN, Docetaxel Injection should be reduced by 20%.
- In case of AST/ALT >5 × ULN and/or AP >5 × ULN Docetaxel Injection should be stopped.
- The dose modifications for cisplatin and fluorouracil in the gastric cancer study are provided below:
Cisplatin dose modifications and delays
Peripheral neuropathy: A neurological examination should be performed before entry into the study, and then at least every 2 cycles and at the end of treatment. In the case of neurological signs or symptoms, more frequent examinations should be performed and the following dose modifications can be made according to NCIC-CTC grade:
- Grade 2: Reduce cisplatin dose by 20%.
- Grade 3: Discontinue treatment.
Ototoxicity: In the case of grade 3 toxicity, discontinue treatment.
Nephrotoxicity: In the event of a rise in serum creatinine ≥grade 2 (>1.5 × normal value) despite adequate rehydration, CrCl should be determined before each subsequent cycle and the following dose reductions should be considered
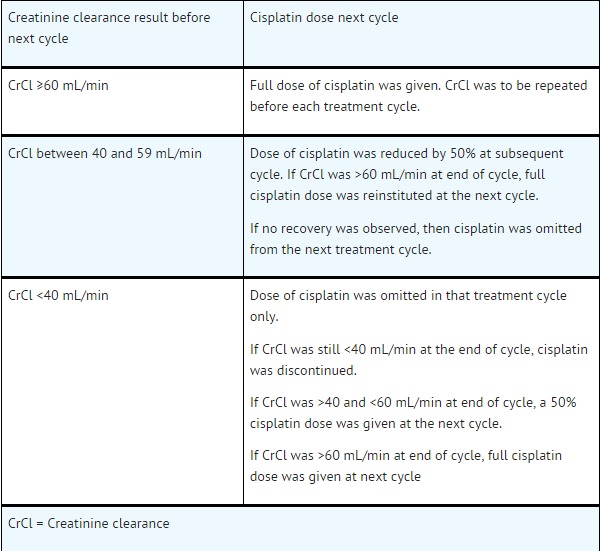
Fluorouracil dose modifications and treatment delays
In the event of grade 2 or greater plantar-palmar toxicity, fluorouracil should be stopped until recovery. The fluorouracil dosage should be reduced by 20%.
For other greater than grade 3 toxicities, except alopecia and anemia, chemotherapy should be delayed (for a maximum of 2 weeks from the planned date of infusion) until resolution to grade ≤1 and then recommenced, if medically appropriate.
For other fluorouracil dosage adjustments, also refer to the manufacturers’ prescribing information.
Combination Therapy with Strong CYP3A4 inhibitors:
Avoid using concomitant strong CYP3A4 inhibitors (e.g., ketoconazole, itraconazole, clarithromycin, atazanavir, indinavir, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin and voriconazole). There are no clinical data with a dose adjustment in patients receiving strong CYP3A4 inhibitors. Based on extrapolation from a pharmacokinetic study with ketoconazole in 7 patients, consider a 50% Docetaxel Injection dose reduction if patients require co-administration of a strong CYP3A4 inhibitor.
IV Compatibility
There is limited information regarding the compatibility of Docetaxel and IV administrations.
Overdosage
There is limited information regarding Docetaxel overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Docetaxel Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Docetaxel Mechanism of Action in the drug label.
Structure
There is limited information regarding Docetaxel Structure in the drug label.
Pharmacodynamics
There is limited information regarding Docetaxel Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Docetaxel Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Docetaxel Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Docetaxel Clinical Studies in the drug label.
How Supplied
There is limited information regarding Docetaxel How Supplied in the drug label.
