Diabetes mellitus
| Diabetes mellitus | ||
 | ||
|---|---|---|
| United Nations blue circle symbol for diabetes.[1] | ||
| ICD-10 | E10–E14 | |
| ICD-9 | 250 | |
| MeSH | C18.452.394.750 | |
|
Diabetes mellitus Main page |
|
Patient Information |
|---|
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-In-Chief: Priyamvada Singh, M.B.B.S. [2]; Cafer Zorkun, M.D., Ph.D. [3]
Overview
Historical Perspective
Pathophysiology
Risk Factors
Screening
Epidemiology and demographics
Natural history, Complications, and Prognosis
Classification
Causes
Differentiating Type page name here from other Disorders
Diagnosis
History and Symptoms | Physical Examination | Laboratory Tests | Electrocardiogram | Chest X Ray | MRI | CT | Echocardiography | Other Imaging Findings | Other Diagnostic Studies
Treatment
Medical: Medical Therapy
Surgical: Surgery
Primary Prevention | Secondary Prevention | Cost-Effectiveness of Therapy | Future or Investigational Therapies
Prevention
Type 1 diabetes risk is known to depend upon a genetic predisposition based on HLA types (particularly types DR3 and DR4), an unknown environmental trigger (suspected to be an infection, although none has proven definitive in all cases), and an uncontrolled autoimmune response that attacks the insulin producing beta cells.[2] Some research has suggested that breastfeeding decreased the risk; [3][4] various other nutritional risk factors are being studied, but no firm evidence has been found. [5] Giving children 2000 IU of Vitamin D during their first year of life is associated with reduced risk of type 1 diabetes. [6]
Type 2 diabetes risk can be reduced in many cases by making changes in diet and increasing physical activity.[7][8] The American Diabetes Association (ADA) recommends maintaining a healthy weight, getting at least 2½ hours of exercise per week (a brisk sustained walk appears sufficient), having a modest fat intake, and eating a good amount of fiber and whole grains. The ADA does not recommend alcohol consumption as a preventative, but it is interesting to note that moderate alcohol intake may reduce the risk (though heavy consumption clearly increases damage to body systems significantly). There is inadequate evidence that eating foods of low glycemic index is clinically helpful.[9]
Some studies have shown delayed progression to diabetes in predisposed patients through prophylactic use of metformin,[8] rosiglitazone,[10] or valsartan.[11] In patients on hydroxychloroquine for rheumatoid arthritis, incidence of diabetes was reduced by 77%.[12] Breastfeeding might also be associated with the prevention of type 2 of the disease in mothers.[13]
It is possible that adequate copper could help prevent insulin dependant diabetes since it does so for ATZ poisoned mice [14] and copper in drinking water has somewhat of a protective affect [15]. It could be that copper produces its effects through super oxidase dismutase (SOD) because metaloporpherin based superoxide dismutase can prevent or delay the onset of the autoimmune cascade in diabetes, using mice [16]. However, there are sufficient differences in human and animal models to indicate this is only a theory at the present time.
Children with antibodies treated with vitamin B-3 (niacin) had less than half the onset of diabetes incidence in a 7-year time span as the general population and even lower incidence relative to those with antibodies as above, but no vitamin B-3 [17]
Treatment and Management
Diabetes mellitus is currently a chronic disease, without a cure, and medical emphasis must necessarily be on managing/avoiding possible short-term as well as long-term diabetes-related problems. There is an exceptionally important role for patient education, dietetic support, sensible exercise, self glucose monitoring, with the goal of keeping both short-term blood glucose levels, and long term levels as well, within acceptable bounds. Careful control is needed to reduce the risk of long term complications. This is theoretically achievable with combinations of diet, exercise and weight loss (type 2), various oral diabetic drugs (type 2 only), and insulin use (type 1 and increasingly for type 2 not responding to oral medications). In addition, given the associated higher risks of cardiovascular disease, lifestyle modifications should be undertaken to control blood pressure[18] and cholesterol by exercising more, smoking cessation, consuming an appropriate diet, wearing diabetic socks, and if necessary, taking any of several drugs to reduce pressure. Many Type 1 treatments include the combination use of regular or NPH insulin, and/or synthetic insulin analogs such as Humalog, Novolog or Apidra; the combination of Lantus/Levemir and Humalog, Novolog or Apidra. Another Type 1 treatment option is the use of the insulin pump with the some of most popular pump brands being: Cozmo, Animas, Medtronic Minimed, and Omnipod.
