COVID-19-associated acute respiratory distress syndrome: Difference between revisions
(English edits) |
|||
| (17 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
{{COVID-19}} | __NOTOC__ | ||
{{Main article|COVID-19}} | |||
{{SI}} | |||
'''For COVID-19 frequently asked inpatient questions, click [[COVID-19 frequently asked inpatient questions|here]]''' | '''For COVID-19 frequently asked inpatient questions, click [[COVID-19 frequently asked inpatient questions|here]]'''<br> | ||
'''For COVID-19 frequently asked outpatient questions, click [[COVID-19 frequently asked outpatient questions|here]]'''<br>'''For COVID-19 patient information, click [[COVID-19 (patient information)|here]]''' | |||
'''For COVID-19 frequently asked outpatient questions, click [[COVID-19 frequently asked outpatient questions|here]]''' | |||
{{CMG}}; {{AE}} {{AyeshaFJ}} {{Usman Ali Akbar}} | {{CMG}}; {{AE}} {{AyeshaFJ}} {{Usman Ali Akbar}} | ||
| Line 9: | Line 10: | ||
==Overview== | ==Overview== | ||
[[Acute respiratory distress syndrome|ARDS]] has | Acute Respiratory Distress Syndrome ([[Acute respiratory distress syndrome|ARDS]]) is a form of non-cardiogenic pulmonary edema attributed to the breakdown of alveolar membrane and capillaries leading to hypoxia. The treatment of ARDS has evolved over the last decade. The management of COVID-19 related ARDS are stratified according to the classification of ARDS. Among patients with COVID-19, ARDS developed in 20% after a median of eight days after onset of symptoms; [[mechanical ventilation]] was implemented in 12.3%. The mortality rate of COVID-19 related ARDS is higher in elderly patients. Given the importance of [[heterogeneity]] in the [[Acute respiratory distress syndrome|ARDS]] profile, appropriate intervention at an appropriate time is needed to help to prevent the deterioration of lung function. Recent advances observed from the RECOVERY trial has further strengthened the notion that the use of [[dexamethasone]] in patients on a ventilator can reduce the mortality rate of patients by one-third. The treatment of COVID-19 related ARDS is evolving with time and different treatment options are now available for the management of [[Acute respiratory distress syndrome|ARDS]]. | ||
[[File:Covid-ards-overview.jpg|800px|center]] | |||
==Historical Perspective== | ==Historical Perspective== | ||
| Line 26: | Line 28: | ||
* [[ARDS]] arises as a [[complication]] of [[COVID-19]] [[infection]] due to [[acute]] [[inflammation]] of the [[alveolar space]] which prevents normal [[gas exchange]]. The increase in [[proinflammatory]] [[cytokines]] within the [[lung]] leads to recruitment of [[leukocytes]], further propagating the local [[inflammatory response]].<ref name="pmidPMID: 32329246">{{cite journal| author=Whyte CS, Morrow GB, Mitchell JL, Chowdary P, Mutch NJ| title=Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. | journal=J Thromb Haemost | year= 2020 | volume= | issue= | pages= | pmid=PMID: 32329246 | doi=10.1111/jth.14872 | pmc=7264738 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32329246 }} </ref> | * [[ARDS]] arises as a [[complication]] of [[COVID-19]] [[infection]] due to [[acute]] [[inflammation]] of the [[alveolar space]] which prevents normal [[gas exchange]]. The increase in [[proinflammatory]] [[cytokines]] within the [[lung]] leads to recruitment of [[leukocytes]], further propagating the local [[inflammatory response]].<ref name="pmidPMID: 32329246">{{cite journal| author=Whyte CS, Morrow GB, Mitchell JL, Chowdary P, Mutch NJ| title=Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. | journal=J Thromb Haemost | year= 2020 | volume= | issue= | pages= | pmid=PMID: 32329246 | doi=10.1111/jth.14872 | pmc=7264738 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32329246 }} </ref> | ||
* The [[cytokine]] storm and the deadly uncontrolled [[systemic]] [[inflammatory]] response | * The [[cytokine]] storm and the deadly uncontrolled [[systemic]] [[inflammatory]] response is caused by the release of large amounts of [[proinflammatory]] [[cytokines]] including [[interferons]] and [[interleukins]] and, [[chemokines]] by [[immune]] [[effector cells|effector cells.]] This results in [[acute]] [[inflammation]] within the [[alveolar space]]. Furthermore, the [[exudate]] containing [[plasma proteins]] including: [[albumin]], [[fibrinogen]], [[proinflammatory]] [[cytokines]] and [[coagulation factors]] will increase [[alveolar]]-[[capillary]] [[permeability]] and decrease the normal [[gas exchange]][[plasma proteins|asma proteins]][[albumin]], [[fibrinogen]]<ref name="pmidPMID: 19801579">{{cite journal| author=Meduri GU, Annane D, Chrousos GP, Marik PE, Sinclair SE| title=Activation and regulation of systemic inflammation in ARDS: rationale for prolonged glucocorticoid therapy. | journal=Chest | year= 2009 | volume= 136 | issue= 6 | pages= 1631-1643 | pmid=PMID: 19801579 | doi=10.1378/chest.08-2408 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19801579 }} </ref> | ||
* | *[[COVID-19]] [[patients]] with [[ARDS]] show elevated levels of [[IL-6]], [[IFN-a]], and [[CCL5]], [[CXCL8]], [[CXCL-10]] in [[serum]] as compared to those with the mild to moderate [[disease]].<ref name="pmidPMID: 32304191">{{cite journal| author=Tezer H, Bedir Demirdağ T| title=Novel coronavirus disease (COVID-19) in children | journal=Turk J Med Sci | year= 2020 | volume= 50 | issue= SI-1 | pages= 592-603 | pmid=PMID: 32304191 | doi=10.3906/sag-2004-174 | pmc=7195991 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32304191 }} </ref> | ||
* This [[inflammatory]] [[process]] leads to the [[fibrin]] deposition in the [[air spaces]] and [[lung]] parenchyma and contributes to [[hyaline-membrane]] formation and subsequent [[alveolar]] [[fibrosis]].<ref name="pmidPMID: 2314423">{{cite journal| author=Bertozzi P, Astedt B, Zenzius L, Lynch K, LeMaire F, Zapol W | display-authors=etal| title=Depressed bronchoalveolar urokinase activity in patients with adult respiratory distress syndrome. | journal=N Engl J Med | year= 1990 | volume= 322 | issue= 13 | pages= 890-7 | pmid=PMID: 2314423 | doi=10.1056/NEJM199003293221304 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=2314423 }} </ref> | * This [[inflammatory]] [[process]] leads to the [[fibrin]] deposition in the [[air spaces]] and [[lung]] parenchyma and contributes to [[hyaline-membrane]] formation and subsequent [[alveolar]] [[fibrosis]].<ref name="pmidPMID: 2314423">{{cite journal| author=Bertozzi P, Astedt B, Zenzius L, Lynch K, LeMaire F, Zapol W | display-authors=etal| title=Depressed bronchoalveolar urokinase activity in patients with adult respiratory distress syndrome. | journal=N Engl J Med | year= 1990 | volume= 322 | issue= 13 | pages= 890-7 | pmid=PMID: 2314423 | doi=10.1056/NEJM199003293221304 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=2314423 }} </ref> | ||
* [[Patients]] [[infected]] with [[COVID‐19]] also exhibit [[coagulation]] [[abnormalities]].This [[procoagulant]] pattern can lead to [[acute respiratory distress syndrome]].<ref name="pmidPMID: 32302448">{{cite journal| author=Ranucci M, Ballotta A, Di Dedda U, Bayshnikova E, Dei Poli M, Resta M | display-authors=etal| title=The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. | journal=J Thromb Haemost | year= 2020 | volume= | issue= | pages= | pmid=PMID: 32302448 | doi=10.1111/jth.14854 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32302448 }} </ref> | * [[Patients]] [[infected]] with [[COVID‐19]] also exhibit [[coagulation]] [[abnormalities]].This [[procoagulant]] pattern can lead to [[acute respiratory distress syndrome]].<ref name="pmidPMID: 32302448">{{cite journal| author=Ranucci M, Ballotta A, Di Dedda U, Bayshnikova E, Dei Poli M, Resta M | display-authors=etal| title=The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. | journal=J Thromb Haemost | year= 2020 | volume= | issue= | pages= | pmid=PMID: 32302448 | doi=10.1111/jth.14854 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32302448 }} </ref> | ||
[[File:Patho covid ards.jpg|600px|center]] | [[File:Patho covid ards-wikidoc-ar.jpg|600px|center]] | ||
==Clinical Features== | ==Clinical Features== | ||