Storage
There is limited information regarding Docetaxel Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Docetaxel |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Docetaxel |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Docetaxel Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Docetaxel interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Docetaxel Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Docetaxel Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
| File:Docetaxel.svg | |
| File:Docetaxel Taxotere.gif | |
| Clinical data | |
|---|---|
| Routes of administration | IV |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | NA |
| Protein binding | >98% |
| Metabolism | Hepatic |
| Elimination half-life | 86 hours |
| Excretion | Biliary |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C43H53NO14 |
| Molar mass | 807.879 g/mol |
|
WikiDoc Resources for Docetaxel |
|
Articles |
|---|
|
Most recent articles on Docetaxel |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Docetaxel at Clinical Trials.gov Clinical Trials on Docetaxel at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Docetaxel
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Docetaxel Discussion groups on Docetaxel Directions to Hospitals Treating Docetaxel Risk calculators and risk factors for Docetaxel
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Docetaxel |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Overview
Docetaxel is a clinically well established anti-mitotic chemotherapy medication used mainly for the treatment of breast, ovarian, and non-small cell lung cancer.[1][2] Docetaxel has an approved claim for treatment of patients who have locally advanced, or metastatic breast or non small-cell lung cancer who have undergone anthracycline-based chemotherapy and failed to stop cancer progression or relapsed.[3] Administered as a one-hour infusion every three weeks generally over a ten cycle course, docetaxel is considered better than doxorubicin, paclitaxel and fluorouracil as a cytotoxic antimicrotubule agent.[1] Docetaxel is marketed under the name Taxotere by Sanofi-Aventis U.S. LLC.[4]
Chemical structure, nature, and composition
Nature
Docetaxel is of the chemotherapy drug class; taxane, and is a semi-synthetic analogue of paclitaxel (Taxol®), an extract from the rare Pacific yew tree Taxus brevifolia.[2] Due to scarcity of paclitaxel, extensive research was carried out leading to the formulation of docetaxel – an esterified product of 10-deacetyl baccatin III, which is extracted from the renewable and readily available European yew tree.
Docetaxel differs from paclitaxel at two positions in its chemical structure. It has a hydroxyl functional group on carbon 10, whereas paclitaxel has an acetate ester and a tert-butyl substitution exists on the phenylpropionate side chain. The carbon 10 functional group change causes docetaxel to be more lipid soluble than paclitaxel.[2]
Formulations and compositions
Docetaxel is a white powder and is the active ingredient available in 20 mg and 80 mg Taxotere single-dose vials of concentrated anhydrous docetaxel in polysorbate 80.[5][2] The solution is a clear brown-yellow containing 40 mg docetaxel and 1040 mg polysorbate 80 per mL.[5] 20 mg Taxotere® is distributed in a blister carton containing one single-dose vial of Taxotere (docetaxel) preparation in 0.5 mL sterile pyrogen-free anhydrous polysorbate 80, and a single dose Taxotere® solvent vial containing 1.5 mL 13% ethanol in saline to be combined and diluted in a 250 mL infusion bag containing 0.9% sodium chloride or 5% glucose for administration.[5] 80 mg Taxotere is supplied identically but with 2.0 mL polysorbate 80 and 6.0 mL 13% ethanol in saline. The docetaxel and solvent vials are combined to give a solution of 10 mg/mL and the required dose is drawn from this solution. Vials have an overfill to compensate for liquid loss during preparation, foaming, adhesion to vial walls and the dead volume. 20 mg vials may be stored for 24 months below 25°C away from light and 80 mg vials for 26 months in the same conditions.[5]
Active regions