In countries using a general practitioner system, such as the United Kingdom, care may take place mainly outside hospitals, with hospital-based specialist care used only in case of complications, difficult blood sugar control, or research projects. In other circumstances, general practitioners and specialists share care of a patient in a team approach. Optometrists, podiatrists/chiropodists, dietitians, physiotherapists, clinical nurse specialists (eg, Certified Diabetes Educators and DSNs (Diabetic Specialist Nurse)), or nurse practitioners may jointly provide multidisciplinary expertise. In countries where patients must provide their own health care, the impact of out-of-pocket costs of diabetic care can be high. In addition to the medications and supplies needed, patients are often advised to receive regular consultation from a physician (e.g., at least every three to six months).
Cure
Cures for type 1 diabetes
There is no practical cure now for type 1 diabetes. The fact that type 1 diabetes is due to the failure of one of the cell types of a single organ with a relatively simple function (i.e. the failure of the islets of Langerhans) has led to the study of several possible schemes to cure this form diabetes mostly by replacing the pancreas or just the beta cells.[19] Only those type 1 diabetics who have received either a pancreas or a kidney-pancreas transplant (when they have developed diabetic nephropathy) and become insulin-independent may now be considered "cured" from their diabetes. A simultaneous pancreas-kidney transplant is a promising solution, showing similar or improved survival rates over a kidney transplant alone. [20]Still, they generally remain on long-term immunosuppressive drugs and there is a possibility that the immune system will mount a host versus graft response against the transplanted organ.[19]
Transplants of exogenous beta cells have been performed experimentally in both mice and humans, but this measure is not yet practical in regular clinical practice. Thus far, like any such transplant, it has provoked an immune reaction and long-term immunosuppressive drugs will be needed to protect the transplanted tissue.[21] An alternative technique has been proposed to place transplanted beta cells in a semi-permeable container, isolating and protecting them from the immune system. Stem cell research has also been suggested as a potential avenue for a cure since it may permit regrowth of Islet cells which are genetically part of the treated individual, thus perhaps eliminating the need for immuno-suppressants.[19] A 2007 trial of 15 newly diagnosed patients with type 1 diabetes treated with stem cells raised from their own bone marrow after immune suppression showed that the majority did not require any insulin treatment for prolonged periods of time.[22]
Microscopic or nanotechnological approaches are under investigation as well, in one proposed case with implanted stores of insulin metered out by a rapid response valve sensitive to blood glucose levels. At least two approaches have been demonstrated in vitro. These are, in some sense, closed-loop insulin pumps.
Cures for type 2 diabetes
Type 2 diabetes can be cured by one type of gastric bypass surgery in 80-100% of severely obese patients. The effect is not due to weight loss because it usually occurs within days of surgery, which is before significant weight loss occurs. The pattern of secretion of gastrointestinal hormones is changed by the bypass and removal of the duodenum and proximal jejunum, which together form the upper (proximal) part of the small intestine.[23] One hypothesis is that the proximal small intestine is dysfunctional in type 2 diabetes; its removal eliminates the source of an unknown hormone that contributes to insulin resistance.[24] This surgery has been widely performed on morbidly obese patients and has the benefit of reducing the death rate from all causes by up to 40%.[25] A small number of normal to moderately obese patients with type 2 diabetes have successfully undergone similar operations.[26][27]
Prognosis
Patient education, understanding, and participation is vital since the complications of diabetes are far less common and less severe in people who have well-controlled blood sugar levels.[28][29] Wider health issues accelerate the deleterious effects of diabetes. These include smoking, elevated cholesterol levels, obesity, high blood pressure, and lack of regular exercise. According to a study, women with high blood pressure have a threefold risk of developing diabetes.