*Clinical presentations of COVID-19 range from [[asymptomatic]] (81.4%), through mildly symptomatic with or without seasonal flu-like symptoms, to severe [[pneumonia]] (13.9%).<ref name="Borges do Nascimento Cacic Abdulazeem von Groote p=941">{{cite journal | last=Borges do Nascimento | first=Israel Júnior | last2=Cacic | first2=Nensi | last3=Abdulazeem | first3=Hebatullah Mohamed | last4=von Groote | first4=Thilo Caspar | last5=Jayarajah | first5=Umesh | last6=Weerasekara | first6=Ishanka | last7=Esfahani | first7=Meisam Abdar | last8=Civile | first8=Vinicius Tassoni | last9=Marusic | first9=Ana | last10=Jeroncic | first10=Ana | last11=Carvas Junior | first11=Nelson | last12=Pericic | first12=Tina Poklepovic | last13=Zakarija-Grkovic | first13=Irena | last14=Meirelles Guimarães | first14=Silvana Mangeon | last15=Luigi Bragazzi | first15=Nicola | last16=Bjorklund | first16=Maria | last17=Sofi-Mahmudi | first17=Ahmad | last18=Altujjar | first18=Mohammad | last19=Tian | first19=Maoyi | last20=Arcani | first20=Diana Maria Cespedes | last21=O’Mathúna | first21=Dónal P. | last22=Marcolino | first22=Milena Soriano | title=Novel Coronavirus Infection (COVID-19) in Humans: A Scoping Review and Meta-Analysis | journal=Journal of Clinical Medicine | publisher=MDPI AG | volume=9 | issue=4 | date=2020-03-30 | issn=2077-0383 | doi=10.3390/jcm9040941 | page=941}}</ref> | *Clinical presentations of COVID-19 range from [[asymptomatic]] (81.4%), through mildly symptomatic with or without seasonal flu-like symptoms, to severe [[pneumonia]] (13.9%).<ref name="Borges do Nascimento Cacic Abdulazeem von Groote p=941">{{cite journal | last=Borges do Nascimento | first=Israel Júnior | last2=Cacic | first2=Nensi | last3=Abdulazeem | first3=Hebatullah Mohamed | last4=von Groote | first4=Thilo Caspar | last5=Jayarajah | first5=Umesh | last6=Weerasekara | first6=Ishanka | last7=Esfahani | first7=Meisam Abdar | last8=Civile | first8=Vinicius Tassoni | last9=Marusic | first9=Ana | last10=Jeroncic | first10=Ana | last11=Carvas Junior | first11=Nelson | last12=Pericic | first12=Tina Poklepovic | last13=Zakarija-Grkovic | first13=Irena | last14=Meirelles Guimarães | first14=Silvana Mangeon | last15=Luigi Bragazzi | first15=Nicola | last16=Bjorklund | first16=Maria | last17=Sofi-Mahmudi | first17=Ahmad | last18=Altujjar | first18=Mohammad | last19=Tian | first19=Maoyi | last20=Arcani | first20=Diana Maria Cespedes | last21=O’Mathúna | first21=Dónal P. | last22=Marcolino | first22=Milena Soriano | title=Novel Coronavirus Infection (COVID-19) in Humans: A Scoping Review and Meta-Analysis | journal=Journal of Clinical Medicine | publisher=MDPI AG | volume=9 | issue=4 | date=2020-03-30 | issn=2077-0383 | doi=10.3390/jcm9040941 | page=941}}</ref> | ||
* Respiratory problems manifest as [[dyspnea]] that | * Respiratory problems manifest as [[dyspnea]] that range from [[Exertional dyspnea|effort dyspnea]] to [[dyspnea]] occurring at rest. | ||
* Patients with dyspnea can revert to an [[Asymptomatic|asymptomatic phase]] or progress to ARDS, requiring positive pressure oxygen therapy and intensive care therapy [18] in 17–19.6% of [[symptomatic]] patients.<ref name="Wang Hu Hu Zhu p=1061">{{cite journal | last=Wang | first=Dawei | last2=Hu | first2=Bo | last3=Hu | first3=Chang | last4=Zhu | first4=Fangfang | last5=Liu | first5=Xing | last6=Zhang | first6=Jing | last7=Wang | first7=Binbin | last8=Xiang | first8=Hui | last9=Cheng | first9=Zhenshun | last10=Xiong | first10=Yong | last11=Zhao | first11=Yan | last12=Li | first12=Yirong | last13=Wang | first13=Xinghuan | last14=Peng | first14=Zhiyong | title=Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China | journal=JAMA | publisher=American Medical Association (AMA) | volume=323 | issue=11 | date=2020-03-17 | issn=0098-7484 | doi=10.1001/jama.2020.1585 | page=1061}}</ref> | * Patients with dyspnea can revert to an [[Asymptomatic|asymptomatic phase]] or progress to ARDS, requiring positive pressure oxygen therapy and intensive care therapy [18] in 17–19.6% of [[symptomatic]] patients.<ref name="Wang Hu Hu Zhu p=1061">{{cite journal | last=Wang | first=Dawei | last2=Hu | first2=Bo | last3=Hu | first3=Chang | last4=Zhu | first4=Fangfang | last5=Liu | first5=Xing | last6=Zhang | first6=Jing | last7=Wang | first7=Binbin | last8=Xiang | first8=Hui | last9=Cheng | first9=Zhenshun | last10=Xiong | first10=Yong | last11=Zhao | first11=Yan | last12=Li | first12=Yirong | last13=Wang | first13=Xinghuan | last14=Peng | first14=Zhiyong | title=Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China | journal=JAMA | publisher=American Medical Association (AMA) | volume=323 | issue=11 | date=2020-03-17 | issn=0098-7484 | doi=10.1001/jama.2020.1585 | page=1061}}</ref> | ||
| Line 62: | Line 64: | ||
|41 | |41 | ||
|29.26 % | |29.26 % | ||
(16.13-45.53) | (16.13-45.53) | ||
|- | |- | ||
|January 29,2020 | |January 29,2020 | ||
|China | |China | ||
|Nanshan Chen et al.<ref name=" | |Nanshan Chen et al.<ref name="Chen Zhou Dong Qu 2020 pp. 507–513">{{cite journal | last=Chen | first=Nanshan | last2=Zhou | first2=Min | last3=Dong | first3=Xuan | last4=Qu | first4=Jieming | last5=Gong | first5=Fengyun | last6=Han | first6=Yang | last7=Qiu | first7=Yang | last8=Wang | first8=Jingli | last9=Liu | first9=Ying | last10=Wei | first10=Yuan | last11=Xia | first11=Jia'an | last12=Yu | first12=Ting | last13=Zhang | first13=Xinxin | last14=Zhang | first14=Li | title=Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study | journal=The Lancet | publisher=Elsevier BV | volume=395 | issue=10223 | year=2020 | issn=0140-6736 | doi=10.1016/s0140-6736(20)30211-7 | pages=507–513}}</ref> | ||
|99 | |99 <br /> | ||
|17.17 % | |17.17 % | ||
(10.33-26.06) | (10.33-26.06) | ||
|- | |- | ||
|March 13, 2020 | |March 13, 2020 | ||
|China | |China | ||
|Chaomin Wu et al. | |Chaomin Wu et al.<ref name="Wu Chen Cai Xia p=934">{{cite journal | last=Wu | first=Chaomin | last2=Chen | first2=Xiaoyan | last3=Cai | first3=Yanping | last4=Xia | first4=Jia’an | last5=Zhou | first5=Xing | last6=Xu | first6=Sha | last7=Huang | first7=Hanping | last8=Zhang | first8=Li | last9=Zhou | first9=Xia | last10=Du | first10=Chunling | last11=Zhang | first11=Yuye | last12=Song | first12=Juan | last13=Wang | first13=Sijiao | last14=Chao | first14=Yencheng | last15=Yang | first15=Zeyong | last16=Xu | first16=Jie | last17=Zhou | first17=Xin | last18=Chen | first18=Dechang | last19=Xiong | first19=Weining | last20=Xu | first20=Lei | last21=Zhou | first21=Feng | last22=Jiang | first22=Jinjun | last23=Bai | first23=Chunxue | last24=Zheng | first24=Junhua | last25=Song | first25=Yuanlin | title=Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China | journal=JAMA Internal Medicine | publisher=American Medical Association (AMA) | volume=180 | issue=7 | date=2020-07-01 | issn=2168-6106 | doi=10.1001/jamainternmed.2020.0994 | page=934}}</ref> | ||
|201 | |201 | ||
|41.8 % | |41.8 % | ||
| Line 87: | Line 89: | ||
* Patients of all age groups may develop [[Acute respiratory distress syndrome|ARDS]]. | * Patients of all age groups may develop [[Acute respiratory distress syndrome|ARDS]]. | ||
* It is more commonly observed among patients aged ≥65 | * It is more commonly observed among patients aged ≥65 years old. | ||
=== Gender === | === Gender === | ||
* Some case studies report that men are more commonly affected by ARDS than women. | * Some case studies report that men are more commonly affected by ARDS than women. | ||
* In | * In a Chinese public data set, the number of men who died from [[COVID-19]] is 2.4 times that of women (70.3 vs. 29.7%, ''P'' = 0.016).<ref name="Jin Bai He Wu p.">{{cite journal | last=Jin | first=Jian-Min | last2=Bai | first2=Peng | last3=He | first3=Wei | last4=Wu | first4=Fei | last5=Liu | first5=Xiao-Fang | last6=Han | first6=De-Min | last7=Liu | first7=Shi | last8=Yang | first8=Jin-Kui | title=Gender Differences in Patients With COVID-19: Focus on Severity and Mortality | journal=Frontiers in Public Health | publisher=Frontiers Media SA | volume=8 | date=2020-04-29 | issn=2296-2565 | doi=10.3389/fpubh.2020.00152 | page=}}</ref> | ||
=== Race=== | === Race=== | ||
| Line 124: | Line 126: | ||
* The exudative phase typically encompasses the first 5 to 7 days of illness after exposure to one or more precipitation factors. | * The exudative phase typically encompasses the first 5 to 7 days of illness after exposure to one or more precipitation factors. | ||