A model based on electron crystallographic density and nuclear magnetic resonance deconvolution has been proposed to explain the binding of docetaxel to β-tubulin.[6] In this T-shaped/butterfly model, a deep hydrophobic cleft exists near the surface of the β-tubulin where three potential hydrogen bonds and multiple hydrophobic contacts bind to docetaxel. The hydrophobic pocket walls contain helices H1, H6, H7 and a loop between H6 and H7 that form hydrophobic interactions with the 3’-benzamido phenyl, 3’-phenyl, and the 2-benzoyl phenyl of docetaxel. 3’-phenyl also has contact with β-sheets B8 and B10. The C-8 methyl of docetaxel has Van der Waal's interactions with two residues, Thr-276 and Gln-281 near the C-terminal end of β-tubulin. Docetaxel’s O-21 experiences electrostatic attraction to Thr-276 and the C-12 methyl has proximity with Leu-371 on the loop between B9 and B10.[6]
Pharmacokinetics
Absorption and distribution
Intravenous administration of docetaxel results in 100% bioavailability and absorption is immediate.[7] Oral bioavailability has been found to be 8% ±6% on its own and when co-administered with cyclosporine, bioavailability increased to 90% ± 44%.[8] In practice, docetaxel is administered intravenously only to increase dose precession.[2][5][9][10] Evaluation of docetaxel pharmacokinetics in phase II and III clinical studies were with 100 mg/m² dosages given over one-hour infusions every three weeks. Docetaxel's plasma protein binding includes lipoproteins, alpha1 acid glycoprotein and albumin. Alpha1 acid glycoprotein is the most variable of these proteins inter-individually, especially in cancer patients and is therefore the main determinant of docetaxel's plasma binding variability.[11] Docetaxel interacted little with erythrocytes and was unaffected by the polysorbate 80 in its storage medium.[2][11]
The concentration-time profile of docetaxel was consistent with a three-compartment pharmacokinetic model.[2][5] An initial, relatively rapid decline, with an α half-life of mean 4.5 minutes is representative of distribution to peripheral compartments from the systemic circulation. A β half-life of mean 38.3 minutes and a relatively slow γ half-life of mean 12.2 hours represent the slow efflux of docetaxel from the peripheral compartment.[2][5]
Administration a 100 mg/m² dose over a one hour infusion gave a mean total body clearance of 21 L/h/m² and a mean steady state volume of distribution of 73.8 L/m² or 123 L based on the mean BSA (body surface area) of 1.68 m².[2][5] Area under the plasma concentration-time curve had a mean value of 2.8 mg.h/L.[2] The Cmax of docetaxel was found to be 4.15 ± 1.35 mg/L.[12] Increased dose resulted in a linear increase of the area under the concentration-time curve and so it is concluded that dose is directly proportional to plasma concentration.[2]
Metabolism and excretion
Docetaxel is mainly metabolised in the liver by the cytochrome P450 CYP3A4 and CYP3A5 subfamilies of isoenzymes.[2][13][14] Metabolism is principally oxidative and at the tert-butylpropionate side chain, resulting first in an alcohol docetaxel (M2), which is then cyclised to three further metabolites (M1, M3 and M4).[14] M1 and M3 are two diasteromeric hydroxyoxazolidinones and M4 is an oxazolidinedione. Phase II trials of 577 patients showed docetaxel clearance to be related to body surface area and; hepatic enzyme and alpha1 acid glycoprotein, plasma levels.[11] The following model is agreed to represent docetaxel clearance in humans:
where CL is total body clearance (L/h), AAG and ALB represent alpha1 acid glycoprotein and albumin plasma concentrations (g/L) respectively, BSA is total body surface area (m²) and AGE is the patients age (years).[2] HEP12 represents a measure of hepatic dysfunction, affecting clearance of docetaxel. This final model accounted for a modest proportion of patients and identified most of the patients varying from the model (population median of CL = 35.6 L/h) as having hepatic dysfunction, indicating hepatic function as the most unpredictable factor with regards to clearance variability.[2]
Patients with significant hepatic dysfunction had an approximately 30% decrease in clearance of docetaxel and were also at a higher risk of toxicity poisoning from docetaxel treatment.[2] Clearance has been shown from population pharmacokinetic studies to decrease significantly with age, increased alpha1 acid glycoprotein and albumin concentrations and decreased body surface area.[2]
