Congenital adrenal hyperplasia
This page contains general information about Congenital adrenal hyperplasia. For more information on specific types, please visit the pages on 21-hydroxylase deficiency, 17 alpha-hydroxylase deficiency, 11β-hydroxylase deficiency, 3 beta-hydroxysteroid dehydrogenase deficiency, cytochrome P450-oxidoreductase (POR) deficiency (ORD), congenital lipoid adrenal hyperplasia.
| Adrenal insufficiency | |
 | |
|---|---|
| Adrenal gland |
|
Congenital adrenal hyperplasia main page |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mehrian Jafarizade, M.D [2]
Synonyms and keywords: Congenital adrenal hyperplasia; CAH; Adrenal hyperplasia.
Overview
Congenital adrenal hyperplasia consists of several disorders resulting from defective enzymes and proteins involved in steroid and cortisol synthesis pathways. Defects in steroid biosynthesis are caused by several genetic mutations and may lead to delayed puberty, precocious puberty or ambiguous genitalia in specific disorders. The decrease in cortisol level leads to the release of the inhibitory feedback on corticotropin (ACTH) production. The high ACTH level causes increase cortisol precursors and overproduction of other hormones. The most common cause of congenital adrenal hyperplasia is a 21-hydroxylase deficiency, which accounts for more than 95% of cases. Other causes include 17 alpha-hydroxylase deficiency, 11β-hydroxylase deficiency, 3 beta-hydroxysteroid dehydrogenase deficiency, Cytochrome P450-oxidoreductase (POR) deficiency (ORD), and congenital lipoid adrenal hyperplasia.
Classification
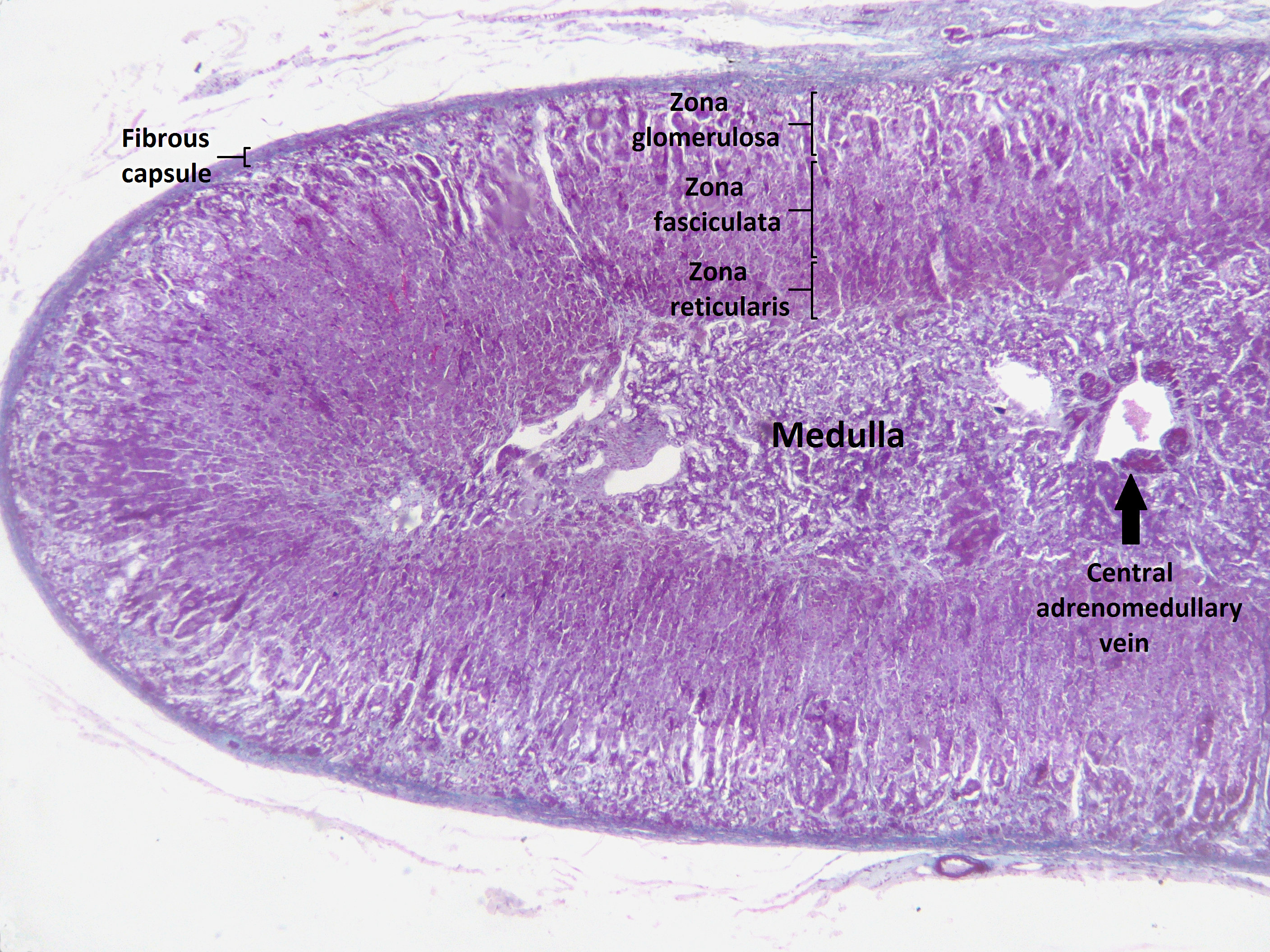
Congenital adrenal hyperplasia (CAH) is classified into seven types based on the genetic causes that lead to hyperplasia and hormonal imbalance. CAH is hyperplasia of different layer of Adrenal cortex. Within the adrenal cortex, there are three layers named zones; each of them has a distinct cell type and secrete specific hormones.