Anecdotal evidence suggests that some of those with type 2 diabetes who exercise regularly, lose weight, and eat healthy diets may be able to keep some of disease or some of the effects of the disease in 'remission.' Certainly these tips can help prevent people predisposed to type 2 diabetes and those at pre-diabetic stages from actually developing the disorder as it helps restore insulin sensitivity. However patients should talk to their doctors about this for real expectations before undertaking it (esp. to avoid hypoglycemia or other complications); few people actually seem to go into total 'remission,' but some may find they need less of their insulin medications since the body tends to have lower insulin requirements during and shortly following exercise. Regardless of whether it works that way or not for an individual, there are certainly other benefits to this healthy lifestyle for both diabetics and nondiabetics.
The way diabetes is managed changes with age. Insulin production decreases due to age-related impairment of pancreatic beta cells. Additionally, insulin resistance increases due to the loss of lean tissue and the accumulation of fat, particularly intra-abdominal fat, and the decreased tissue sensitivity to insulin. Glucose tolerance progressively declines with age, leading to a high prevalence of type 2 diabetes and postchallenge hyperglycemia in the older population.[30] Age-related glucose intolerance in humans is often accompanied by insulin resistance, but circulating insulin levels are similar to those of younger people. [31] Treatment goals for older patients with diabetes vary with the individual, and take into account health status, as well as life expectancy, level of dependence, and willingness to adhere to a treatment regimen.[32]
Acute complications
- Diabetic ketoacidosis
Diabetic ketoacidosis (DKA) is an acute and dangerous complication that is always a medical emergency. Lack of insulin causes the liver to turn fat into ketone bodies, a fuel mainly used by the brain. Elevated levels of ketone bodies in the blood decrease the blood's pH, leading to most of the symptoms of DKA. On presentation at hospital, the patient in DKA is typically dehydrated and is breathing rapidly and deeply. Abdominal pain is common and may be severe. The level of consciousness is typically normal until late in the process, when lethargy may progress to coma. Ketoacidosis can become severe enough to cause hypotension, shock, and death. Analysis of the urine reveals significant levels of ketone bodies present (which spill over from the blood when the kidneys filter blood). Prompt proper treatment usually results in full recovery, though death can result from inadequate or delayed treatment, or from complications. Ketoacidosis is much more common in type 1 diabetes than type 2.
- Nonketotic hyperosmolar coma
The hyperosmolar nonketotic state (HNS) is an acute complication with many symptoms in common with DKA, but an entirely different cause and different treatment. In a person with very high blood glucose levels (usually considered to be above 300 mg/dl (16 mmol/l)), water is drawn out of cells into the blood by osmosis and the kidneys dump glucose into the urine. This results in loss of water and an increase in blood osmolality. If fluid is not replaced (by mouth or intravenously), the osmotic effect of high glucose levels combined with the loss of water will eventually lead to dehydration. The body's cells become progressively dehydrated as water is taken from them and excreted. Electrolyte imbalances are also common and dangerous. As with DKA, urgent medical treatment is necessary, especially volume replacement. Lethargy may ultimately progress to a coma, which is more common in type 2 diabetes than type 1.
- Hypoglycemia
Hypoglycemia, or abnormally low blood glucose, is a complication of several diabetes treatments. It may develop if the glucose intake does not cover the treatment. The patient may become agitated, sweaty, and have many symptoms of sympathetic activation of the autonomic nervous system resulting in feelings similar to dread and immobilized panic. Consciousness can be altered or even lost in extreme cases, leading to coma, seizures, or even brain damage and death. In patients with diabetes, this may be caused by several factors, such as too much or incorrectly timed insulin, too much or incorrectly timed exercise (exercise decreases insulin requirements) or not enough food (specifically glucose-producing carbohydrates), but this is an over-simplification.
