Congestive heart failure pathophysiology
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]; Saleh El Dassouki, M.D [3], Atif Mohammad, MD
Overview
Heart failure is a complex syndrome whereby there is inadequate output of the heart to meet the metabolic demands of the body. Abnormal function of different anatomic parts of the heart cause heart failure including the pericardium, the myocardium, the endocardium, the heart valves and the great vessels. Symptoms of heart failure are due to a lack of both forward blood flow to the body, and backward flow into the lungs.
Underlying Anatomic Abnormalities
Heart failure may result from an abnormality or dysfunction of any one of the anatomical structures of the heart:
- Pericardium
- Myocardium
- Endocardium
- Valvular heart disease or
- Disorders of the great vessels (e.g. pulmonary hypertension)
Mechanism by Which Anatomic Structures are Damaged
- Pericardium: Can be damaged by infiltration of inflammatory cells (e.g. pericarditis) or fluid (pericardial effusion).
- Myocardium: Can be damaged by ischemic injury (e.g. acute MI), infiltration by inflammatory cells (e.g. myocarditis), pressure overload (e.g. hypertension or aortic stenosis), metabolic derangements, or volume overload (aortic insufficiency or mitral insufficiency).
- Endocardium: Can be damaged by infiltration
- Valvular heart disease: Can be damaged or dysfuncitonal from birth (bicuspid aortic valve) or can be acquired (e.g. endocarditis or rheumatic heart disease).
- Disorders of the great vessels: Can be stiffened by atherosclerosis (e.g. hypertension) or can be chronically constricted (e.g. pulmonary hypertension)
Systolic versus Diastolic Dysfunction
Heart failure was once thought to be secondary to a depressed left ventricular ejection fraction. However, studies have shown that approximately 50% of patients who are diagnosed with heart failure have a normal ejection fraction (diastolic dysfunction). Patients may be broadly classified as having heart failure with depressed left ventricular ejection fraction (systolic dysfunction) or normal/preserved ejection fraction (diastolic dysfunction). Systolic and diastolic dysfunction commonly occur in conjunction with each other.
Pathophysiology
- In systolic heart failure, there is reduced contractility of the heart and a reduced left ventricular ejection fraction.
- In systolic heart failure, there is a failure of blood to move forward (forward failure) and a tendency of blood to move back ward (backward failure).
- Normally, blood flows from the lungs, into the pulmonary veins, into the left atrium, through the mitral valve, and finally into the left ventricle. When the left ventricle cannot be normally filled during diastole in both diastolic and systolic heart failure, blood will back up into the left atrium and, eventually, into the lungs. The result is a higher than normal pressure of blood within the vessels of the lung. As a result of hydrostatic forces, this high pressure leads to leaking of fluid (i.e. transudate) from the lung's blood vessels into the air-spaces (alveoli) of the lungs. The result is pulmonary edema, a condition characterized by difficulty breathing, inadequate oxygenation of blood, and, if severe and untreated, death.
- A reduced stroke volume, as a result of a failure of systole, diastole or both. Increased end systolic volume is usually caused by reduced contractility. Decreased end diastolic volume results from impaired ventricular filling – as occurs when the compliance of the ventricle falls (i.e. when the walls stiffen in diastolic dysfunciton).
Compensatory Mechanisms
- Left ventricular systolic dysfunction is associated with a reduction in stroke volume, the amount of blood the heart ejects with each heart beat.
- The reduction in stroke volume leads to a reduction in cardiac output which is the stroke volume multiplied by the heart rate.
- There are two compensatory mechanisms to preserve forward cardiac output (the product of stroke volume and heart rate):
- Dilation of the left ventricle to increase the stroke volume and
- Increase in heart rate
- Dilation of the left ventricle increases volume and increases contractility up to a point (see Frank-Starling law of the heart). As further LV dilation occurs, however, contractility drops. This is due to reduced ability to cross-link actin and myosin filaments in over-stretched heart muscle.[1].