Adrenal cortex zones based on the hormonal synthesis ability and location:
- Zona glumerulosa:
- The outer layer of adrenal cortex.
- Laying directly under the adrenal capsule.
- Secretion: Aldosterone synthesis.
- Zona fasciculate:
- The middle part of adrenal cortex
- Laying under zona glumerulosa
- Secretion: Cortisol synthesis
- Zona reticularis:
- The inner part of adrenal cortex
- Laying between zona fasciculate and adrenal medulla
- Secretion: Androgen synthesis
Impairment of each pathway and enzyme may lead to a specific subtype of congenital adrenal hyperplasia include:
- 21-hydroxylase deficiency
- 17 alpha-hydroxylase deficiency
- 11β-hydroxylase deficiency
- 3 beta-hydroxysteroid dehydrogenase deficiency
- Cytochrome P450-oxidoreductase (POR) deficiency (ORD)
- Congenital lipoid adrenal hyperplasia
Adrenal steroid synthesis pathways in adrenal cortex and related enzymes
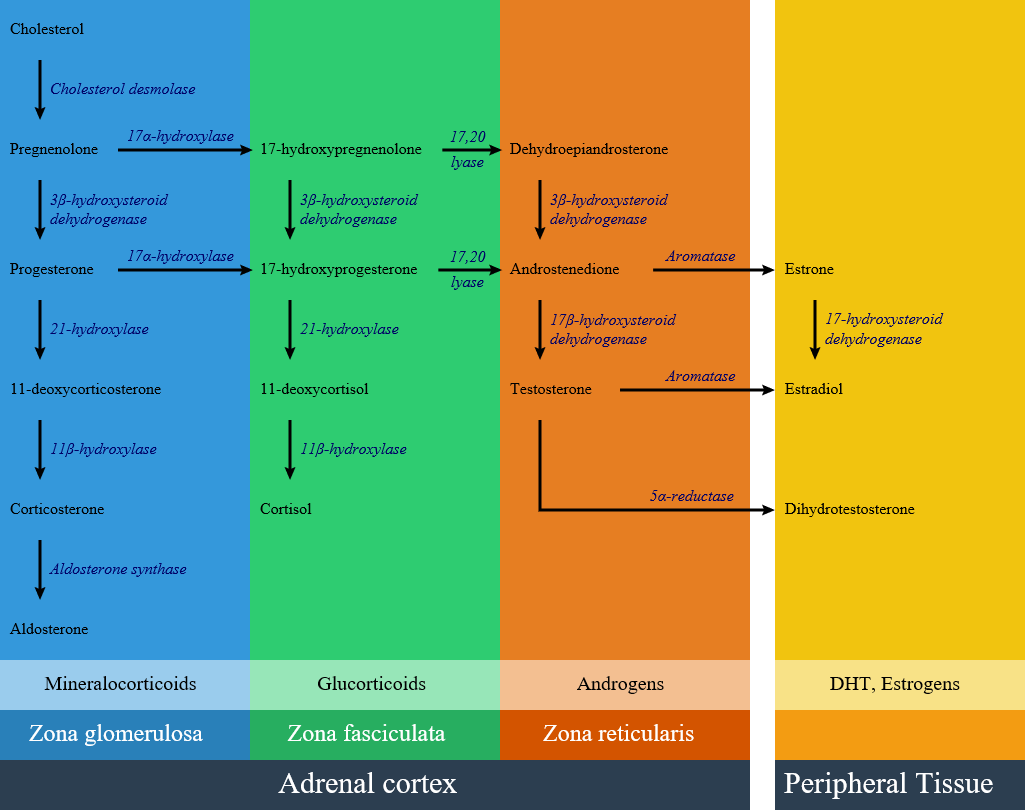
Summary and important Characteristics of the different congenital adrenal hyperplasia subtypes: [2][3][4]
| Disease | History and symptoms | Laboratory findings | Defective gene | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Blood pressure | Genitalia | Cortisol | Aldosterone | Androgens | Estrogens | Increased hormone precursors | Potassium levels | |||
| 21-hydroxylase deficiency | Classic type, salt-wasting | ↓ |
|
↓ | ↓↓ | ↑ | Relatively low | ↑ | ||
| Classic type, non-salt-wasting | Normal |
|
↓ | ↓ | ↑ | Relatively low | Normal | CYP21A1 and CYP21A2 gene | ||
| Non-classic type | Normal |
|
Normal | Normal | ↑ | Relatively low |
|
Normal | ||
| 17-α hydroxylase deficiency | ↑ |
|
Normal corticosterone
Normal cortisol |
↑ | ↓ | ↓ | ↓ | CYP17A1 | ||
| 11-β hydroxylase deficiency | ↑ |
|
↓ | ↑ | ↑ | Relatively low |
|
↓ | CYP11B1 | |
| 3 beta-hydroxysteroid dehydrogenase deficiency | ↓ |
|
↓ | ↓ | Male:↓
Female:↑ |
↓ | ↑ | HSD3B2 | ||
| Cytochrome P450-oxidoreductase (POR) deficiency (ORD) | Normal |
|
↓ | Normal | ↓ | ↓ | Normal | Mutations in the flavoprotein co-factor of the enzymes CYP17A1, CYP21A2, and CYP19A1 (aromatase). | ||
| Congenital lipoid adrenal hyperplasia | ↓ |
|
↓ | ↓ | ↓ | ↓ | None of precursors increased | ↓ | Gene mutations on chromosome 8, codes for a protein called steroid acute regulatory protein (StAR) | |
Differential Diagnosis
Congenital adrenal hyperplasia can present with different symptoms such as:
- Ambiguous genitalia
- Virilization and hirsutism in female
- Primary amenorrhea
- Low reninemic hypertension
Differential diagnosis for each of these symptoms are described in below tables.
Congenital adrenal hyperplasia must be differentiated from diseases that cause ambiguous genitalia:[5][6]
| Disease | Steroid status | Important clinical findings | |
|---|---|---|---|