It is more accurate to note that iatogenic (caused by medical treatment) hypoglycemia is typically the result of the interplay of absolute (or relative) insulin excess and compromised glucose counterregulation in type 1 and advanced type 2 diabetes. Decrements in insulin, increments in glucagon, and, absent the latter, increments in epinephrine stand high in the hierarchy of redundant glucose counterregulatory factors that normally prevent or rapidly correct hypoglycemia. In insulin-deficient diabetes (exogenous) insulin levels do not decrease as glucose levels fall, and the combination of deficient glucagon and epinephrine responses causes defective glucose counterregulation.
Furthermore, reduced sympathoadrenal responses can cause hypoglycemia unawareness. The concept of hypoglycemia-associated autonomic failure (HAAF) in diabetes posits that recent incidents of hypoglycemia causes both defective glucose counterregulation and hypoglycemia unawareness. By shifting glycemic thresholds for the sympathoadrenal (including epinephrine) and the resulting neurogenic responses to lower plasma glucose concentrations, antecedent hypoglycemia leads to a vicious cycle of recurrent hypoglycemia and further impairment of glucose counterregulation. In many cases (but not all), short-term avoidance of hypoglycemia reverses hypoglycemia unawareness in most affected patients, although this is easier in theory than it is in practice.
In most cases, hypoglycemia is treated with sugary drinks or food. In severe cases, an injection of glucagon (a hormone with the opposite effects of insulin) or an intravenous infusion of dextrose is used for treatment, but usually only if the person is unconscious. In hospitals, intravenous dextrose is often used.
Chronic complications
- Vascular disease
Chronic elevation of blood glucose level leads to damage of blood vessels (angiopathy). The endothelial cells lining the blood vessels take in more glucose than normal, since they don't depend on insulin. They then form more surface glycoproteins than normal, and cause the basement membrane to grow thicker and weaker. In diabetes, the resulting problems are grouped under "microvascular disease" (due to damage to small blood vessels) and "macrovascular disease" (due to damage to the arteries).
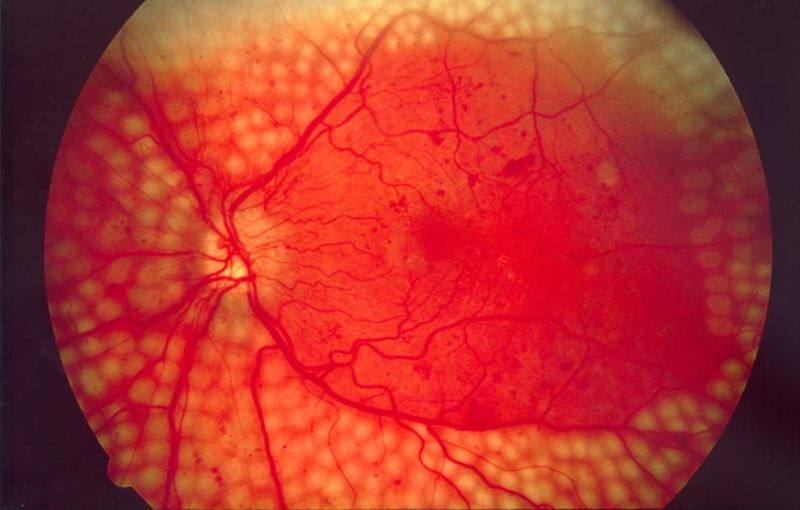
The damage to small blood vessels leads to a microangiopathy, which can cause one or more of the following:
- Diabetic retinopathy, growth of friable and poor-quality new blood vessels in the retina as well as macular edema (swelling of the macula), which can lead to severe vision loss or blindness. Retinal damage (from microangiopathy) makes it the most common cause of blindness among non-elderly adults in the US.
- Diabetic neuropathy, abnormal and decreased sensation, usually in a 'glove and stocking' distribution starting with the feet but potentially in other nerves, later often fingers and hands. When combined with damaged blood vessels this can lead to diabetic foot (see below). Other forms of diabetic neuropathy may present as mononeuritis or autonomic neuropathy. Diabetic amyotrophy is muscle weakness due to neuropathy.