- As further dilation occurs, functional mitral regurgitation (MR) may develop despite an anatomically normal mitral valve.
- Left ventricular enlargement and lack of forward cardiac output can lead to left atrial enlargement. Left atrial dilation may lead to atrial fibrillation which occurs in 20% of patients with congestive heart failure. Atrial fibrillation diminishes left ventricular filling through the loss of the atrial kick (the atrial contraction) and due to an increase in the heart rate which reduces the time available for the left ventricle to fill.
Pulmonary Edema
- The reduction in forward cardiac output leads to a rise in the pulmonary capillary wedge pressure. Rales usually develop if the pulmonary capillary wedge pressure is >25 mm Hg. Rales may not be present in the patient with chronic heart failure. Rales may develop at even lower pressures if LV function deteriorates suddenly.
- Dyspnea and orthopnea occur due to interstitial edema within the lungs at lower pressures.
End Organ Hypoperfusion
- The reduction in forward cardiac output leads to hypoperfusion at rest which is suggested by
- Cool extremities
- Confusion and altered mentation
- Impaired renal perfusion and declining renal function
- Reduced spare capacity. As the heart works harder to meet normal metabolic demands, the amount cardiac output can increase in times of increased oxygen demand (e.g. exercise) is reduced. This contributes to the exercise intolerance commonly seen in heart failure. This translates to the loss of one's cardiac reserve. The cardiac reserve refers to the ability of the heart to work harder during exercise or strenuous activity. Since the heart has to work harder to meet the normal metabolic demands, it is incapable of meeting the metabolic demands of the body during exercise.
- Increased heart rate, stimulated by increased sympathetic activity[2] in order to maintain cardiac output. Initially, this helps compensate for heart failure by maintaining blood pressure and perfusion, but places further strain on the myocardium, increasing coronary perfusion requirements, which can lead to worsening of ischemic heart disease. Sympathetic activity may also cause potentially fatal arrhythmias.
- Hypertrophy (an increase in physical size) of the myocardium, caused by the terminally differentiated heart muscle fibres increasing in size in an attempt to improve contractility. This may contribute to the increased stiffness and decreased ability to relax during diastole.
- Enlargement of the ventricles, contributing to the enlargement and spherical shape of the failing heart. The increase in ventricular volume also causes a reduction in stroke volume due to mechanical and contractile inefficiency.[3]
The general effect is one of reduced cardiac output and increased strain on the heart. This increases the risk of cardiac arrest (specifically due to ventricular dysrhythmias), and reduces blood supply to the rest of the body. In chronic disease the reduced cardiac output causes a number of changes in the rest of the body, some of which are physiological compensations, some of which are part of the disease process:
- Arterial blood pressure falls. This destimulates baroreceptors in the carotid sinus and aortic arch which link to the nucleus tractus solitarius. This center in the brain increases sympathetic activity, releasing catecholamines into the blood stream. Binding to alpha-1 receptors results in systemic arterial vasoconstriction. This helps restore blood pressure but also increases the total peripheral resistance, increasing the workload of the heart. Binding to beta-1 receptors in the myocardium increases the heart rate and make contractions more forceful, in an attempt to increase cardiac output. This also, however, increases the amount of work the heart has to perform.
- Increased sympathetic stimulation also causes the hypothalamus to secrete vasopressin (also known as antidiuretic hormone or ADH), which causes fluid retention at the kidneys. This increases the blood volume and blood pressure.
- Reduced perfusion (blood flow) to the kidneys stimulates the release of renin – an enzyme which catalyses the production of the potent vasopressor angiotensin. Angiotensin and its metabolites cause further vasocontriction, and stimulate increased secretion of the steroid aldosterone from the adrenal glands. This promotes salt and fluid retention at the kidneys, also increasing the blood volume.