| Increased | Decreased | ||
| Classic type of 21-hydroxylase deficiency |
|
| |
| 11-β hydroxylase deficiency |
|
| |
| 17-α hydroxylase deficiency |
| ||
| 3 beta-hydroxysteroid dehydrogenase deficiency |
| ||
| Gestational hyperandrogenism |
|
| |
Congenital adrenal hyperplasia must be differentiated from diseases that cause virilization and hirsutism in female:[7][6][8]
| Disease | Steroid status | Other laboratory | Important clinical findings |
|---|---|---|---|
| Non-classic type of 21-hydroxylase deficiency | Increased:
|
|
|
| 11-β hydroxylase deficiency | Increased:
Decreased: |
|
|
| 3 beta-hydroxysteroid dehydrogenase deficiency | Increased:
Decreased: |
|
|
| Polycystic ovary syndrome |
|
|
|
| Adrenal tumors |
|
|
|
| Ovarian virilizing tumor |
|
|
|
| Cushing's syndrome |
|
||
| Hyperprolactinemia |
|
|
Some types of congenital adrenal hyperplasia must be differentiated from diseases with primary amenorrhea:[9][2][3][4][10][11][12][13]
| Disease | Cause | Differentiating | ||||||
|---|---|---|---|---|---|---|---|---|
| Findings | Uterus | Breast development | Testosterone | LH | FSH | Karyotyping | ||
| 3 beta-hydroxysteroid dehydrogenase deficiency |
|
Yes in female |
Yes in female |
↓ |
Normal |
Normal |
||
| 17-alpha-hydroxylase deficiency |
|
No |
No |
↓ |
Normal |
Normal |
||
| Gonadal dysgenesis |
|
|
Yes |
Yes |
↓ |
↑ |
↑ |
|
| Testicular regression syndrome |
|
|
No |
No |
↓ |
↑ |
↑ |
|
| LH receptor defects |
|
No |
No |
↓ |
↑ |
↑ |
||
| 5-alpha-reductase type 2 deficiency |
|
No |
No |
Normal male range |
High to normal |
High to normal |
||
| Androgen insensitivity syndrome |
|
|
No |
Yes |
Normal male range |
Normal |
Normal |
|
| Mullerian agenesis |
|
No |
Yes |
Normal female range |
Normal |
Normal |
||
| Primary ovarian insufficiency |
|
|
Yes |
Yes |
Normal female range |
↑ |
↑ |
|
| Hypogonadotropic hypogonadism |
|
|
Yes |
No |
Normal female range |
Low |
Normal |
|
|
|
Yes |
Yes |
Normal female range |
↑ |
↑ |
||
17 alpha-hydroxylase deficiency and 11β-hydroxylase deficiency can cause low reninemic hypertension and should be differentiate from other causes of pseudohyperaldosteronism (low renin):
| Pseudohyperaldosteronism causes | Disease | Etiology | Clinical features | Labratory | Treatment | |||
|---|---|---|---|---|---|---|---|---|
| Elevated mineralocorticoid | Renin | Aldosterone | Other | |||||
| Endogenous causes | 17 alpha-hydroxylase deficiency | Mutations in the CYP17A1 gene |
|
Deoxycorticosterone (DOC) | ↓ | ↓ | Cortisol ↓ | Corticosteroids |
| 11β-hydroxylase deficiency | Mutations in the CYP11B1 gene |
|
Cortisol ↓ | |||||
| Apparent mineralocorticoid excess syndrome (AME) | Genetic or acquired defect of 11-HSD gene
|
|
Cortisol has mineralocorticoid effects | ↓ | ↓ | Urinary free cortisone ↓↓ | Dexamethasone and/or mineralocorticoid blockers | |