- Diabetic nephropathy, damage to the kidney which can lead to chronic renal failure, eventually requiring dialysis. Diabetes mellitus is the most common cause of adult kidney failure worldwide in the developed world.
Macrovascular disease leads to cardiovascular disease, to which accelerated atherosclerosis is a contributor:
- Coronary artery disease, leading to angina or myocardial infarction ("heart attack")
- Stroke (mainly the ischemic type)
- Peripheral vascular disease, which contributes to intermittent claudication (exertion-related leg and foot pain) as well as diabetic foot.
- Diabetic myonecrosis ('muscle wasting')
Diabetic foot, often due to a combination of neuropathy and arterial disease, may cause skin ulcer and infection and, in serious cases, necrosis and gangrene. It is why diabetics are prone to leg and foot infections and why it takes longer for them to heal from leg and foot wounds. It is the most common cause of adult amputation, usually of toes and or feet, in the developed world.
Carotid artery stenosis does not occur more often in diabetes, and there appears to be a lower prevalence of abdominal aortic aneurysm. However, diabetes does cause higher morbidity, mortality and operative risks with these conditions.[33]
Social issues
The 1989 Declaration of St Vincent was the result of international efforts to improve the care accorded to those with diabetes. Doing so is important both in terms of quality of life and life expectancy but also economically - expenses to diabetes have been shown to be a major drain on health- and productivity-related resources for healthcare systems and governments.
Several countries established more and less successful national diabetes programmes to improve treatment of the disease.[34]
A study shows that diabetic patients with neuropathic symptoms such as numbness or tingling in feet or hands are twice more likely to be unemployed than those without the symptoms.[35]
See also
References
- ↑ "IDF Chooses Blue Circle to Represent UN Resolution Campaign". Unite for Diabetes. 17 March 2006.
- ↑ Daneman D (2006). "Type 1 diabetes". Lancet. 367 (9513): 847–58. PMID 16530579.
- ↑ Borch-Johnsen K, Joner G, Mandrup-Poulsen T, Christy M, Zachau-Christiansen B, Kastrup K, Nerup J (1984). "Relation between breast-feeding and incidence rates of insulin-dependent diabetes mellitus. A hypothesis". Lancet. 2 (8411): 1083–6. PMID 6150150.
- ↑ Naim Shehadeh, Raanan Shamir, Moshe Berant, Amos Etzioni (2001). "Insulin in human milk and the prevention of type 1 diabetes". Pediatric Diabetes. 2 (4): 175–177.
- ↑ Virtanen S, Knip M (2003). "Nutritional risk predictors of beta cell autoimmunity and type 1 diabetes at a young age". Am J Clin Nutr. 78 (6): 1053–67. PMID 14668264.
- ↑ Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM (2001). "Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study". Lancet. PMID 11705562.
- ↑ Lindström J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson J, Hemiö K, Hämäläinen H, Härkönen P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Mannelin M, Paturi M, Sundvall J, Valle T, Uusitupa M, Tuomilehto J (2006). "Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study". Lancet. 368 (9548): 1673–9. PMID 17098085.
- ↑ 8.0 8.1 Knowler W, Barrett-Connor E, Fowler S, Hamman R, Lachin J, Walker E, Nathan D (2002). "Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin". N Engl J Med. 346 (6): 393–403. PMID 11832527.
- ↑ Bantle JP, Wylie-Rosett J, Albright AL; et al. (2006). "Nutrition recommendations and interventions for diabetes--2006: a position statement of the American Diabetes Association". Diabetes Care. 29 (9): 2140–57. doi:10.2337/dc06-9914. PMID 16936169.
- ↑ Gerstein H, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman R (2006). "Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial". Lancet. 368 (9541): 1096–105. PMID 16997664.