* Histopathologically, loss of integrity of the alveolar barrier results in influx of proteinaceous fluid into the air place and formation of hyaline membrane. [[Pulmonary edema]] and [[atelectasis]] with reduced [[pulmonary compliance]] ensue, leading to the development of [[pulmonary shunt]] and [[hypoxemia]]. | * Histopathologically, loss of integrity of the alveolar barrier results in the influx of proteinaceous fluid into the air place, and formation of the hyaline membrane. [[Pulmonary edema]] and [[atelectasis]] with reduced [[pulmonary compliance]] ensue, leading to the development of [[pulmonary shunt]] and [[hypoxemia]]. | ||
* In this phase, patients experience respiratory symptoms including [[dyspnea]], [[tachypnea]], and [[Labored breathing|increased work of breathing]] that eventually result in [[respiratory failure]] requiring ventilator support. If left untreated, approximately 70% of patients with ARDS may progress to [[mortality]].<ref>National Heart and Lung Institute. Task Force on Research in Respiratory Diseases, and National Heart and Lung Institute. Lung Program. Respiratory Diseases; Task Force Report on Problems, Research Approaches, Needs. The Lung Program, National Heart and Lung Institute. [Bethesda, Md., U.S. Dept. of Health, Education, and Welfare, National Institutes of Health] for sale by the Supt. of Docs., U.S. Govt. Print. Off., Washington, 1972. http://archive.org/details/respiratorydisea00nati.</ref> | * In this phase, patients experience respiratory symptoms including [[dyspnea]], [[tachypnea]], and [[Labored breathing|increased work of breathing]] that eventually result in [[respiratory failure]] requiring ventilator support. If left untreated, approximately 70% of patients with ARDS may progress to [[mortality]].<ref>National Heart and Lung Institute. Task Force on Research in Respiratory Diseases, and National Heart and Lung Institute. Lung Program. Respiratory Diseases; Task Force Report on Problems, Research Approaches, Needs. The Lung Program, National Heart and Lung Institute. [Bethesda, Md., U.S. Dept. of Health, Education, and Welfare, National Institutes of Health] for sale by the Supt. of Docs., U.S. Govt. Print. Off., Washington, 1972. http://archive.org/details/respiratorydisea00nati.</ref> | ||
* Among non-survivors, approximately 50% patients die within a week of the onset with exudative change as the predominant histopathological feature.<ref>Thille, Arnaud W., Andrés Esteban, Pilar Fernández-Segoviano, José-María Rodriguez, José-Antonio Aramburu, Patricio Vargas-Errázuriz, Ana Martín-Pellicer, José A. Lorente, and Fernando Frutos-Vivar. “Chronology of Histological Lesions in Acute Respiratory Distress Syndrome with Diffuse Alveolar Damage: A Prospective Cohort Study of Clinical Autopsies.” The Lancet. Respiratory Medicine 1, no. 5 (July 2013): 395–401. doi:10.1016/S2213-2600(13)70053-5.</ref> | * Among non-survivors, approximately 50% patients die within a week of the onset with exudative change as the predominant histopathological feature.<ref>Thille, Arnaud W., Andrés Esteban, Pilar Fernández-Segoviano, José-María Rodriguez, José-Antonio Aramburu, Patricio Vargas-Errázuriz, Ana Martín-Pellicer, José A. Lorente, and Fernando Frutos-Vivar. “Chronology of Histological Lesions in Acute Respiratory Distress Syndrome with Diffuse Alveolar Damage: A Prospective Cohort Study of Clinical Autopsies.” The Lancet. Respiratory Medicine 1, no. 5 (July 2013): 395–401. doi:10.1016/S2213-2600(13)70053-5.</ref> | ||
| Line 143: | Line 145: | ||
:* ARDS is complicated by VAP in approximately 37% to 60% of cases.<ref>Delclaux, C., E. Roupie, F. Blot, L. Brochard, F. Lemaire, and C. Brun-Buisson. “Lower Respiratory Tract Colonization and Infection during Severe Acute Respiratory Distress Syndrome: Incidence and Diagnosis.” American Journal of Respiratory and Critical Care Medicine 156, no. 4 Pt 1 (October 1997): 1092–98. doi:10.1164/ajrccm.156.4.9701065.</ref><ref>Markowicz, P., M. Wolff, K. Djedaïni, Y. Cohen, J. Chastre, C. Delclaux, J. Merrer, et al. “Multicenter Prospective Study of Ventilator-Associated Pneumonia during Acute Respiratory Distress Syndrome. Incidence, Prognosis, and Risk Factors. ARDS Study Group.” American Journal of Respiratory and Critical Care Medicine 161, no. 6 (June 2000): 1942–48. doi:10.1164/ajrccm.161.6.9909122.</ref><ref>Meduri, G. U., R. C. Reddy, T. Stanley, and F. El-Zeky. “Pneumonia in Acute Respiratory Distress Syndrome. A Prospective Evaluation of Bilateral Bronchoscopic Sampling.” American Journal of Respiratory and Critical Care Medicine 158, no. 3 (September 1998): 870–75. doi:10.1164/ajrccm.158.3.9706112.</ref><ref>Chastre, J., J. L. Trouillet, A. Vuagnat, M. L. Joly-Guillou, H. Clavier, M. C. Dombret, and C. Gibert. “Nosocomial Pneumonia in Patients with Acute Respiratory Distress Syndrome.” American Journal of Respiratory and Critical Care Medicine 157, no. 4 Pt 1 (April 1998): 1165–72. doi:10.1164/ajrccm.157.4.9708057.</ref> | :* ARDS is complicated by VAP in approximately 37% to 60% of cases.<ref>Delclaux, C., E. Roupie, F. Blot, L. Brochard, F. Lemaire, and C. Brun-Buisson. “Lower Respiratory Tract Colonization and Infection during Severe Acute Respiratory Distress Syndrome: Incidence and Diagnosis.” American Journal of Respiratory and Critical Care Medicine 156, no. 4 Pt 1 (October 1997): 1092–98. doi:10.1164/ajrccm.156.4.9701065.</ref><ref>Markowicz, P., M. Wolff, K. Djedaïni, Y. Cohen, J. Chastre, C. Delclaux, J. Merrer, et al. “Multicenter Prospective Study of Ventilator-Associated Pneumonia during Acute Respiratory Distress Syndrome. Incidence, Prognosis, and Risk Factors. ARDS Study Group.” American Journal of Respiratory and Critical Care Medicine 161, no. 6 (June 2000): 1942–48. doi:10.1164/ajrccm.161.6.9909122.</ref><ref>Meduri, G. U., R. C. Reddy, T. Stanley, and F. El-Zeky. “Pneumonia in Acute Respiratory Distress Syndrome. A Prospective Evaluation of Bilateral Bronchoscopic Sampling.” American Journal of Respiratory and Critical Care Medicine 158, no. 3 (September 1998): 870–75. doi:10.1164/ajrccm.158.3.9706112.</ref><ref>Chastre, J., J. L. Trouillet, A. Vuagnat, M. L. Joly-Guillou, H. Clavier, M. C. Dombret, and C. Gibert. “Nosocomial Pneumonia in Patients with Acute Respiratory Distress Syndrome.” American Journal of Respiratory and Critical Care Medicine 157, no. 4 Pt 1 (April 1998): 1165–72. doi:10.1164/ajrccm.157.4.9708057.</ref> | ||
:* [[VAP]] usually develops 5 to 7 days after the initial exposure to the precipitating factor.<ref>Delclaux, C., E. Roupie, F. Blot, L. Brochard, F. Lemaire, and C. Brun-Buisson. “Lower Respiratory Tract Colonization and Infection during Severe Acute Respiratory Distress Syndrome: Incidence and Diagnosis.” American Journal of Respiratory and Critical Care Medicine 156, no. 4 Pt 1 (October 1997): 1092–98. doi:10.1164/ajrccm.156.4.9701065.</ref> | :* [[VAP]] usually develops 5 to 7 days after the initial exposure to the precipitating factor.<ref>Delclaux, C., E. Roupie, F. Blot, L. Brochard, F. Lemaire, and C. Brun-Buisson. “Lower Respiratory Tract Colonization and Infection during Severe Acute Respiratory Distress Syndrome: Incidence and Diagnosis.” American Journal of Respiratory and Critical Care Medicine 156, no. 4 Pt 1 (October 1997): 1092–98. doi:10.1164/ajrccm.156.4.9701065.</ref> | ||
:* The most likely microorganisms of [[VAP]] | :* The most likely microorganisms of [[VAP]] includes non-fermenting [[Gram-negative bacilli]], [[methicillin]]-resistant ''[[Staphylococcus aureus]]'', and ''[[Enterobacteriaceae]]''.<ref>Chastre, J., J. L. Trouillet, A. Vuagnat, M. L. Joly-Guillou, H. Clavier, M. C. Dombret, and C. Gibert. “Nosocomial Pneumonia in Patients with Acute Respiratory Distress Syndrome.” American Journal of Respiratory and Critical Care Medicine 157, no. 4 Pt 1 (April 1998): 1165–72. doi:10.1164/ajrccm.157.4.9708057.</ref> | ||
* [[Barotrauma]] (e.g., [[pneumothorax]], [[pneumomediastinum]], and [[subcutaneous emphysema]]) | * [[Barotrauma]] (e.g., [[pneumothorax]], [[pneumomediastinum]], and [[subcutaneous emphysema]]) | ||
| Line 183: | Line 185: | ||
==== Vital Signs ==== | ==== Vital Signs ==== | ||
*The presence of the following signs of [[shock]] or [[infection]] on physical examination | *The presence of the following signs of [[shock]] or [[infection]] on physical examination is highly suggestive of ARDS: | ||
**[[Temperature|'''Temperature (Temp, T)''']]: [[Hyperpyrexia]] ≥ 38°C or 100.4°F) or low temperature < 36°C or 96.8°F. | **[[Temperature|'''Temperature (Temp, T)''']]: [[Hyperpyrexia]] ≥ 38°C or 100.4°F) or low temperature < 36°C or 96.8°F. | ||
** [[Blood pressure|'''Blood pressure (BP)''']]: inappropriately low, with a low [[Mean arterial pressure|mean arterial pressure (MAP)]]. | ** [[Blood pressure|'''Blood pressure (BP)''']]: inappropriately low, with a low [[Mean arterial pressure|mean arterial pressure (MAP)]]. | ||
| Line 228: | Line 230: | ||