Renal impairment is unlikely to affect metabolism or excretion of docetaxel as renal excretion contributes less than 5% of elimination.[2] Limited data is available for docetaxel use in children with dosage between 55 and 75 mg/m². Two paediatric studies have taken place that show a mean clearance of 33 L/h/m² and concentration-time profiles best fitted by a two-compartmental model of distribution and elimination. Mean distribution half-life was 0.09 hours and mean elimination half-life was 1.4 hours in paediatric studies.[2]
Biodistribution of 14C-labelled docetaxel in three patients showed the bulk of the drug to be metabolised and excreted in bile to the faeces.[2] Of the radioactively labelled docetaxel administered, 80% was eliminated to the faeces with 5% in the urine over seven days, an indication that urinary excretion of docetaxel is minimal. Saliva contributed minimal excretion and no excretion was detected through pulmonary means.[2] The terminal half-life of docetaxel was determined as approximately 86 hours, through prolonged plasma sampling, contrary to the clinically stated terminal half-life of 10-18 hours.[8][12]
Mechanism of action
Molecular target
Docetaxel binds to microtubules reversibly with high affinity and has a maximum stoichiometry of 1 mole docetaxel per mole tubulin in microtubules.[15] This binding stabilises microtubules and prevents depolymerisation from calcium ions, decreased temperature and dilution, preferentially at the plus end of the microtubule.[15] Docetaxel has been found to accumulate to higher concentration in ovarian adenocarcinoma cells than kidney carcinoma cells, which may contribute to the more effective treatment of ovarian cancer by docetaxel.[1][15] It has also been found to lead to the phosphorylation of oncoprotein bcl-2, which is apoptosis blocking in its oncoprotein form.[1]
Modes of action
The cytotoxic activity of docetaxel is exerted by promoting and stabilising microtubule assembly, while preventing physiological microtubule depolymerisation/disassembly in the absence of GTP.[1][16][17] This leads to a significant decrease in free tubulin, needed for microtubule formation and results in inhibition of mitotic cell division between metaphase and anaphase, preventing further cancer cell progeny.[1][5][15]
Because microtubules do not disassemble in the presence of docetaxel, they accumulate inside the cell and cause initiation of apoptosis.[15] Apoptosis is also encouraged by the blocking of apoptosis-blocking bcl-2 oncoprotein.[1] Both in vitro and in vivo analysis show the anti-neoplastic activity of docetaxel to; be effective against a wide range of known cancer cells, cooperate with other anti-neoplastic agents activity, and have greater cytotoxicity than paclitaxel, possibly due to its more rapid intracellular uptake.[1]
The main mode of therapeutic action of docetaxel is the suppression of microtubule dynamic assembly and disassembly, rather than microtubule bundling leading to apoptosis, or the blocking of bcl-2.[1][15]
Cellular responses
Docetaxel exhibits cytotoxic activity on breast, colorectal, lung, ovarian, gastric, renal and prostate cancer cells.[1] Docetaxel does not block disassembly of interphase microtubules and so does not prevent entry into the mitotic cycle, but does block mitosis by inhibiting mitotic spindle assembly.[15] Resistance to paclitaxel or anthracycline doxorubicin does not necessarily indicate resistance to docetaxel.[1] Microtubules formed in the presence of docetaxel are of a larger size than those formed in the presence of paclitaxel, which may result in improved cytotoxic efficacy.[17] Abundant formation of microtubules and the prevention to replicate caused by the presence of docetaxel leads to apoptosis of tumour cells and is the basis of docetaxel use as a cancer treatment.[17] It is unknown if pathophysiological interactions with docetaxel exist at this stage, however tumour type has been shown to have efficacy on cellular activity.[1] Docetaxel activity is significantly greater in ovarian and breast tumours than for lung tumours.[1]
Therapeutic Applications and Effects
Therapeutic applications
The main use of docetaxel is the treatment of a variety of cancers after the failure of anthracycline-based chemotherapy.[3] Marketing of docetaxel as Taxotere® is mainly towards the treatment of breast, prostate and other non-small cell cancers.[4] Clinical data has shown docetaxel to have cytotoxic activity against breast, colorectal, lung, ovarian, prostate, liver, renal and gastric cancer and melanoma cells.[1][5]