- The chronically high levels of circulating neuroendocrine hormones such as catecholamines, renin, angiotensin, and aldosterone affects the myocardium directly, causing structural remodelling of the heart over the long term. Many of these remodelling effects seem to be mediated by transforming growth factor beta (TGF-beta), which is a common downstream target of the signal transduction cascade initiated by catecholamines[4] and angiotensin II,[5] and also by epidermal growth factor (EGF), which is a target of the signaling pathway activated by aldosterone[6]
- Reduced perfusion of skeletal muscle causes atrophy of the muscle fibres. This can result in weakness, increased fatigueability and decreased peak strength - all contributing to exercise intolerance.[7]
The increased peripheral resistance and greater blood volume place further strain on the heart and accelerates the process of damage to the myocardium. Vasoconstriction and fluid retention produce an increased hydrostatic pressure in the capillaries. This shifts the balance of forces in favour of interstitial fluid formation as the increased pressure forces additional fluid out of the blood, into the tissue. This results in edema (fluid build-up) in the tissues. In right-sided heart failure this commonly starts in the ankles where venous pressure is high due to the effects of gravity (although if the patient is bed-ridden, fluid accumulation may begin in the sacral region.) It may also occur in the abdominal cavity, where the fluid build-up is called ascites. In left-sided heart failure edema can occur in the lungs - this is called cardiogenic pulmonary edema. This reduces spare capacity for ventilation, causes stiffening of the lungs and reduces the efficiency of gas exchange by increasing the distance between the air and the blood. The consequences of this are shortness of breath, orthopnea and paroxysmal nocturnal dyspnea.
In severe cardiomyopathy, the effects of decreased cardiac output and poor perfusion become more apparent, and patients will manifest with cold and clammy extremities, cyanosis, claudication, generalized weakness, dizziness, and syncope.