- ↑ Kjeldsen SE, Julius S, Mancia G, McInnes GT, Hua T, Weber MA, Coca A, Ekman S, Girerd X, Jamerson K, Larochelle P, Macdonald TM, Schmieder RE, Schork MA, Stolt P, Viskoper R, Widimsky J, Zanchetti A; for the VALUE Trial Investigators (2006). "Effects of valsartan compared to amlodipine on preventing type 2 diabetes in high-risk hypertensive patients: the VALUE trial". J Hypertens. 24 (7): 1405–1412. PMID 16794491.
- ↑ Wasko MC, Hubert HB, Lingala VB; et al. (2007). "Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis". JAMA. 298 (2): 187–93. doi:10.1001/jama.298.2.187. PMID 17622600.
- ↑ Stuebe AM, Rich-Edwards JW, Willett WC, Manson JE, Michels KB (2005). "Duration of lactation and incidence of type 2 diabetes". JAMA. 294 (20): 2601&ndash, 10. PMID 16304074.
- ↑ Sitasawad S, Deshpande M, Katdare M, Tirth S, Parab P. (2001) Beneficial effect of supplementation with copper sulfate on STZ diabetic mice (IDDM). Diabetes Res Clin Pract May;52(2):77-84.
- ↑ Zhao HX, Mold MD, Stenhouse EA, Bird SC, Wright DE, Demaine AG, Millward BA. (2001) Drinking water composition and childhood-onset Type 1 diabetes mellitus in Devon and Cornwall, England. Diabetic Med 18(9) p709-717.This article modified in November 2007.
- ↑ Haskins K, et al (2003) "Immunology of diabetes II. Pathogenesis from mouse to man." Ann. N.Y. Academy of Sciences 1005: 43. doi. 10.1196/annals.1288.006.
- ↑ Elliott RB Pilcher CC Fergusson DM Stewart AW 1996 A population based strategy to prevent insulin-dependent diabetes using nicotinamide. J Pediatr Endocrinol Metab. 1996 Sep-Oct;9(5):501-9.
- ↑ Adler, A.I. (2000). "Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study". BMJ. 321 (7258): 412–419. ISSN 0959-8146. PMID 10938049. Unknown parameter
|coauthors=ignored (help) - ↑ 19.0 19.1 19.2 Vinik AI, Fishwick DT, Pittenger G (2004). "Advances in diabetes for the millennium: toward a cure for diabetes". MedGenMed : Medscape general medicine. 6 (3 Suppl): 12. PMID 15647717.
- ↑ Stratta RJ, Alloway RR. (1998). "Pancreas transplantation for diabetes mellitus: a guide to recipient selection and optimum immunosuppression". BioDrugs. 10 (5): 347–357. PMID 18020607.
- ↑ Shapiro AM, Ricordi C, Hering BJ; et al. (2006). "International trial of the Edmonton protocol for islet transplantation". N. Engl. J. Med. 355 (13): 1318–30. doi:10.1056/NEJMoa061267. PMID 17005949.
- ↑ Voltarelli, JC (2007). "Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus". JAMA. 297 (14): 1568–76. PMID 17426276. Unknown parameter
|coauthors=ignored (help) - ↑ Rubino, F (2002). "Potential of surgery for curing type 2 diabetes mellitus". Ann. Surg. 236 (5): 554–9. ISSN 0003-4932. PMID 12409659. Unknown parameter
|coauthors=ignored (help) - ↑ Rubino, F (2006). "The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes". Ann. Surg. 244 (5): 741–9. PMID 17060767. Unknown parameter
|coauthors=ignored (help) - ↑ Adams, TD (2007). "Long-term mortality after gastric bypass surgery". N. Engl. J. Med. 357 (8): 753–61. doi:10.1056/NEJMoa066603. ISSN 0028-4793. PMID 17715409. Unknown parameter
|coauthors=ignored (help) - ↑ Cohen, RV (2007). "Duodenal-jejunal bypass for the treatment of type 2 diabetes in patients with body mass index of 22-34 kg/m2: a report of 2 cases". Surg Obes Relat Dis. 3 (2): 195–7. doi:10.1016/j.soard.2007.01.009. PMID 17386401. Unknown parameter
|coauthors=ignored (help) - ↑ Vasonconcelos, Alberto (2007-09-01). "Could type 2 diabetes be reversed using surgery?". New Scientist (2619): 11–13. Retrieved 2007-09-26. Check date values in:
|date=(help) - ↑ Nathan, D.M. (2005). "Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes". N. Engl. J. Med. 353 (25): 2643–53. doi:10.1056/NEJMoa052187. PMID 16371630. Unknown parameter
|coauthors=ignored (help) - ↑ The Diabetes Control and Complications Trial Research Group (1995). "The effect of intensive diabetes therapy on the development and progression of neuropathy". Annals of Internal Medicine. 122 (8): 561–568. ISSN 0003-4819. PMID 7887548.