* Later, these opacities progress to more extensive consolidation that is diffuse, and they are often asymmetrical. | * Later, these opacities progress to more extensive consolidation that is diffuse, and they are often asymmetrical. | ||
[[File:Covid-19-rapidly-progressive-acute-respiratory-distress-syndrome-ards.jpg|thumb|300px|none|Bilateral alveolar consolidation with panlobar change, with typical radiological findings of ARDS | [[File:Covid-19-rapidly-progressive-acute-respiratory-distress-syndrome-ards.jpg|thumb|300px|none|Bilateral alveolar consolidation with panlobar change, with typical radiological findings of ARDS. [https://radiopaedia.org/cases/covid-19-rapidly-progressive-acute-respiratory-distress-syndrome-ards?lang=us Source: Dr. Edgar Lorente]]] | ||
==== Chest CT-Scan ==== | ==== Chest CT-Scan ==== | ||
| Line 235: | Line 237: | ||
*[[Lung]] biopsy shows [[fibrin]] deposition.<ref name="pmidPMID: 32329246">{{cite journal| author=Whyte CS, Morrow GB, Mitchell JL, Chowdary P, Mutch NJ| title=Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. | journal=J Thromb Haemost | year= 2020 | volume= | issue= | pages= | pmid=PMID: 32329246 | doi=10.1111/jth.14872 | pmc=7264738 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32329246 }} </ref><ref name="pmidPMID: 32031570">{{cite journal| author=Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J | display-authors=etal| title=Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. | journal=JAMA | year= 2020 | volume= | issue= | pages= | pmid=PMID: 32031570 | doi=10.1001/jama.2020.1585 | pmc=7042881 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32031570 }} </ref> | *[[Lung]] biopsy shows [[fibrin]] deposition.<ref name="pmidPMID: 32329246">{{cite journal| author=Whyte CS, Morrow GB, Mitchell JL, Chowdary P, Mutch NJ| title=Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. | journal=J Thromb Haemost | year= 2020 | volume= | issue= | pages= | pmid=PMID: 32329246 | doi=10.1111/jth.14872 | pmc=7264738 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32329246 }} </ref><ref name="pmidPMID: 32031570">{{cite journal| author=Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J | display-authors=etal| title=Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. | journal=JAMA | year= 2020 | volume= | issue= | pages= | pmid=PMID: 32031570 | doi=10.1001/jama.2020.1585 | pmc=7042881 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32031570 }} </ref> | ||
[[File:Covid-19-pneumonia-ards-45.jpg|thumb|300px|none|Multifocal ground glass, mainly in the periphery of both lungs. | [[File:Covid-19-pneumonia-ards-45.jpg|thumb|300px|none|Multifocal ground glass, mainly in the periphery of both lungs. [https://radiopaedia.org/cases/covid-19-pneumonia-45?lang=us Source: Dr. Elshan Abdullayev]]] | ||
==Treatment== | ==Treatment== | ||
| Line 245: | Line 245: | ||
* Studies have shown that in [[ARDS]], [[conservative]] [[fluid management]] may help [[patients]] by reducing edema formation.<ref name="pmidPMID 16714767">{{cite journal| author=National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D | display-authors=etal| title=Comparison of two fluid-management strategies in acute lung injury. | journal=N Engl J Med | year= 2006 | volume= 354 | issue= 24 | pages= 2564-75 | pmid=PMID 16714767 | doi=10.1056/NEJMoa062200 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16714767 }} [https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=&cmd=prlinks&id=17080981 Review in: ACP J Club. 2006 Nov-Dec;145(3):69] </ref><ref name="pmidPMID 25599463">{{cite journal| author=Grissom CK, Hirshberg EL, Dickerson JB, Brown SM, Lanspa MJ, Liu KD | display-authors=etal| title=Fluid management with a simplified conservative protocol for the acute respiratory distress syndrome*. | journal=Crit Care Med | year= 2015 | volume= 43 | issue= 2 | pages= 288-95 | pmid=PMID 25599463 | doi=10.1097/CCM.0000000000000715 | pmc=4675623 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=25599463 }} </ref> | * Studies have shown that in [[ARDS]], [[conservative]] [[fluid management]] may help [[patients]] by reducing edema formation.<ref name="pmidPMID 16714767">{{cite journal| author=National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D | display-authors=etal| title=Comparison of two fluid-management strategies in acute lung injury. | journal=N Engl J Med | year= 2006 | volume= 354 | issue= 24 | pages= 2564-75 | pmid=PMID 16714767 | doi=10.1056/NEJMoa062200 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16714767 }} [https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=&cmd=prlinks&id=17080981 Review in: ACP J Club. 2006 Nov-Dec;145(3):69] </ref><ref name="pmidPMID 25599463">{{cite journal| author=Grissom CK, Hirshberg EL, Dickerson JB, Brown SM, Lanspa MJ, Liu KD | display-authors=etal| title=Fluid management with a simplified conservative protocol for the acute respiratory distress syndrome*. | journal=Crit Care Med | year= 2015 | volume= 43 | issue= 2 | pages= 288-95 | pmid=PMID 25599463 | doi=10.1097/CCM.0000000000000715 | pmc=4675623 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=25599463 }} </ref> | ||
* Conservative [[fluid]] [[management]] with [[buffered]] or [[non-buffered]] [[crystalloid]] is recommended for [[ARDS]] [[patients]]. | * Conservative [[fluid]] [[management]] with [[buffered]] or [[non-buffered]] [[crystalloid]] is recommended for [[ARDS]] [[patients]]. | ||
* The [[conservative]] [[fluid]] strategy results in an increased number of [[ventilator]]-free days and a decreased length of [[ICU]] stay. However, | * The [[conservative]] [[fluid]] strategy results in an increased number of [[ventilator]]-free days and a decreased length of [[ICU]] stay. However, the effect on [[mortality]] remains uncertain.<ref name="pmidPMID 27734109">{{cite journal| author=Silversides JA, Major E, Ferguson AJ, Mann EE, McAuley DF, Marshall JC | display-authors=etal| title=Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. | journal=Intensive Care Med | year= 2017 | volume= 43 | issue= 2 | pages= 155-170 | pmid=PMID 27734109 | doi=10.1007/s00134-016-4573-3 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27734109 }} </ref> | ||
==== Corticosteroids ==== | ==== Corticosteroids ==== | ||
* Recent [[studies]] have shown that the [[corticosteroid]] [[dexamethasone]] may reduce [[mortality]] | * Recent [[studies]] have shown that the [[corticosteroid]] [[dexamethasone]] may reduce [[mortality]] in severe [[COVID-19]] [[patients]].<ref name="pmidPMID: 32551464">{{cite journal| author=Theoharides TC, Conti P| title=Dexamethasone for COVID-19? Not so fast. | journal=J Biol Regul Homeost Agents | year= 2020 | volume= 34 | issue= 3 | pages= | pmid=PMID: 32551464 | doi=10.23812/20-EDITORIAL_1-5 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32551464 }} </ref> | ||
*In England, a non-peer-reviewed [[randomized]] [[trial]] was issued as a press release which suggested that [[dexamethasone]] has a potential survival benefit in [[hospitalized]] [[COVID-19]] [[patients]] requiring [[oxygen]].<ref>{{cite web |url=https://www.gov.uk/government/news/world-first-coronavirus-treatment-approved-for-nhs-use-by-government |title=World first coronavirus treatment approved for NHS use by government - GOV.UK |format= |work= |accessdate=}}</ref> | *In England, a non-peer-reviewed [[randomized]] [[trial]] was issued as a press release which suggested that [[dexamethasone]] has a potential survival benefit in [[hospitalized]] [[COVID-19]] [[patients]] requiring [[oxygen]].<ref>{{cite web |url=https://www.gov.uk/government/news/world-first-coronavirus-treatment-approved-for-nhs-use-by-government |title=World first coronavirus treatment approved for NHS use by government - GOV.UK |format= |work= |accessdate=}}</ref> | ||