The resultant hypoxia caused by pulmonary edema causes vasoconstriction in the pulmonary circulation, which results in pulmonary hypertension. Since the right ventricle generates far lower pressures than the left ventricle (approximately 20 mmHg versus around 120 mmHg, respectively, in the healthy individual) but nonetheless generates cardiac output exactly equal to the left ventricle, this means that a small increase in pulmonary vascular resistance causes a large increase in amount of work the right ventricle must perform. However, the main mechanism by which left-sided heart failure causes right-sided heart failure is actually not well understood. Some theories invoke mechanisms that are mediated by neurohormonal activation.[8] Mechanical effects may also contribute. As the left ventricle distends, the intraventricular septum bows into the right ventricle, decreasing the capacity of the right ventricle.
Pathology
Microscopic Pathology
-
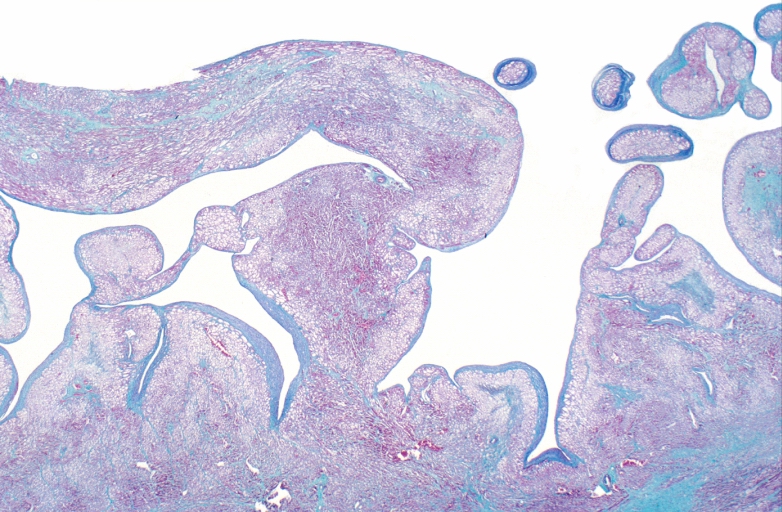
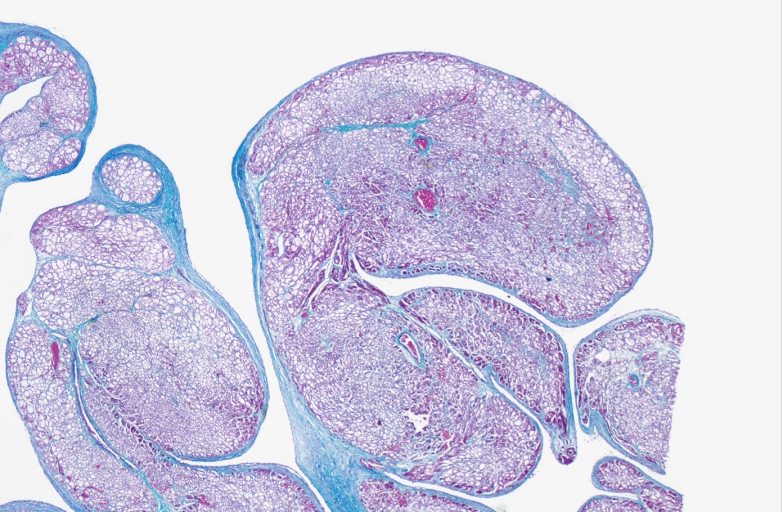
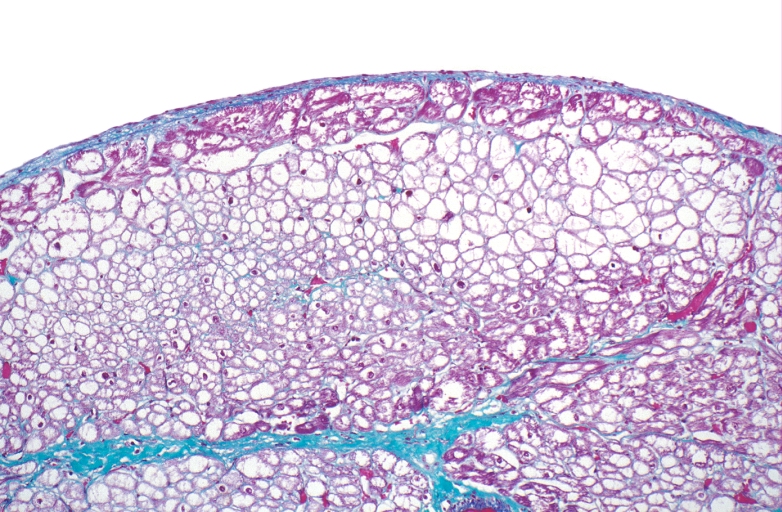
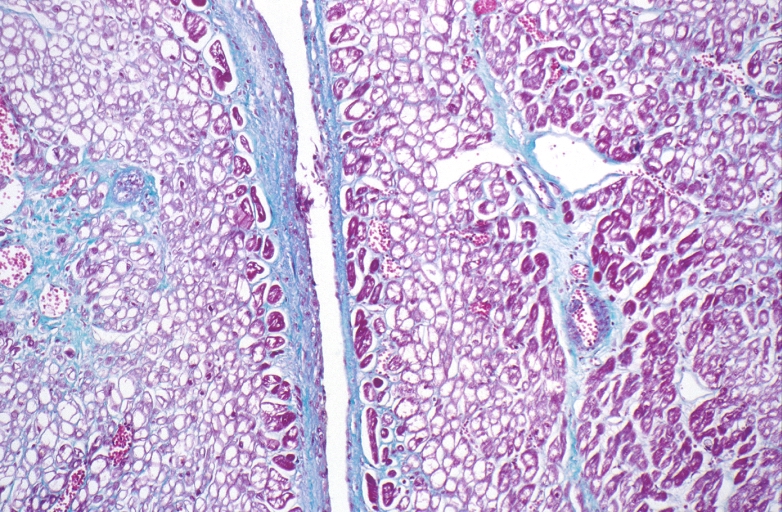
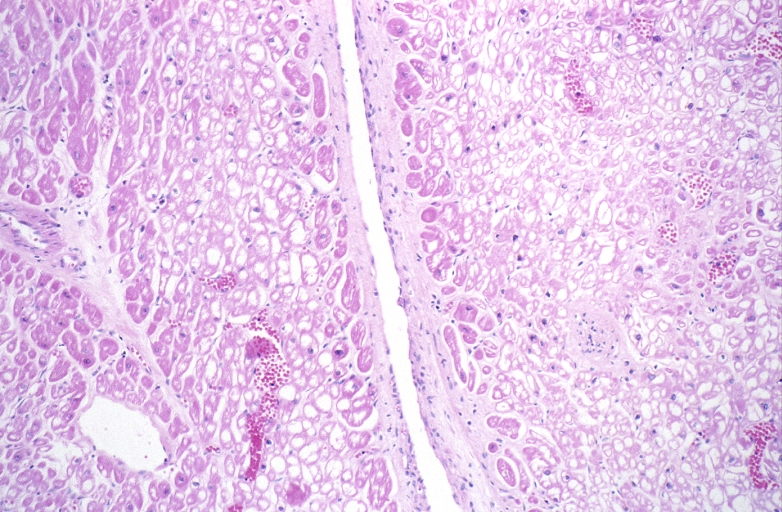