- ↑
- ↑ Annette M. Chang and Jeffrey B. Halter (2003). "Aging and insulin secretion". AJP - Endocrinology and Metabolism. Retrieved 2007-05-14.
- ↑ "Diabetes and Aging". Diabetes Dateline. National Institute of Diabetes and Digestive and Kidney Diseases. 2002. Retrieved 2007-05-14.
- ↑ Weiss J, Sumpio B (2006). "Review of prevalence and outcome of vascular disease in patients with diabetes mellitus". Eur J Vasc Endovasc Surg. 31 (2): 143–50. PMID 16203161.
- ↑ Dubois, HFW and Bankauskaite, V (2005). "Type 2 diabetes programmes in Europe" (PDF). Euro Observer. 7 (2): 5&ndash, 6.
- ↑ Stewart WF, Ricci JA, Chee E, Hirsch AG, Brandenburg NA (2007). "Lost productive time and costs due to diabetes and diabetic neuropathic pain in the US workforce". J. Occup. Environ. Med. 49 (6): 672–9. doi:10.1097/JOM.0b013e318065b83a. PMID 17563611.
Data from the Report of the Expert Committee on the diagnosis and classification of diabetes mellitus
External links
- American Diabetes Association
- Diabetes
- Diabetes Australia-NSW
- Canadian Diabetes Association
- Diet, Nutrition and the prevention of chronic diseases (including diabetes) by a Joint WHO/FAO Expert consultation (2003)
- Centers for Disease Control Diabetes Section
- Diabetes UK
- Diabetes Health Institute
- Diabetes Institute for Immunology and Transplantion
- The Immunology of Diabetes Society
- International Diabetes Federation
- Juvenile Diabetes Research Foundation
- MedlinePlus Diabetes from the U.S. National Library of Medicine
- National Diabetes Education Program
- National Diabetes Information Clearinghouse
- Primary Care Diabetes Europe
- World Health Organization fact sheet on diabetes
- World Health Organization—The Diabetes Programme
- Diabetic Medical ID Tag
Template:SIB af:Diabetes mellitus ar:مرض السكري ast:Diabetes zh-min-nan:Thn̂g-jiō-pēⁿ bs:Diabetes mellitus bg:Диабет ca:Diabetis mellitus cs:Diabetes mellitus cy:Clefyd y siwgr da:Sukkersyge de:Diabetes mellitus et:Suhkurtõbi el:Διαβήτης eo:Diabeto eu:Diabete fa:مرض قند gl:Diabetes mellitus ko:당뇨병 hi:मधुमेह hr:Diabetes mellitus id:Diabetes mellitus is:Sykursýki it:Diabete mellito he:סוכרת pam:Diabetes mellitus ka:დიაბეტი lb:Diabetes mellitus hu:Cukorbetegség ml:പ്രമേഹം ms:Penyakit kencing manis nl:Diabetes mellitus ne:मधुमेह new:मधुमेह no:Diabetes mellitus nn:Diabetes mellitus om:Diabetes qu:Misk'i unquy sq:Diabetes mellitus simple:Diabetes mellitus sk:Cukrovka sl:Sladkorna bolezen sr:Шећерна болест Template:Link FA sh:Dijabetes fi:Diabetes sv:Diabetes ta:நீரிழிவு நோய் te:మధుమేహం th:เบาหวาน uk:Цукровий діабет yi:דיעביטיס