* The Society of Critical Care Medicine (SCCM) provided a weak conditional [[recommendation]] in the favor of [[glucocorticoids]] in [[patients]] with [[COVID-19]] who have severe [[ARDS]] with a [[partial]] [[arterial pressure]] of [[oxygen]]/[[fraction]] of [[inspired]] [[oxygen]] [[PaO2]]:[[FiO2]]] <100 mmHg). This recommendation suggests benefit in [[patients]] with moderate to severe [[ARDS]] which is [[refractory]] to low [[tidal volume]] [[ventilation]].<ref name="pmidPMID 28940011">{{cite journal| author=Annane D, Pastores SM, Rochwerg B, Arlt W, Balk RA, Beishuizen A | display-authors=etal| title=Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. | journal=Intensive Care Med | year= 2017 | volume= 43 | issue= 12 | pages= 1751-1763 | pmid=PMID 28940011 | doi=10.1007/s00134-017-4919-5 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28940011 }} </ref> | * The Society of Critical Care Medicine (SCCM) provided a weak conditional [[recommendation]] in the favor of [[glucocorticoids]] in [[patients]] with [[COVID-19]] who have severe [[ARDS]] with a [[partial]] [[arterial pressure]] of [[oxygen]]/[[fraction]] of [[inspired]] [[oxygen]] [[PaO2]]:[[FiO2]]] <100 mmHg). This recommendation suggests benefit in [[patients]] with moderate to severe [[ARDS]] which is [[refractory]] to low [[tidal volume]] [[ventilation]].<ref name="pmidPMID 28940011">{{cite journal| author=Annane D, Pastores SM, Rochwerg B, Arlt W, Balk RA, Beishuizen A | display-authors=etal| title=Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. | journal=Intensive Care Med | year= 2017 | volume= 43 | issue= 12 | pages= 1751-1763 | pmid=PMID 28940011 | doi=10.1007/s00134-017-4919-5 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28940011 }} </ref> | ||
| Line 266: | Line 266: | ||
* The best way to prevent being infected by COVID-19 is to avoid being exposed to this [[virus]] by adopting the following practices for [[infection]] control: | * The best way to prevent being infected by COVID-19 is to avoid being exposed to this [[virus]] by adopting the following practices for [[infection]] control: | ||
** | ** Wash hands often with [[soap]] and [[water]] for at least 20 seconds. | ||
** Use an [[Hand sanitizer|alcohol-based hand sanitizer]] containing at least 60% [[alcohol]] in case [[soap]] and [[water]] are not available. | ** Use an [[Hand sanitizer|alcohol-based hand sanitizer]] containing at least 60% [[alcohol]] in case [[soap]] and [[water]] are not available. | ||
** Avoid touching the [[Eye|eyes]], [[nose]], and [[mouth]] without washing hands. | ** Avoid touching the [[Eye|eyes]], [[nose]], and [[mouth]] without washing hands. | ||
| Line 279: | Line 279: | ||
* The secondary prevention measures of Coronavirus disease 2019 (COVID-19) constitute protective measures to make sure that an infected individual does not transfer the disease to others by maintaining self-isolation at home or designated [[quarantine]] facilities. | * The secondary prevention measures of Coronavirus disease 2019 (COVID-19) constitute protective measures to make sure that an infected individual does not transfer the disease to others by maintaining self-isolation at home or designated [[quarantine]] facilities. | ||
* The [[ARDS]] [[patients]] have an increased [[risk]] of [[hospital]]-associated [[venous thromboembolism]] ([[VTE]]).<ref>{{cite web |url=https://b-s-h.org.uk/media/18171/th-and-covid-25-march-2020-final.pdf |title=b-s-h.org.uk |format= |work= |accessdate=}}</ref> | * The [[ARDS]] [[patients]] have an increased [[risk]] of [[hospital]]-associated [[venous thromboembolism]] ([[VTE]]).<ref>{{cite web |url=https://b-s-h.org.uk/media/18171/th-and-covid-25-march-2020-final.pdf |title=b-s-h.org.uk |format= |work= |accessdate=}}</ref> | ||
* | * It is advised that patients with no contraindications take [[low molecular weight heparin]] ([[LMWH]]) [[prophylactically]]. Studies have shown that the [[heparin]], either unfractionated or [[LMWH]], can also reduce [[inflammatory]] [[biomarkers]] and could help in reducing the [[inflammation]].<ref name="pmidPMID: 32338827">{{cite journal| author=Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M | display-authors=etal| title=ISTH interim guidance on recognition and management of coagulopathy in COVID-19. | journal=J Thromb Haemost | year= 2020 | volume= 18 | issue= 5 | pages= 1023-1026 | pmid=PMID: 32338827 | doi=10.1111/jth.14810 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32338827 }} </ref> | ||
==References== | ==References== | ||
{{Reflist|2}} | {{Reflist|2}} | ||
[[Category:Up-To-Date]] | |||
{{WikiDoc Help Menu}} | |||
{{WikiDoc Sources}} | |||
Latest revision as of 18:26, 29 July 2020
For COVID-19 frequently asked inpatient questions, click here
For COVID-19 frequently asked outpatient questions, click here
For COVID-19 patient information, click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ayesha Javid, MBBS[2] Usman Ali Akbar, M.B.B.S.[3]
Overview
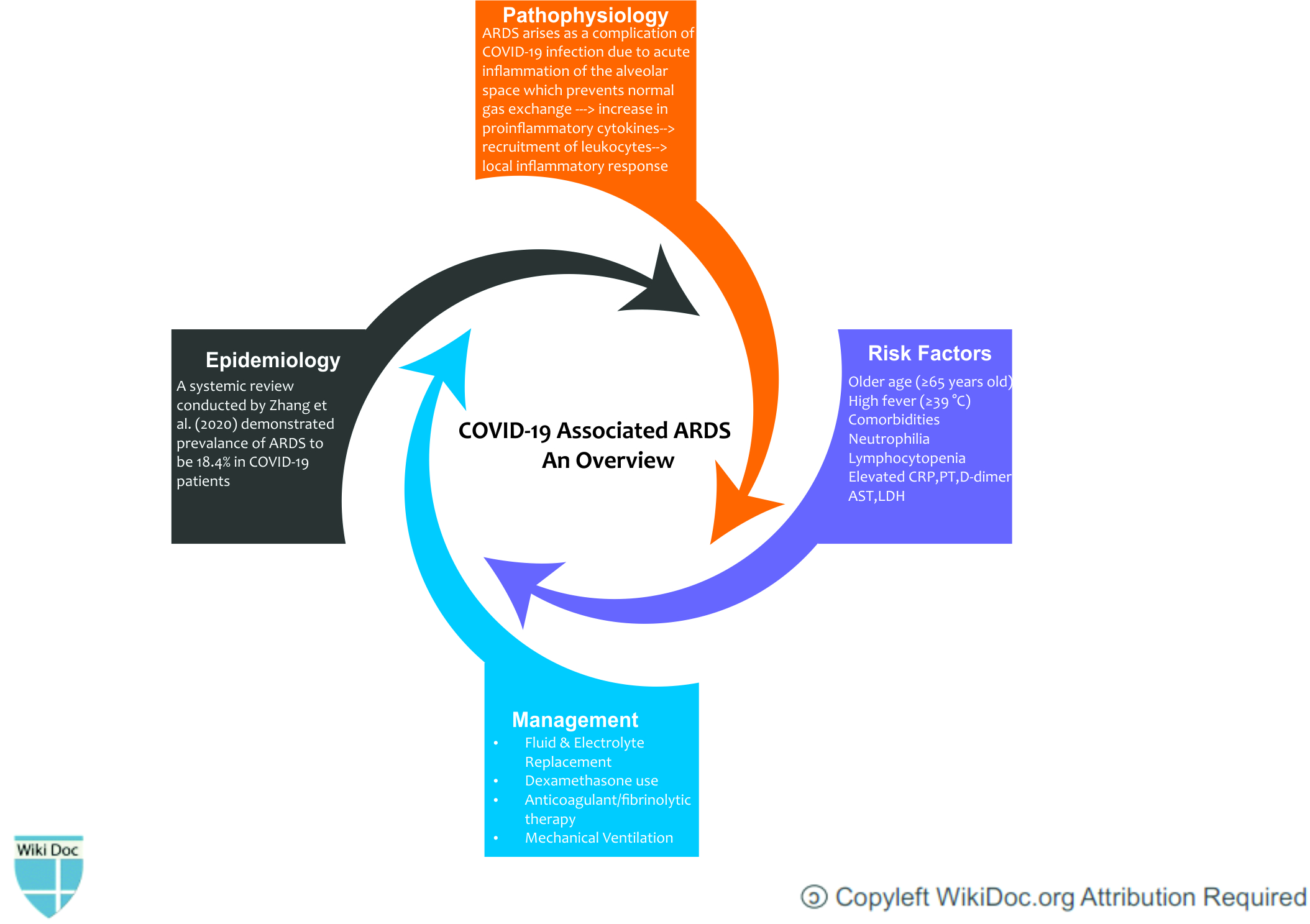
Acute Respiratory Distress Syndrome (ARDS) is a form of non-cardiogenic pulmonary edema attributed to the breakdown of alveolar membrane and capillaries leading to hypoxia. The treatment of ARDS has evolved over the last decade. The management of COVID-19 related ARDS are stratified according to the classification of ARDS. Among patients with COVID-19, ARDS developed in 20% after a median of eight days after onset of symptoms; mechanical ventilation was implemented in 12.3%. The mortality rate of COVID-19 related ARDS is higher in elderly patients. Given the importance of heterogeneity in the ARDS profile, appropriate intervention at an appropriate time is needed to help to prevent the deterioration of lung function. Recent advances observed from the RECOVERY trial has further strengthened the notion that the use of dexamethasone in patients on a ventilator can reduce the mortality rate of patients by one-third. The treatment of COVID-19 related ARDS is evolving with time and different treatment options are now available for the management of ARDS.
Historical Perspective
- In 2002–2003, the novel coronavirus was named as the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) due to its high similarity to SARS-CoV, which caused acute respiratory distress syndrome (ARDS).
- SARS-CoV-2 virus primarily affects the respiratory system causing a wide variety of respiratory symptoms which can range from symptoms of lower respiratory tract infection to severe hypoxia to acute respiratory distress syndrome within a very short span of time.
- The acute respiratory distress syndrome (ARDS) is a common cause of morbidity and mortality in critically ill COVID-19 infected patients. It is defined by the acute onset of noncardiogenic pulmonary edema, hypoxemia and the need for mechanical ventilation.
Classification
Several authors in a case report highlighted the nonuniformity of patients with COVID-19-associated ARDS and proposed the existence of two primary phenotypes:[1]
- Type L (low values of elastance, pulmonary ventilation/ perfusion ratio, lung weight, and recruitability).
- Type H (high values of elastance, right-to-left shunt, lung weight, and recruitability), more consistent with typical severe ARDS.
Pathophysiology
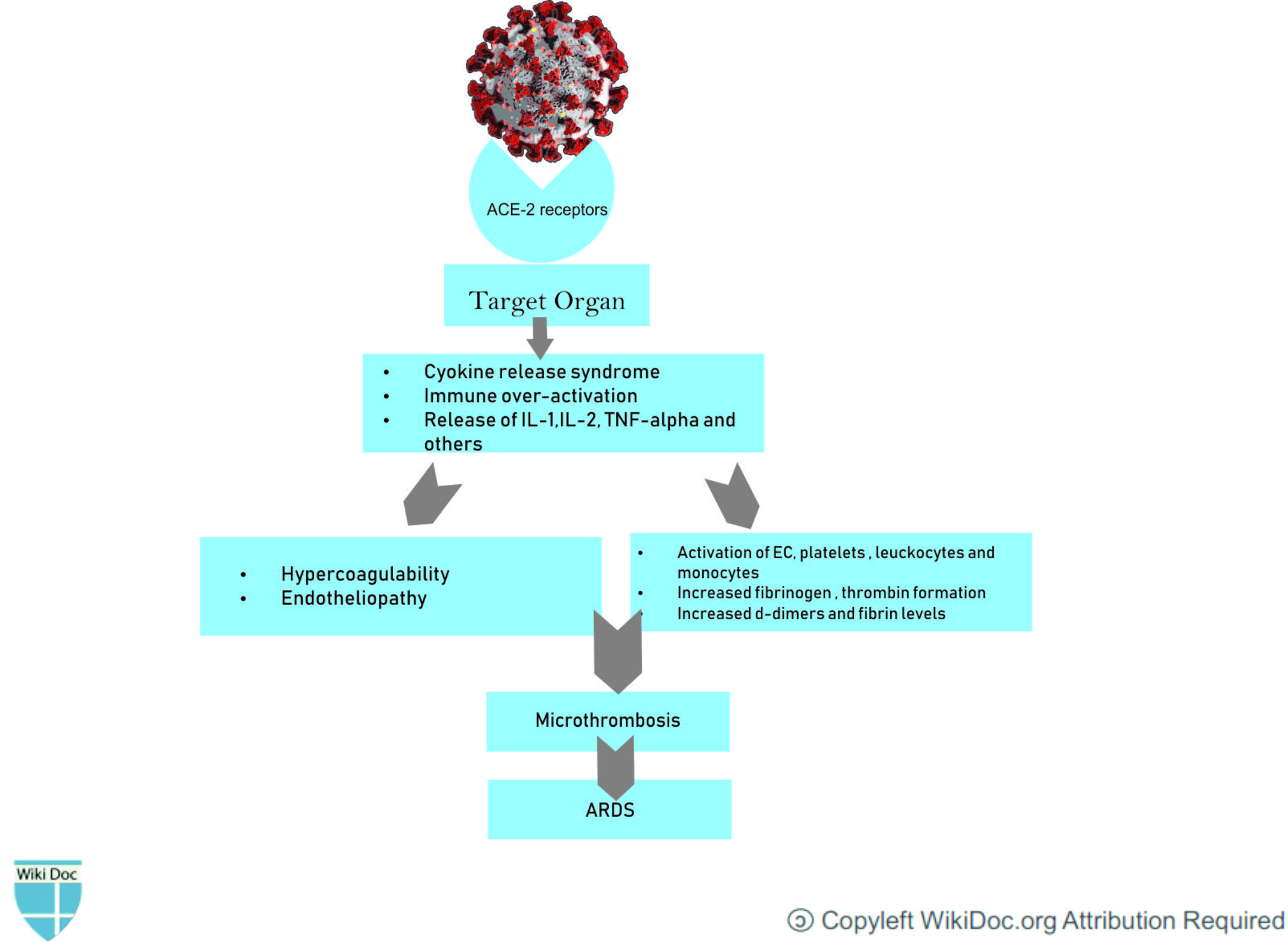
- ARDS arises as a complication of COVID-19 infection due to acute inflammation of the alveolar space which prevents normal gas exchange. The increase in proinflammatory cytokines within the lung leads to recruitment of leukocytes, further propagating the local inflammatory response.[2]
- The cytokine storm and the deadly uncontrolled systemic inflammatory response is caused by the release of large amounts of proinflammatory cytokines including interferons and interleukins and, chemokines by immune effector cells. This results in acute inflammation within the alveolar space. Furthermore, the exudate containing plasma proteins including: albumin, fibrinogen, proinflammatory cytokines and coagulation factors will increase alveolar-capillary permeability and decrease the normal gas exchangeasma proteinsalbumin, fibrinogen[3]
- COVID-19 patients with ARDS show elevated levels of IL-6, IFN-a, and CCL5, CXCL8, CXCL-10 in serum as compared to those with the mild to moderate disease.[4]
- This inflammatory process leads to the fibrin deposition in the air spaces and lung parenchyma and contributes to hyaline-membrane formation and subsequent alveolar fibrosis.[5]
- Patients infected with COVID‐19 also exhibit coagulation abnormalities.This procoagulant pattern can lead to acute respiratory distress syndrome.[6]
Clinical Features
- Clinical presentations of COVID-19 range from asymptomatic (81.4%), through mildly symptomatic with or without seasonal flu-like symptoms, to severe pneumonia (13.9%).[7]
- Respiratory problems manifest as dyspnea that range from effort dyspnea to dyspnea occurring at rest.