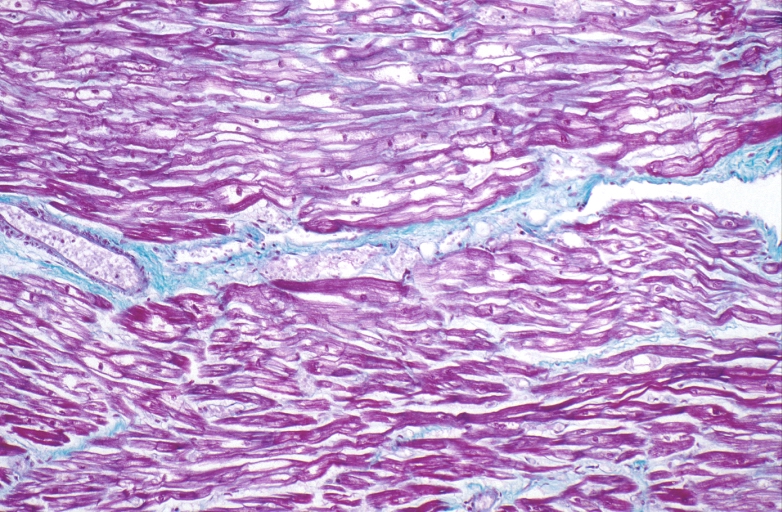
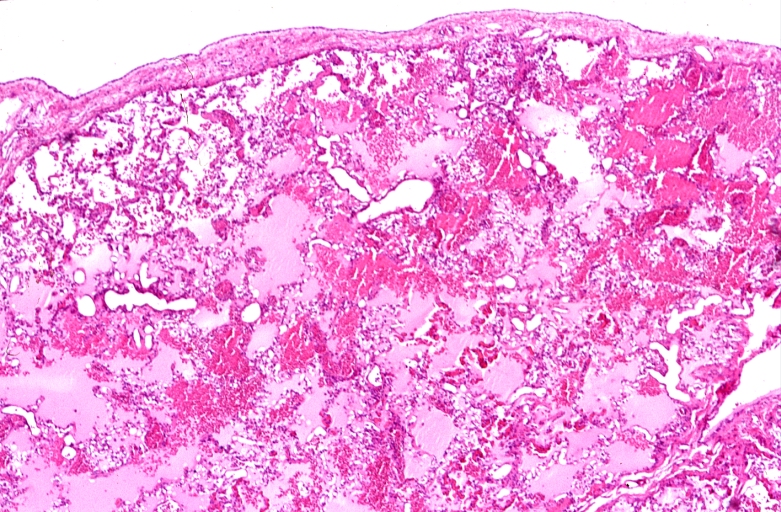
HEART: Congestive heart failure, hydropic change
-
HEART: Congestive heart failure, hydropic change
-
HEART: Congestive heart failure, hydropic change
-
HEART: Congestive heart failure, hydropic change
-
HEART: Congestive heart failure, hydropic change
-
HEART: Congestive heart failure, hydropic change
-
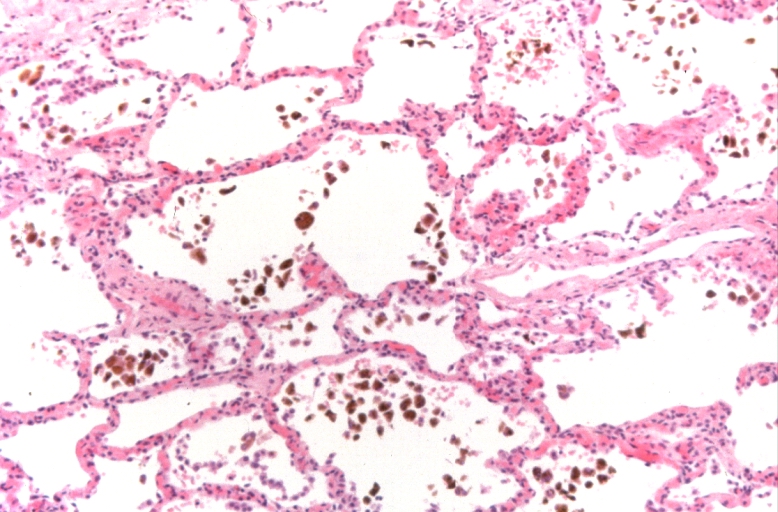
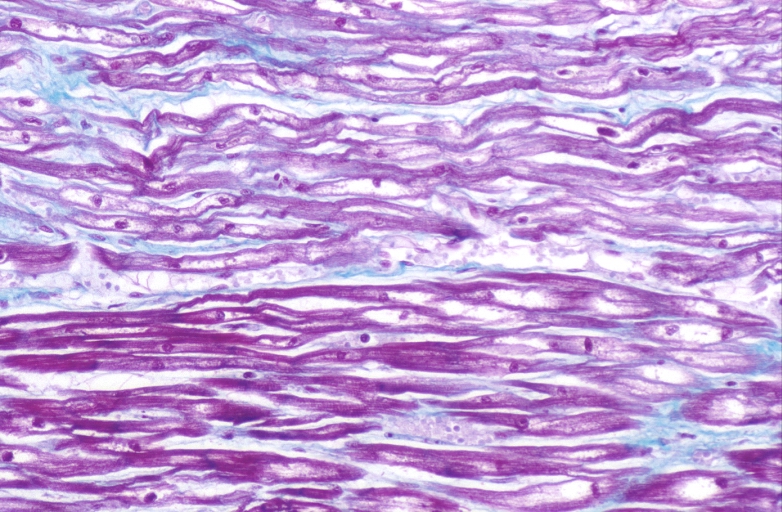
Lung, congestion, heart failure cells (hemosiderin laden macrophages)
-
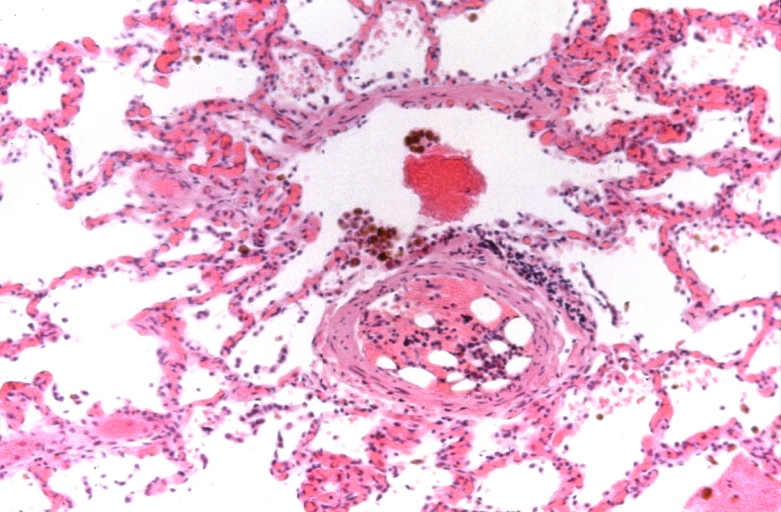
Lung, Congestive Heart Failure, bone marrow embolus
-
Lung, pulmonary edema in patient with congestive heart failure due to heart transplant rejection
-
HEART: Congestive heart failure, hydropic change
-
HEART Congestive heart failure, hydropic change
-
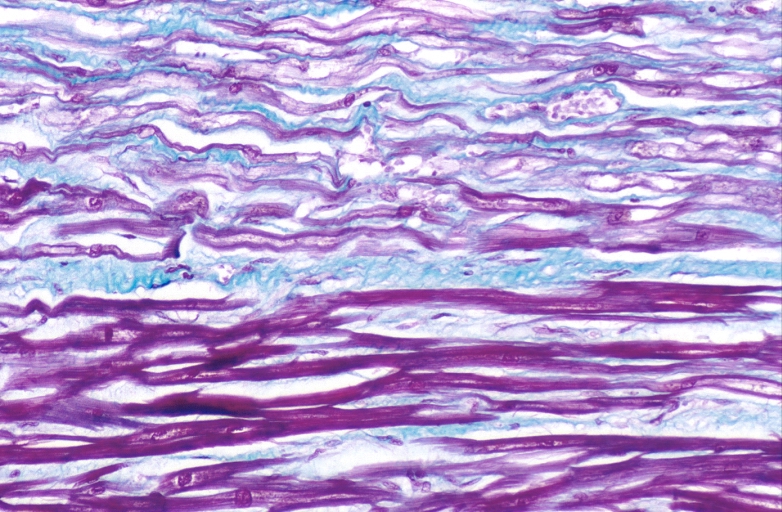
Spleen, congestion, congestive heart failure
References
- ↑ Boron and Boulpaep 2005 Medical Physiology Updated Edition p533 ISBN 0-7216-3256-4
- ↑ Rang HP (2003). Pharmacology. Edinburgh: Churchill Livingstone. p. 127. ISBN 0-443-07145-4.