| Liddle’s syndrome (Pseudohyperaldosteronism type 1) | Mutation of the epithelial sodium channels (ENaC) gene in the distal renal tubules | No extra mineralocorticoid presents, and mutations in Na channels mimic aldosterone mechanism | ↓ | ↓ | Cortisol ↓ | Amiloride or triamterene | ||
| Cushing’s syndrome |
of cortisol which saturates 11-HSD2 activity,
|
Rapid weight gain, particularly of the trunk and face with limbs sparing (central obesity)
|
Cortisol has mineralocorticoid effects | ↓ |
|
Urinary free cortisol markedly ↑↑ |
| |
| Insensitivity to glucocorticoids (Chrousos syndrome) | Mutations in glucocorticoid receptor (GR) gene |
|
Deoxycorticosterone (DOC) | ↓ | ↓ | Cortisol | Dexamethasone | |
| Cortisol-secreting adrenocortical carcinoma | Multifactorial |
Rapid weight gain, particularly of the trunk and face with limbs sparing (central obesity)
|
Cortisol has mineralocorticoid effects | ↓ |
|
Urinary free cortisol markedly ↑↑ | Surgery | |
| Geller’s syndrome | Mutation of mineralocorticoid (MR) receptor that alters its specificity and allows progesterone to bind MR | Severe hypertension particularly during pregnancy | Progesterone has mineralocorticoid effects | ↓ | ↓ | - | mineralocorticoid blockers | |
| Gordon’s syndrome (Pseudohypoaldosteronism type 2) | Mutations of at least four genes have been identified, including WNK1 and WNK4 |
|
No excess mineralocorticoid; an increased activity of the thiazide-sensitive Na–Cl co-transporter in the distal tubule | ↓ | Normal | Hyperkalemia | thiazide diuretics and/or dietary sodium restriction | |
| Exogenous causes | Corticosteroids with mineralocorticoid activity | Fludrocortisone or fluoroprednisolone can mimic the action of aldosterone, | Medications such as fludrocortisone | ↓ | ↓ | - | Change the treatment | |
| Licorice ingestion | Glycyrrhetinic acid that binds mineralocorticoid receptor and blocks 11-HSD2 at the level of classical target tissues of aldosterone | - | ↓ | ↓ | Urinary free cortisol Moderate ↑ | Discontinue licorice | ||
| Grapefruit | High assumption of naringenin, a component of grapefruit, can also block 11-HSD | - | ↓ | ↓ | - | Discontinue grapefruit | ||
| Estrogens | Estrogens can retain sodium and water by different mechanisms, causing:
|
- | ↓ | ↓ | - | Discontinue estrogens | ||
CAH must be differentiated from other causes of irregular menses and hirsutism:
| Disease | Differentiating Features |
|---|---|
| Pregnancy |
|
| Hypothalamic amenorrhea |
|
| Primary amenorrhea |
|
| Cushing syndrome |
|
| Hyperprolactinemia |
|
| Ovarian or adrenal tumor |
|
| Congenital adrenal hyperplasia |
|
| Anabolic steroid abuse |
|
| Hirsutism |
|
References
- ↑ "File:Adrenal Steroids Pathways.svg - Wikimedia Commons".