- Patients with dyspnea can revert to an asymptomatic phase or progress to ARDS, requiring positive pressure oxygen therapy and intensive care therapy [18] in 17–19.6% of symptomatic patients.[8]
Differentiating COVID-associated ARDS from other Diseases
COVID-19 associated ARDS must be differentiated from other diseases that also cause ARDS by the following parameters:
- Positive SARS-CoV-2 infection on PCR
- COVID-19 ARDS causes the typical ARDS pathological changes of diffuse alveolar damage in the lung.[9]
- As patients move through the course of their illness, the longer term outcomes of ARDS are being reported, with lung fibrosis appearing as part of COVID-19 ARDS.[10]
- Pulmonary thrombosis is also associated with COVID-19 related ARDS.
- Deaths from COVID-19 associated ARDS have been reported due to thrombotic disseminated intravascular coagulation.[11]
Epidemiology and Demographics
| Date of publication | Country | Author | Total Number of patients | Prevalence |
|---|---|---|---|---|
| January 24,2020 | China | Huang C et al.[12] | 41 | 29.26 %
(16.13-45.53) |
| January 29,2020 | China | Nanshan Chen et al.[13] | 99 |
17.17 %
(10.33-26.06) |
| March 13, 2020 | China | Chaomin Wu et al.[14] | 201 | 41.8 % |
| May 14, 2020 | Multiple | Zhang et al.[15] | 4203 | 18.4% |
Age
- Patients of all age groups may develop ARDS.
- It is more commonly observed among patients aged ≥65 years old.
Gender
- Some case studies report that men are more commonly affected by ARDS than women.
- In a Chinese public data set, the number of men who died from COVID-19 is 2.4 times that of women (70.3 vs. 29.7%, P = 0.016).[16]
Race
- A large study in the United States reported that that African Americans were at a higher risk of ARDS than white individuals.[17]
Risk Factors
- In a retrospective study conducted in China, following risk factors were the main predisposing factors for the development of ARDS:[14]
- Older age (≥65 years old)
- High fever (≥39 °C)
- Comorbidities (eg, hypertension, diabetes)
- Neutrophilia
- Lymphocytopenia (as well as lower CD3 and CD4 T-cell counts)
- Elevated end-organ related indices (eg, AST, urea, LDH)
- Elevated inflammation-related indices (high-sensitivity C-reactive protein and serum ferritin)
- Elevated coagulation function–related indicators (PT and D-dimer)
Natural History, Complications and Prognosis
Natural History
The natural history of ARDS is hallmarked by three histopathological phases—exudative, proliferative, and fibrotic phase—each correlated to distinctive clinical manifestations.[18]
| Exudative Phase | Proliferative Phase | Fibrotic Phase |
|---|---|---|
|
|
|
Complications
Common complications include:
- ARDS is complicated by VAP in approximately 37% to 60% of cases.[21][22][23][24]
- VAP usually develops 5 to 7 days after the initial exposure to the precipitating factor.[25]
- The most likely microorganisms of VAP includes non-fermenting Gram-negative bacilli, methicillin-resistant Staphylococcus aureus, and Enterobacteriaceae.[26]
- Barotrauma (e.g., pneumothorax, pneumomediastinum, and subcutaneous emphysema)
- Barotrauma occurs as a consequence of inappropriate positive airway pressure in regions with reduced pulmonary compliance and may complicate ARDS in approximately 10% of cases.[27][28][29]
Other complications include:
- Significant weakness due to critical illness myoneuropathy and muscle atrophy as a result of long-term immobilization
- Impaired lung function
- Chronic ventilator dependency due to advanced weakness and atrophy of the muscles of respiration
- Pulmonary fibrosis and restrictive lung disease
- Psychiatric illness, including post-traumatic stress disorder (PTSD), anxiety, and depression
- Impaired cognition
- Persistent vegetative state or brain death due to prolonged hypoxemia
Prognosis
- The survival rate for patients with COVID-19 with ARDS is approximately 25%.[30]
- Factors associated with increased mortality in patients with COVID-19 pneumonia included age ≥65 years, presence of cardiovascular or cerebrovascular disease, lymphopenia, and elevation in troponin I levels.[31]
- Despite major progress in the care of patients with ARDS, survivors are at high risk for cognitive decline, depression, post-traumatic stress disorder, and physical deconditioning.[32]
Diagnosis
Diagnostic Criteria
- COVID-19 ARDS is diagnosed when someone with confirmed COVID-19 infection meets the Berlin 2012 ARDS diagnostic criteria of:[33]
- Acute hypoxemic respiratory failure.
- Presentation within 1 week of worsening respiratory symptoms.
- Bilateral airspace disease on chest x-ray, computed tomography (CT) or ultrasound that is not fully explained by effusions, lobar or lung collapse, or nodules.
- Cardiac failure is not the primary cause of acute hypoxemic respiratory failure.
Symptoms
Physical Examination
- The physical exam findings of a patient with ARDS vary according to the underlying cause and typically develop within 24 to 48 hours of the precipitating illness or injury and progress over the course of 1 to 2 weeks. However, ARDS related to COVID-19 has sometimes late-onset. Common physical findings include:
Vital Signs
- The presence of the following signs of shock or infection on physical examination is highly suggestive of ARDS:
- Temperature (Temp, T): Hyperpyrexia ≥ 38°C or 100.4°F) or low temperature < 36°C or 96.8°F.
- Blood pressure (BP): inappropriately low, with a low mean arterial pressure (MAP).
- Heart rate (HR): rapid (tachycardia > 100 beats/minute), normal, or slow (bradycardia <60 beats/minute).
- Respiratory rate (RR): Tachypnea > 20 breaths/minute or bradypnea <12 breaths/minute.
- Peripheral capillary oxygen saturation (SpO2): low (< 90% on ambient air or a fraction of inspired oxygen, (FIO2) of 21% at sea level).
Skin
- Cyanosis due to poor oxygenation.
- Pallor due to poor perfusion.
Lungs
- Tachypnea
- Dyspnea
- Coarse breath sounds, rhonchi, crackles, or decreased breath sounds
Heart
- Tachycardia or bradycardia on heart auscultation.
Extremities
- Cyanosis
- Cool extremities or reduced peripheral pulses due to poor perfusion.
Laboratory Findings
- Blood plasma has elevated levels of IL-6, IL-1, tumour necrosis factor-α (TNF α) and C-reactive protein.[38]
- Thrombocytopenia.[39]
- Increased D-dimer levels. The elevated level of D-dimer is strongly associated with a higher mortality rate.[40]
- Increased fibrin degradation products.[40]
- Increased fibrinogen.[40][41]
- Prothrombin time and activated partial thromboplastin time may be slightly elevated.[40]
Imaging Findings
Chest-X ray
On Chest X-ray following findings can be seen.
- Ground-glass opacification and consolidation
- Early findings on the chest radiograph include normal or diffuse alveolar opacities (consolidation), which are often bilateral and which obscure the pulmonary vascular markings.
- Later, these opacities progress to more extensive consolidation that is diffuse, and they are often asymmetrical.

Chest CT-Scan
- Chest CT scan shows characteristic ground-glass opacities (GCO). This indicates the presence of exudate in the bronchoalveolar airspace.[2]
- Lung biopsy shows fibrin deposition.[2][42]

Treatment
Medical Therapy
Fluid and electrolytes management
- Studies have shown that in ARDS, conservative fluid management may help patients by reducing edema formation.[43][44]
- Conservative fluid management with buffered or non-buffered crystalloid is recommended for ARDS patients.
- The conservative fluid strategy results in an increased number of ventilator-free days and a decreased length of ICU stay. However, the effect on mortality remains uncertain.[45]
Corticosteroids
- Recent studies have shown that the corticosteroid dexamethasone may reduce mortality in severe COVID-19 patients.[46]
- In England, a non-peer-reviewed randomized trial was issued as a press release which suggested that dexamethasone has a potential survival benefit in hospitalized COVID-19 patients requiring oxygen.[47]
- The Society of Critical Care Medicine (SCCM) provided a weak conditional recommendation in the favor of glucocorticoids in patients with COVID-19 who have severe ARDS with a partial arterial pressure of oxygen/fraction of inspired oxygen PaO2:FiO2] <100 mmHg). This recommendation suggests benefit in patients with moderate to severe ARDS which is refractory to low tidal volume ventilation.[48]
Mechanical Ventilation
- Mechanical ventilation along with supportive therapies are the mainstay of treatment of ARDS.[49]
- Invasive mechanical ventilation (ie, ventilation via an endotracheal tube or tracheostomy with breaths delivered by a mechanical ventilator) is preferred for patients with ARDS, particularly those with moderate or severe ARDS (ie, arterial oxygen tension/fraction of inspired oxygen PaO2/FiO2 ≤200 mmHg on positive end-expiratory pressure (PEEP) ≥5 cm H2O).[50][8]
- It is recommended to use low tidal volume ventilation (LTVV) with 4 to 8 mL/kg predicted body weight [PBW]. Several meta-analyses and randomized trials that report a mortality benefit from LTVV in patients with ARDS.[51]
- The aim is to maintain oxygen saturation between 90% to 96%. The severe hypoxemia of the COVID-19 ARDS best responds when Positive end-expiratory pressure (PEEP) is high with Pplat ≤30 cm H2O. It is beneficial if the physician starts with higher than usual levels of PEEP (10 to 15 cm H2O).[52]
Anticoagulant or thrombolytic therapy
- Fibrinolytic drugs such as tissue-type plasminogen activator (tPA) degrade pre-existing fibrin in the lungs.[40]
- Nebulizer plasminogen activators may provide more targeted therapy to degrade fibrin and improving oxygenation in critically ill patients. It is in Phase II of the clinical trial.
Prevention
Primary Prevention
- The best way to prevent being infected by COVID-19 is to avoid being exposed to this virus by adopting the following practices for infection control:
- Wash hands often with soap and water for at least 20 seconds.
- Use an alcohol-based hand sanitizer containing at least 60% alcohol in case soap and water are not available.
- Avoid touching the eyes, nose, and mouth without washing hands.
- Avoid being in close contact with people sick with COVID-19 infection.