- ↑ Template:GPnotebook
- ↑ Shigeyama J, Yasumura Y, Sakamoto A; et al. (2005). "Increased gene expression of collagen Types I and III is inhibited by beta-receptor blockade in patients with dilated cardiomyopathy". Eur. Heart J. 26 (24): 2698–705. doi:10.1093/eurheartj/ehi492. PMID 16204268. Unknown parameter
|month=ignored (help) - ↑ Tsutsui H, Matsushima S, Kinugawa S; et al. (2007). "Angiotensin II type 1 receptor blocker attenuates myocardial remodeling and preserves diastolic function in diabetic heart" (– Scholar search). Hypertens. Res. 30 (5): 439–49. doi:10.1291/hypres.30.439. PMID 17587756. Unknown parameter
|month=ignored (help)[dead link] - ↑ Krug AW, Grossmann C, Schuster C; et al. (2003). "Aldosterone stimulates epidermal growth factor receptor expression". J. Biol. Chem. 278 (44): 43060–6. doi:10.1074/jbc.M308134200. PMID 12939263. Unknown parameter
|month=ignored (help) - ↑ Template:GPnotebook
- ↑ Hunter JG, Boon NA, Davidson S, Colledge NR, Walker B (2006). Davidson's principles & practice of medicine. Elsevier/Churchill Livingstone. p. 544. ISBN 0-443-10057-8.