In the treatment of breast cancer, eight phase II studies were carried out in patients with either locally advanced or metastatic breast cancer.[5] A total of 283 previously untreated and treated patients underwent the following dose allocations;
| Dosage | 75 mg/m² | 100 mg/m² | Total |
|---|---|---|---|
| Previously Untreated | 55 | 117 | 172 |
| Previously Treated | - | 111 | 111 |
| 283 |
Taxotere® was administered over a one-hour infusion every three weeks for these trials.[5] The 75 mg/m² cohort showed an overall response rate of 47% and 9% complete responses. Duration of response and the time to progression (treatment failure) had median values of 34 weeks and 22 weeks, respectively. Patients with two or fewer organs involved had a response rate of 58.6%, whereas patients with three or more organs involved showed 29.4% response.[5]
Previously untreated patients in the 100 mg/m² cohort had an overall response rate of 56% and 9.4% complete responses.[5] The previously treated population had an overall response of 48.6% and 3.6% complete responses. Median duration of response and time to progression was 30 weeks and 21 weeks for the previously untreated population and 28 weeks and 19 weeks for the previously treated patients. The 100 mg/m² cohort showed higher toxicity. Previously untreated patients with three or more organs involved had a 54.3% response rate and previously treated patients had a 55.8% response rate.[5]
Two randomised phase III studies of 326 alkylating agent failure and 392 anthracycline failure metastatic breast cancer patients have been carried out with 100 mg/m² dosages administered over a one-hour infusion every three weeks for seven and ten cycles respectively.[5] While no significant differences in median time to progression or survival were observed between docetaxel and doxorubicin in alkylating agent failure patients, anthracycline failure patients showed increased response rate to docetaxel. Median time to progression and median overall survival were also improved with docetaxel.[5]
The following table is the results of an unpublished, non-peer reviewed, comparative, open-label, randomised phase III study of docetaxel and paclitaxel assigned randomly to 449 patients with advanced breast cancer.[5] Docetaxel was administered as a one-hour infusion of 100 mg/m² Taxotere® every three weeks and paclitaxel as a three-hour infusion of 175 mg/m² paclitaxel every three weeks.
| Endpoint | Docetaxel 100 mg/m² n=225 | Paclitaxel 175 mg/m² n=224 | p-value |
|---|---|---|---|
| Median survival (months) | 15.3 | 12.7 | 0.03 |
| Median time to progression (weeks) | 24.6 | 15.6 | <0.01 |
| Overall response rate (%) | 32.0 | 25.0 | 0.10 |
| Overall response rate in evaluable population (%) | 37.0 | 26.0 | 0.01 |
Clinical studies have taken place for the treatment of non-small cell lung cancer and prostate cancer.[5] Patients treated for non-small cell lung cancer in phase II studies with 100 mg/m² docetaxel showed an overall response rate of 26.9% for previously untreated patients (n=160) and 17% for previously treated patients (n=88). Median survival time for previously untreated patients was nine months and for previously treated patients, eight months.[5]
The TAX 327 trial was a phase III study that showed significant survival benefit from docetaxel in androgen-independent metastatic prostate cancer.[18] Compared with mitoxantrone treatment, docetaxel treated patients showed a 12% overall response rate and mitoxantrone showed a 7% overall response rate. Another large advantage of docetaxel was increased quality of life. Docetaxel showed a 22% response and mitoxantrone had a 13% response. Used in conjunction with prednisone for pain management, docetaxel had a 35% response and Mitoxantrone had a 22% response. This trial leads docetaxel to be a preferred method of treatment to Mitoxantrone where possible.[18]
Specific outcomes and benefits of treatment