- Stay home while being symptomatic to prevent spread to others.
- Cover mouth while coughing or sneezing with a tissue paper, and then throw the tissue in the trash.
- Clean and disinfect the objects and surfaces which are touched frequently.
- There is currently no vaccine available to prevent COVID-19.
Secondary Prevention
- The secondary prevention measures of Coronavirus disease 2019 (COVID-19) constitute protective measures to make sure that an infected individual does not transfer the disease to others by maintaining self-isolation at home or designated quarantine facilities.
- The ARDS patients have an increased risk of hospital-associated venous thromboembolism (VTE).[53]
- It is advised that patients with no contraindications take low molecular weight heparin (LMWH) prophylactically. Studies have shown that the heparin, either unfractionated or LMWH, can also reduce inflammatory biomarkers and could help in reducing the inflammation.[54]
References
- ↑ Gattinoni, Luciano; Chiumello, Davide; Caironi, Pietro; Busana, Mattia; Romitti, Federica; Brazzi, Luca; Camporota, Luigi (2020-04-14). "COVID-19 pneumonia: different respiratory treatments for different phenotypes?". Intensive Care Medicine. Springer Science and Business Media LLC. 46 (6): 1099–1102. doi:10.1007/s00134-020-06033-2. ISSN 0342-4642.
- ↑ 2.0 2.1 2.2 Whyte CS, Morrow GB, Mitchell JL, Chowdary P, Mutch NJ (2020). "Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19". J Thromb Haemost. doi:10.1111/jth.14872. PMC 7264738 Check
|pmc=value (help). PMID 32329246 PMID: 32329246 Check|pmid=value (help). - ↑ Meduri GU, Annane D, Chrousos GP, Marik PE, Sinclair SE (2009). "Activation and regulation of systemic inflammation in ARDS: rationale for prolonged glucocorticoid therapy". Chest. 136 (6): 1631–1643. doi:10.1378/chest.08-2408. PMID 19801579 PMID: 19801579 Check
|pmid=value (help). - ↑ Tezer H, Bedir Demirdağ T (2020). "Novel coronavirus disease (COVID-19) in children". Turk J Med Sci. 50 (SI-1): 592–603. doi:10.3906/sag-2004-174. PMC 7195991 Check
|pmc=value (help). PMID 32304191 PMID: 32304191 Check|pmid=value (help). - ↑ Bertozzi P, Astedt B, Zenzius L, Lynch K, LeMaire F, Zapol W; et al. (1990). "Depressed bronchoalveolar urokinase activity in patients with adult respiratory distress syndrome". N Engl J Med. 322 (13): 890–7. doi:10.1056/NEJM199003293221304. PMID 2314423 PMID: 2314423 Check
|pmid=value (help). - ↑ Ranucci M, Ballotta A, Di Dedda U, Bayshnikova E, Dei Poli M, Resta M; et al. (2020). "The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome". J Thromb Haemost. doi:10.1111/jth.14854. PMID 32302448 PMID: 32302448 Check
|pmid=value (help). - ↑ Borges do Nascimento, Israel Júnior; Cacic, Nensi; Abdulazeem, Hebatullah Mohamed; von Groote, Thilo Caspar; Jayarajah, Umesh; Weerasekara, Ishanka; Esfahani, Meisam Abdar; Civile, Vinicius Tassoni; Marusic, Ana; Jeroncic, Ana; Carvas Junior, Nelson; Pericic, Tina Poklepovic; Zakarija-Grkovic, Irena; Meirelles Guimarães, Silvana Mangeon; Luigi Bragazzi, Nicola; Bjorklund, Maria; Sofi-Mahmudi, Ahmad; Altujjar, Mohammad; Tian, Maoyi; Arcani, Diana Maria Cespedes; O’Mathúna, Dónal P.; Marcolino, Milena Soriano (2020-03-30). "Novel Coronavirus Infection (COVID-19) in Humans: A Scoping Review and Meta-Analysis". Journal of Clinical Medicine. MDPI AG. 9 (4): 941. doi:10.3390/jcm9040941. ISSN 2077-0383.
- ↑ 8.0 8.1 Wang, Dawei; Hu, Bo; Hu, Chang; Zhu, Fangfang; Liu, Xing; Zhang, Jing; Wang, Binbin; Xiang, Hui; Cheng, Zhenshun; Xiong, Yong; Zhao, Yan; Li, Yirong; Wang, Xinghuan; Peng, Zhiyong (2020-03-17). "Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China". JAMA. American Medical Association (AMA). 323 (11): 1061. doi:10.1001/jama.2020.1585. ISSN 0098-7484.
- ↑ Tian, Sufang; Xiong, Yong; Liu, Huan; Niu, Li; Guo, Jianchun; Liao, Meiyan; Xiao, Shu-Yuan (2020-04-14). "Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies". Modern Pathology. Springer Science and Business Media LLC. 33 (6): 1007–1014. doi:10.1038/s41379-020-0536-x. ISSN 0893-3952.
- ↑ Chen, Jing-Yu; Qiao, Kun; Liu, Feng; Wu, Bo; Xu, Xin; Jiao, Guo-Qing; Lu, Rong-Guo; Li, Hui-Xing; Zhao, Jin; Huang, Jian; Yang, Yi; Lu, Xiao-Jie; Li, Jia-Shu; Jiang, Shu-Yun; Wang, Da-Peng; Hu, Chun-Xiao; Wang, Gui-Long; Huang, Dong-Xiao; Jiao, Guo-Hui; Wei, Dong; Ye, Shu-Gao; Huang, Jian-An; Zhou, Li; Zhang, Xiao-Qin; He, Jian-Xing (2020-04-01). "Lung transplantation as therapeutic option in acute respiratory distress syndrome for COVID-19-related pulmonary fibrosis". Chinese Medical Journal. Ovid Technologies (Wolters Kluwer Health). Publish Ahead of Print. doi:10.1097/cm9.0000000000000839. ISSN 0366-6999.
- ↑ Wang, Janice; Hajizadeh, Negin; Moore, Ernest E.; McIntyre, Robert C.; Moore, Peter K.; Veress, Livia A.; Yaffe, Michael B.; Moore, Hunter B.; Barrett, Christopher D. (2020-05-11). "Tissue plasminogen activator (tPA) treatment for COVID‐19 associated acute respiratory distress syndrome (ARDS): A case series". Journal of Thrombosis and Haemostasis. Wiley. 18 (7): 1752–1755. doi:10.1111/jth.14828. ISSN 1538-7933.
- ↑ "Redirecting". Page Redirection. Retrieved 2020-07-15.
- ↑ Chen, Nanshan; Zhou, Min; Dong, Xuan; Qu, Jieming; Gong, Fengyun; Han, Yang; Qiu, Yang; Wang, Jingli; Liu, Ying; Wei, Yuan; Xia, Jia'an; Yu, Ting; Zhang, Xinxin; Zhang, Li (2020). "Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study". The Lancet. Elsevier BV. 395 (10223): 507–513. doi:10.1016/s0140-6736(20)30211-7. ISSN 0140-6736.
- ↑ 14.0 14.1 Wu, Chaomin; Chen, Xiaoyan; Cai, Yanping; Xia, Jia’an; Zhou, Xing; Xu, Sha; Huang, Hanping; Zhang, Li; Zhou, Xia; Du, Chunling; Zhang, Yuye; Song, Juan; Wang, Sijiao; Chao, Yencheng; Yang, Zeyong; Xu, Jie; Zhou, Xin; Chen, Dechang; Xiong, Weining; Xu, Lei; Zhou, Feng; Jiang, Jinjun; Bai, Chunxue; Zheng, Junhua; Song, Yuanlin (2020-07-01). "Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China". JAMA Internal Medicine. American Medical Association (AMA). 180 (7): 934. doi:10.1001/jamainternmed.2020.0994. ISSN 2168-6106.
- ↑ Zhang, John J Y; Lee, Keng Siang; Ang, Li Wei; Leo, Yee Sin; Young, Barnaby Edward (2020-05-14). "Risk Factors of Severe Disease and Efficacy of Treatment in Patients Infected with COVID-19: A Systematic Review, Meta-Analysis and Meta-Regression Analysis". Clinical Infectious Diseases. Oxford University Press (OUP). doi:10.1093/cid/ciaa576. ISSN 1058-4838.
- ↑ Jin, Jian-Min; Bai, Peng; He, Wei; Wu, Fei; Liu, Xiao-Fang; Han, De-Min; Liu, Shi; Yang, Jin-Kui (2020-04-29). "Gender Differences in Patients With COVID-19: Focus on Severity and Mortality". Frontiers in Public Health. Frontiers Media SA. 8. doi:10.3389/fpubh.2020.00152. ISSN 2296-2565.
- ↑ Template:Cite
- ↑ Ware, Lorraine B. “Autopsy in ARDS: Insights into Natural History.” The Lancet. Respiratory Medicine 1, no. 5 (July 2013): 352–54. doi:10.1016/S2213-2600(13)70093-6.
- ↑ National Heart and Lung Institute. Task Force on Research in Respiratory Diseases, and National Heart and Lung Institute. Lung Program. Respiratory Diseases; Task Force Report on Problems, Research Approaches, Needs. The Lung Program, National Heart and Lung Institute. [Bethesda, Md., U.S. Dept. of Health, Education, and Welfare, National Institutes of Health] for sale by the Supt. of Docs., U.S. Govt. Print. Off., Washington, 1972. http://archive.org/details/respiratorydisea00nati.
- ↑ Thille, Arnaud W., Andrés Esteban, Pilar Fernández-Segoviano, José-María Rodriguez, José-Antonio Aramburu, Patricio Vargas-Errázuriz, Ana Martín-Pellicer, José A. Lorente, and Fernando Frutos-Vivar. “Chronology of Histological Lesions in Acute Respiratory Distress Syndrome with Diffuse Alveolar Damage: A Prospective Cohort Study of Clinical Autopsies.” The Lancet. Respiratory Medicine 1, no. 5 (July 2013): 395–401. doi:10.1016/S2213-2600(13)70053-5.
- ↑ Delclaux, C., E. Roupie, F. Blot, L. Brochard, F. Lemaire, and C. Brun-Buisson. “Lower Respiratory Tract Colonization and Infection during Severe Acute Respiratory Distress Syndrome: Incidence and Diagnosis.” American Journal of Respiratory and Critical Care Medicine 156, no. 4 Pt 1 (October 1997): 1092–98. doi:10.1164/ajrccm.156.4.9701065.