- ↑ 2.0 2.1 Moreira AC, Leal AM, Castro M (1990). "Characterization of adrenocorticotropin secretion in a patient with 17 alpha-hydroxylase deficiency". J. Clin. Endocrinol. Metab. 71 (1): 86–91. doi:10.1210/jcem-71-1-86. PMID 2164530.
- ↑ 3.0 3.1 Heremans GF, Moolenaar AJ, van Gelderen HH (1976). "Female phenotype in a male child due to 17-alpha-hydroxylase deficiency". Arch. Dis. Child. 51 (9): 721–3. PMC 1546244. PMID 999330.
- ↑ 4.0 4.1 Biglieri EG (1979). "Mechanisms establishing the mineralocorticoid hormone patterns in the 17 alpha-hydroxylase deficiency syndrome". J. Steroid Biochem. 11 (1B): 653–7. PMID 226795.
- ↑ Hughes IA, Nihoul-Fékété C, Thomas B, Cohen-Kettenis PT (2007). "Consequences of the ESPE/LWPES guidelines for diagnosis and treatment of disorders of sex development". Best Pract. Res. Clin. Endocrinol. Metab. 21 (3): 351–65. doi:10.1016/j.beem.2007.06.003. PMID 17875484.
- ↑ 6.0 6.1 White PC, Speiser PW (2000). "Congenital adrenal hyperplasia due to 21-hydroxylase deficiency". Endocr. Rev. 21 (3): 245–91. doi:10.1210/edrv.21.3.0398. PMID 10857554.
- ↑ Hohl A, Ronsoni MF, Oliveira M (2014). "Hirsutism: diagnosis and treatment". Arq Bras Endocrinol Metabol. 58 (2): 97–107. PMID 24830586. Vancouver style error: initials (help)
- ↑ Melmed, Shlomo (2016). Williams textbook of endocrinology. Philadelphia, PA: Elsevier. ISBN 978-0323297387.=
- ↑ Maimoun L, Philibert P, Cammas B, Audran F, Bouchard P, Fenichel P, Cartigny M, Pienkowski C, Polak M, Skordis N, Mazen I, Ocal G, Berberoglu M, Reynaud R, Baumann C, Cabrol S, Simon D, Kayemba-Kay's K, De Kerdanet M, Kurtz F, Leheup B, Heinrichs C, Tenoutasse S, Van Vliet G, Grüters A, Eunice M, Ammini AC, Hafez M, Hochberg Z, Einaudi S, Al Mawlawi H, Nuñez CJ, Servant N, Lumbroso S, Paris F, Sultan C (2011). "Phenotypical, biological, and molecular heterogeneity of 5α-reductase deficiency: an extensive international experience of 55 patients". J. Clin. Endocrinol. Metab. 96 (2): 296–307. doi:10.1210/jc.2010-1024. PMID 21147889.
- ↑ Saenger P (1996). "Turner's syndrome". N. Engl. J. Med. 335 (23): 1749–54. doi:10.1056/NEJM199612053352307. PMID 8929268.
- ↑ Bastian C, Muller JB, Lortat-Jacob S, Nihoul-Fékété C, Bignon-Topalovic J, McElreavey K, Bashamboo A, Brauner R (2015). "Genetic mutations and somatic anomalies in association with 46,XY gonadal dysgenesis". Fertil. Steril. 103 (5): 1297–304. doi:10.1016/j.fertnstert.2015.01.043. PMID 25813279.
- ↑ Imperato-McGinley J, Guerrero L, Gautier T, Peterson RE (1974). "Steroid 5alpha-reductase deficiency in man: an inherited form of male pseudohermaphroditism". Science. 186 (4170): 1213–5. PMID 4432067.
- ↑ Schnitzer JJ, Donahoe PK (2001). "Surgical treatment of congenital adrenal hyperplasia". Endocrinol. Metab. Clin. North Am. 30 (1): 137–54. PMID 11344932.