Treatment with docetaxel has the specific outcome of increasing survival time in patients with certain types of cancer.[1][2][4][5] While some clinical trials show median survival times to be increased by approximately only three months, the range of survival time is large.[5] Many patients survive beyond five years with treatment from docetaxel, however it is difficult to attribute these findings directly to treatment with docetaxel.[18] Improved median survival time and response indicates that docetaxel slows metastatic cancer progression and can lead to disease-free survival.[5][9][18] Conjunctive treatment of prednisone with docetaxel has been shown to lead to improved survival rate as well as improved quality of life and reduction of pain compared with treatments with mitoxantrone.[18] Docetaxel has been shown to improve survival as an adjuvant therapy with doxorubicin and cyclophosphamide for the treatment of node-positive breast cancer and so docetaxel has the benefit of aiding other treatments.[5]
Monitoring and combination with other drugs
Docetaxel is administered via a one-hour infusion every three weeks over ten or more cycles.[5] Treatment is given under supervision from an oncologist and takes place in a hospital, where vital signs are monitored during infusion. Strict monitoring of blood cell counts, liver function, serum electrolytes, serum creatinine, heart function and fluid retention is required to track the progression of tumour cells, response, adverse reactions and toxicity so that treatment can be modified or terminated if necessary.[5][8]
Premedication with corticosteroids is recommended before each administration of docetaxel to reduce fluid retention and hypersensitive reactions.[5] Oral dexamethasone is given before docetaxel treatment for prostate cancer. Docetaxel is typically used for the treatment of carcinoma on its own. Other medications will often be given to aid pain management and other symptoms. The treatment of breast cancer with doxorubicin and cyclophosphamide is enhanced by adjuvant treatment with docetaxel. Docetaxel is also used in combination with capecitabine, a DNA synthesis inhibitor.[5][19]
Side-effects/contraindications/drug interactions
Positive side-effects
As well as inhibiting mitosis, the presence of docetaxel has been found to lead to the phosphorylation of the oncoprotein bcl-2, which leads to apoptosis of cancer cells that had previously blocked the apoptotic inducing mechanism, leading to tumour regression.[1] Enhanced effects of radiation therapy when combined with docetaxel has been observed in mice.[1] Docetaxel has also been found to have greater cellular uptake and is retained longer intracellularly than paclitaxel allowing docetaxel treatment to be effective with a smaller dose, leading to fewer and less severe adverse effects.[17]
Adverse effects
Docetaxel is a chemotherapeutic agent and is a cytotoxic compound and so is effectively a biologically damaging drug.[1][13] As with all chemotherapy, adverse effects are common and many varying side-effects have been documented.[5][9] Because docetaxel is a cell cycle specific agent, it is cytotoxic to all dividing cells in the body.[20] This includes tumour cells as well as hair follicles, bone marrow and other germ cells. For this reason, common chemotherapy side effects such as alopecia occur.[20]
Haematological adverse effects include Neutropenia (95.5%), Anaemia (90.4%), Febrile neutropenia (11.0%) and Thrombocytopenia (8.0%).[5][9] Deaths due to toxicity accounted for 1.7% of the 2045 patients and incidence was increased (9.8%) in patients with elevated baseline liver function tests (liver dysfunction).[5][9]
Observations of severe side effects in the above 40 phase II and phase III studies were also recorded.
Many more side effects have been reported for conjunctive and adjuvant treatment with docetaxel as well as rare post-marketing events.[5]
Contraindications and patient factors
Docetaxel is contraindicated for use with patients with; a baseline neutrophil count less than 1.5x109 cells/L, a history of severe hypersensitivity reactions to docetaxel or polysorbate 80, severe liver impairment and pregnant or breast-feeding women.[5][9]
Side effects are experienced more frequently by patients of 65 years or older, but dosage is usually not decreased.[5][8] Renal failure is thought not to be a significant factor for docetaxel dosage adjustment.[8] Patients with hepatic insufficiency resulting in serum bilirubin greater than the upper limit of normal (ULN) should not be administered docetaxel, though this is not a stated contraindication. Dosage should be reduced by 20% in patients who suffer from; grade 3 or 4 diarrhoea following exposure to docetaxel, hepatotoxicity defined by liver enzymes at levels greater than five times the ULN, and grade 2 palmer-planter toxicity.[8]