- ↑ Markowicz, P., M. Wolff, K. Djedaïni, Y. Cohen, J. Chastre, C. Delclaux, J. Merrer, et al. “Multicenter Prospective Study of Ventilator-Associated Pneumonia during Acute Respiratory Distress Syndrome. Incidence, Prognosis, and Risk Factors. ARDS Study Group.” American Journal of Respiratory and Critical Care Medicine 161, no. 6 (June 2000): 1942–48. doi:10.1164/ajrccm.161.6.9909122.
- ↑ Meduri, G. U., R. C. Reddy, T. Stanley, and F. El-Zeky. “Pneumonia in Acute Respiratory Distress Syndrome. A Prospective Evaluation of Bilateral Bronchoscopic Sampling.” American Journal of Respiratory and Critical Care Medicine 158, no. 3 (September 1998): 870–75. doi:10.1164/ajrccm.158.3.9706112.
- ↑ Chastre, J., J. L. Trouillet, A. Vuagnat, M. L. Joly-Guillou, H. Clavier, M. C. Dombret, and C. Gibert. “Nosocomial Pneumonia in Patients with Acute Respiratory Distress Syndrome.” American Journal of Respiratory and Critical Care Medicine 157, no. 4 Pt 1 (April 1998): 1165–72. doi:10.1164/ajrccm.157.4.9708057.
- ↑ Delclaux, C., E. Roupie, F. Blot, L. Brochard, F. Lemaire, and C. Brun-Buisson. “Lower Respiratory Tract Colonization and Infection during Severe Acute Respiratory Distress Syndrome: Incidence and Diagnosis.” American Journal of Respiratory and Critical Care Medicine 156, no. 4 Pt 1 (October 1997): 1092–98. doi:10.1164/ajrccm.156.4.9701065.
- ↑ Chastre, J., J. L. Trouillet, A. Vuagnat, M. L. Joly-Guillou, H. Clavier, M. C. Dombret, and C. Gibert. “Nosocomial Pneumonia in Patients with Acute Respiratory Distress Syndrome.” American Journal of Respiratory and Critical Care Medicine 157, no. 4 Pt 1 (April 1998): 1165–72. doi:10.1164/ajrccm.157.4.9708057.
- ↑ “Ventilation with Lower Tidal Volumes as Compared with Traditional Tidal Volumes for Acute Lung Injury and the Acute Respiratory Distress Syndrome. The Acute Respiratory Distress Syndrome Network.” The New England Journal of Medicine 342, no. 18 (May 4, 2000): 1301–8. doi:10.1056/NEJM200005043421801.
- ↑ Weg, J. G., A. Anzueto, R. A. Balk, H. P. Wiedemann, E. N. Pattishall, M. A. Schork, and L. A. Wagner. “The Relation of Pneumothorax and Other Air Leaks to Mortality in the Acute Respiratory Distress Syndrome.” The New England Journal of Medicine 338, no. 6 (February 5, 1998): 341–46. doi:10.1056/NEJM199802053380601.
- ↑ Stewart, T. E., M. O. Meade, D. J. Cook, J. T. Granton, R. V. Hodder, S. E. Lapinsky, C. D. Mazer, et al. “Evaluation of a Ventilation Strategy to Prevent Barotrauma in Patients at High Risk for Acute Respiratory Distress Syndrome. Pressure- and Volume-Limited Ventilation Strategy Group.” The New England Journal of Medicine 338, no. 6 (February 5, 1998): 355–61. doi:10.1056/NEJM199802053380603.
- ↑ Yang, Xiaobo; Yu, Yuan; Xu, Jiqian; Shu, Huaqing; Xia, Jia'an; Liu, Hong; Wu, Yongran; Zhang, Lu; Yu, Zhui; Fang, Minghao; Yu, Ting; Wang, Yaxin; Pan, Shangwen; Zou, Xiaojing; Yuan, Shiying; Shang, You (2020). "Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study". The Lancet Respiratory Medicine. Elsevier BV. 8 (5): 475–481. doi:10.1016/s2213-2600(20)30079-5. ISSN 2213-2600.
- ↑ Du, Rong-Hui; Liang, Li-Rong; Yang, Cheng-Qing; Wang, Wen; Cao, Tan-Ze; Li, Ming; Guo, Guang-Yun; Du, Juan; Zheng, Chun-Lan; Zhu, Qi; Hu, Ming; Li, Xu-Yan; Peng, Peng; Shi, Huan-Zhong (2020-04-08). "Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study". European Respiratory Journal. European Respiratory Society (ERS). 55 (5): 2000524. doi:10.1183/13993003.00524-2020. ISSN 0903-1936.
- ↑ Vittori, Alessandro; Lerman, Jerrold; Cascella, Marco; Gomez-Morad, Andrea D.; Marchetti, Giuliano; Marinangeli, Franco; Picardo, Sergio G. (2020-04-27). "COVID-19 Pandemic Acute Respiratory Distress Syndrome Survivors: Pain After the Storm?". Anesthesia & Analgesia. Ovid Technologies (Wolters Kluwer Health). 131 (1): 117–119. doi:10.1213/ane.0000000000004914. ISSN 0003-2999.
- ↑ "Acute Respiratory Distress Syndrome". JAMA. American Medical Association (AMA). 307 (23). 2012-06-20. doi:10.1001/jama.2012.5669. ISSN 0098-7484.
- ↑ Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H; et al. (2020). "Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study". Lancet Respir Med. 8 (5): 475–481. doi:10.1016/S2213-2600(20)30079-5. PMC 7102538 Check
|pmc=value (help). PMID 32105632 PMID: 32105632 Check|pmid=value (help). - ↑ Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J; et al. (2020). "Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China". JAMA. doi:10.1001/jama.2020.1585. PMC 7042881 Check
|pmc=value (help). PMID 32031570 PMID 32031570 Check|pmid=value (help). - ↑ Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D (2020). "COVID-19 Does Not Lead to a "Typical" Acute Respiratory Distress Syndrome". Am J Respir Crit Care Med. 201 (10): 1299–1300. doi:10.1164/rccm.202003-0817LE. PMC 7233352 Check
|pmc=value (help). PMID 32228035 PMID: 32228035 Check|pmid=value (help). - ↑ Repessé X, Vieillard-Baron A (2017). "Hypercapnia during acute respiratory distress syndrome: the tree that hides the forest!". J Thorac Dis. 9 (6): 1420–1425. doi:10.21037/jtd.2017.05.69. PMC 5506150. PMID 28740647 PMID: 28740647 Check
|pmid=value (help). - ↑ Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A; et al. (2019). "Acute respiratory distress syndrome". Nat Rev Dis Primers. 5 (1): 18. doi:10.1038/s41572-019-0069-0. PMC 6709677 Check
|pmc=value (help). PMID 30872586 PMID: 30872586 Check|pmid=value (help). - ↑ Lippi G, Plebani M, Henry BM (2020). "Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis". Clin Chim Acta. 506: 145–148. doi:10.1016/j.cca.2020.03.022. PMC 7102663 Check
|pmc=value (help). PMID 32178975 PMID: 32178975 Check|pmid=value (help). - ↑ 40.0 40.1 40.2 40.3 40.4 Tang N, Li D, Wang X, Sun Z (2020). "Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia". J Thromb Haemost. 18 (4): 844–847. doi:10.1111/jth.14768. PMC 7166509 Check
|pmc=value (help). PMID 32073213 PMID: 32073213 Check|pmid=value (help). - ↑ Dowton SB, Colten HR (1988). "Acute phase reactants in inflammation and infection". Semin Hematol. 25 (2): 84–90. PMID 2455348 PMID: 2455348 Check
|pmid=value (help). - ↑ Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J; et al. (2020). "Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China". JAMA. doi:10.1001/jama.2020.1585. PMC 7042881 Check
|pmc=value (help). PMID 32031570 PMID: 32031570 Check|pmid=value (help). - ↑ National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D; et al. (2006). "Comparison of two fluid-management strategies in acute lung injury". N Engl J Med. 354 (24): 2564–75. doi:10.1056/NEJMoa062200. PMID 16714767 PMID 16714767 Check
|pmid=value (help). Review in: ACP J Club. 2006 Nov-Dec;145(3):69 - ↑ Grissom CK, Hirshberg EL, Dickerson JB, Brown SM, Lanspa MJ, Liu KD; et al. (2015). "Fluid management with a simplified conservative protocol for the acute respiratory distress syndrome*". Crit Care Med. 43 (2): 288–95. doi:10.1097/CCM.0000000000000715. PMC 4675623. PMID 25599463 PMID 25599463 Check
|pmid=value (help). - ↑ Silversides JA, Major E, Ferguson AJ, Mann EE, McAuley DF, Marshall JC; et al. (2017). "Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis". Intensive Care Med. 43 (2): 155–170. doi:10.1007/s00134-016-4573-3. PMID 27734109 PMID 27734109 Check
|pmid=value (help). - ↑ Theoharides TC, Conti P (2020). "Dexamethasone for COVID-19? Not so fast". J Biol Regul Homeost Agents. 34 (3). doi:10.23812/20-EDITORIAL_1-5. PMID 32551464 PMID: 32551464 Check
|pmid=value (help). - ↑ "World first coronavirus treatment approved for NHS use by government - GOV.UK".
- ↑ Annane D, Pastores SM, Rochwerg B, Arlt W, Balk RA, Beishuizen A; et al. (2017). "Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017". Intensive Care Med. 43 (12): 1751–1763. doi:10.1007/s00134-017-4919-5. PMID 28940011 PMID 28940011 Check
|pmid=value (help). - ↑ Fanelli V, Vlachou A, Ghannadian S, Simonetti U, Slutsky AS, Zhang H (2013). "Acute respiratory distress syndrome: new definition, current and future therapeutic options". J Thorac Dis. 5 (3): 326–34. doi:10.3978/j.issn.2072-1439.2013.04.05. PMC 3698298. PMID 23825769 PMID: 23825769 Check
|pmid=value (help). - ↑ "www.who.int" (PDF).
- ↑ Weiss CH, Baker DW, Weiner S, Bechel M, Ragland M, Rademaker A; et al. (2016). "Low Tidal Volume Ventilation Use in Acute Respiratory Distress Syndrome". Crit Care Med. 44 (8): 1515–22. doi:10.1097/CCM.0000000000001710. PMC 4949102. PMID 27035237 PMID: 27035237 Check
|pmid=value (help). - ↑ "www.who.int" (PDF).
- ↑ "b-s-h.org.uk" (PDF).
- ↑ Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M; et al. (2020). "ISTH interim guidance on recognition and management of coagulopathy in COVID-19". J Thromb Haemost. 18 (5): 1023–1026. doi:10.1111/jth.14810. PMID 32338827 PMID: 32338827 Check
|pmid=value (help).