Paediatric trials of docetaxel have been limited and so safety of use in patients under 16 years has not been established.[2][8]
Drug interactions
Drug interactions may be the result of altered pharmacokinetics or pharmacodynamics due to one of the drugs involved.[2] Cisplatin, dexamethasone, doxorubicin, etoposide and vinblastine are all potentially co-administered with docetaxel and did not modify docetaxel plasma binding in phase II studies.[11] Cisplatin is known to have a complex interaction with some CYPs and has in some events been shown to reduce docetaxel clearance by up to 25%.[2] Anticonvulsants induce some metabolic pathways relevant to docetaxel. CYP450 and CYP3A show increased expression in response to the use of anticonvulsants and the metabolism of docetaxel metabolite M4 is processed by these CYPs. A corresponding increase in clearance of M4 by 25% is observed in patients taking phenytoin and phenobarbital, common anticonvulsants.[2]
| Drug Interacting with Docetaxel | Adverse Effects from Interaction |
|---|---|
| Cisplatin | increased risk of delayed neuropathy |
| Cyclosporine, Dalfopristin, Erythromycin, Itraconazole, Ketoconazole, Quinupristin, Terfenadine, Troleandomycin | increased risk of docetaxel toxicity including some or all of; anaemia, leucopoenia, thrombocytopenia, fever, diarrhoea |
| Doxorubicin Hydrochloride | cholestatic jaundice and pseudomembranous colitis |
| Doxorubicin Hydrochloride Liposome | increased doxorubicin exposure |
| Vaccinations for; Bacillus of Calmette and Guerin, Measles, Mumps, Poliovirus, Rotavirus, Rubella, Smallpox, Typhoid, Varicella, Yellow Fever | increased risk of infection by live vaccine |
| Thalidomide | increased risk of venous thromboembolism |
Erythromycin, ketoconazole and cyclosporine are CYP3A4 inhibitors and therefore inhibit the metabolic pathway of docetaxel.[2] When used with anticonvulsants, which induce CYP3A4, an increased dose of docetaxel may be required.[2]
Pre-treatment with corticosteroids has been used to decrease hypersensitivity reactions and oedema in response to docetaxel and has shown no effect on the pharmacokinetics of docetaxel.[2] The efficacy of docetaxel was improved by treatment with oral capecitabine and after more than 27 months follow-up, the survival benefit has been confirmed.[1] Doxorubicin was combined with docetaxel in one study of 24 patients and resulted in an increased AUC of docetaxel by 50 to 70%, indicating doxorubicin may affect the disposition of docetaxel.[2] Etoposide has also been shown to decrease docetaxel clearance, thought patient numbers for this observation have been low.[2]
Prednisone given with docetaxel led to improved survival, quality of life and pain management in patients with hormone-refractory prostate cancer.[18]
Discovery, Regulation and Marketing
Taxotere was developed by Rhône-Poulenc Rorer (now Aventis) following from the discoveries of Pierre Potier at CNRS at Gif-sur-Yvette during his work on improvements to the production of Taxol.[21]
Docetaxel is currently protected by patents (U.S. patent 4814470, European patent no EP 253738, due to expire in 2010) which are owned by Sanofi-Aventis, and so is available only under the Taxotere® brand name internationally.[22]
Clinical Trials
MD Anderson Cancer Center: A phase I/II study of Docetaxel, 5-Fluorouracil and Oxaliplatin (D-FOX) in patients with untreated locally unresectable or metastatic adenocarcinoma of the stomach or gastroesophageal junction.
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 Lyseng-Williamson KA, Fenton C. Docetaxel: a review of its use in metastatic breast cancer. Drugs 2005;65(17):2513-31.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 2.20 2.21 2.22 2.23 2.24 2.25 2.26 2.27 2.28 2.29 Clarke SJ, Rivory LP. Clinical pharmacokinetics of docetaxel. Clin Pharmacokinet 1999;36(2):99-114
- ↑ 3.0 3.1 Anonymous. Oncology Tools: Approved Claims for microtubule inhibitors. US Food and Drug Administration. http://www.accessdata.fda.gov/scripts/cder/onctools/classlist.cfm?Class=microtubule%20inhibitor (17 Sep 2006). Last modified 22 Jun 1998.
- ↑ 4.0 4.1 4.2 Anonymous. Taxotere.com for Healthcare Professionals: About. Sanofi-aventis U.S. LLC. http://www.taxotere.com/professional/about/index.do (17 Sep 2006). Last modified Jul 2005.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 5.16 5.17 5.18 5.19 5.20 5.21 5.22 5.23 5.24 5.25 5.26 5.27 5.28 5.29 5.30 5.31 5.32 5.33 Anonymous. Taxotere Docetaxel concentrate for infusion. Medsafe. http://www.medsafe.govt.nz/profs/Datasheet/t/taxotereinf.htm (25 Sep 2006). Last modified 6 Feb 2006.
- ↑ 6.0 6.1 Snyder JP, Nettles JH, Cornett B, Downing KH, Nogales E. The binding conformation of Taxol in b-tubulin: A model based on electron crystallographic density. PNAS. 2001;98(9):5312-16.
- ↑ Rang HP, Dale MM, Ritter JM, Moore PK. Pharmacology. 5th ed. London: Churchill Livingstone; 2003. p. 100-1
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 Anonymous. Drugdex Evaluations: Docetaxel. Thomson MICROMEDEX. http://www.library.auckland.ac.nz/databases/learn_database/public.asp?record=micromedex#Drugdex (26 Sep 2006). Last modified 2006.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 Anonymous. Taxotere.com for Healthcare Professionals: Efficacy and Safety. Sanofi-aventis U.S. LLC. http://www.taxotere.com/professional/about/efficacy_safety.do (24 Sep 2006). Last modified Jul 2005.
- ↑ Anonymous. New Zealand Pharmaceutical Schedule. Wellington: PHARMAC; 2006. p. 133.
- ↑ 11.0 11.1 11.2 11.3
- ↑ 12.0 12.1 Baker SD, Zhao M, Lee CKK, Verweij J, Zabelina Y, Brahmer JR, et al. Comparative pharmacokinetics of weekly and every-three-weeks docetaxel. Clin Cancer Res. 2004;10(6):1976-83.
- ↑ 13.0 13.1 Anonymous. Taxotere.com for Healthcare Professionals: Pharmacokinetics. Sanofi-aventis U.S. LLC. http://www.taxotere.com/professional/about/pharmacokinetics.do (23 Sep 2006). Last modified Jul 2005.
- ↑ 14.0 14.1 Guitton J, Cohen S, Tranchand B, Vignal B, Droz JP, Guillaumont M, et al. Quantification of docetaxel and its main metabolites in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2005;19(17):2419-26.
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 15.6 Yvon AC, Wadsworth P, Jordan MA. Taxol Suppresses Dynamics of Individual Microtubules in Living Human Tumor Cells. The American Society for Cell Biology. 1999;10:947-959.
- ↑ Anonymous. Docetaxel: Clinical Pharmacology. RxList. http://www.rxlist.com/cgi/generic3/docetaxel_cp.htm (24 Sep 2006). Last modified 29 Jun 2006.
- ↑ 17.0 17.1 17.2 17.3 Eisenhauer EA, Vermorken JB. The taxoids: Comparative clinical pharmacology and therapeutic potential. Drugs. 1998;55(1):5-30.
- ↑ 18.0 18.1 18.2 18.3 18.4 18.5 Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502-12.
- ↑ Goyle S, Maraveyas A. Chemotherapy for colorectal cancer. Dig Surg. 2005;22(6):401-14.
- ↑ 20.0 20.1 Rang HP, Dale MM, Ritter JM, Moore PK. Pharmacology. 5th ed. London: Churchill Livingstone; 2003. p. 694-8.
- ↑ http://www.cnrs.fr/cw/en/pres/compress/mistpotier.html Pierre Potier, chemist, 1998 CNRS Gold Medalist
- ↑ Vogel CL, Bellet RE, inventors; Aventis Pharma S.A., assignee. Use of docetaxel for treating cancers. United States patent US20016333348. 2001 Dec 25.
External links
- Taxotere (manufacturer's prescribing information)
- Taxotere (manufacturer's website)
- Docetaxel (patient information)
- Pages with script errors
- Pages with reference errors
- Pages with broken file links
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Chemotherapeutic agents
- Alkaloids