Breast cancer: Difference between revisions
No edit summary |
(No difference)
|
Revision as of 14:14, 23 January 2009
| Breast cancer | |
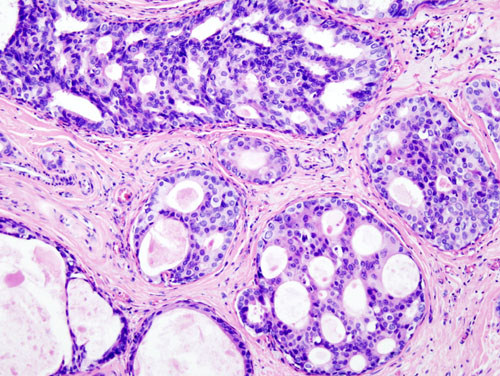 | |
|---|---|
| Histopathologic image from ductal cell carcinoma in situ (DCIS) of breast. Hematoxylin-eosin stain. | |
| ICD-10 | C50 |
| ICD-9 | 174-175 |
| OMIM | 114480 |
| DiseasesDB | 1598 |
| MedlinePlus | 000913 |
| eMedicine | med/2808 |
| MeSH | D001943 |
|
WikiDoc Resources for Breast cancer |
|
Articles |
|---|
|
Most recent articles on Breast cancer Most cited articles on Breast cancer |
|
Media |
|
Powerpoint slides on Breast cancer |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Breast cancer at Clinical Trials.gov Trial results on Breast cancer Clinical Trials on Breast cancer at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Breast cancer NICE Guidance on Breast cancer
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Breast cancer Discussion groups on Breast cancer Patient Handouts on Breast cancer Directions to Hospitals Treating Breast cancer Risk calculators and risk factors for Breast cancer
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Breast cancer |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [2] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Breast cancer is a cancer of the glandular breast tissue.
Worldwide, breast cancer is the fifth most common cause of cancer death (after lung cancer, stomach cancer, liver cancer, and colon cancer).[1] In 2005, breast cancer caused 502,000 deaths (7% of cancer deaths; almost 1% of all deaths) worldwide.[1] Among women worldwide, breast cancer is the most common cause of cancer death.[1]
In the United States, breast cancer is the third most common cause of cancer death (after lung cancer and colon cancer). In 2007, breast cancer is expected to cause 40,910 deaths (7% of cancer deaths; almost 2% of all deaths) in the U.S.[2] Among women in the U.S., breast cancer is the most common cancer and the second- most common cause of cancer death (after lung cancer).[2] Women in the U.S. have a 1 in 8 lifetime chance of developing invasive breast cancer and a 1 in 33 chance of breast cancer causing their death.[3] A U.S. study conducted in 2005 by the Society for Women's Health Research indicated that breast cancer remains the most feared disease,[4] even though heart disease is a much more common cause of death among women.[5]
The number of cases has significantly increased since the 1970s, a phenomenon partly blamed on modern lifestyles in the Western world.[6][7] Because the breast is composed of identical tissues in males and females, breast cancer also occurs in males, though it is less common.[8]
Classification
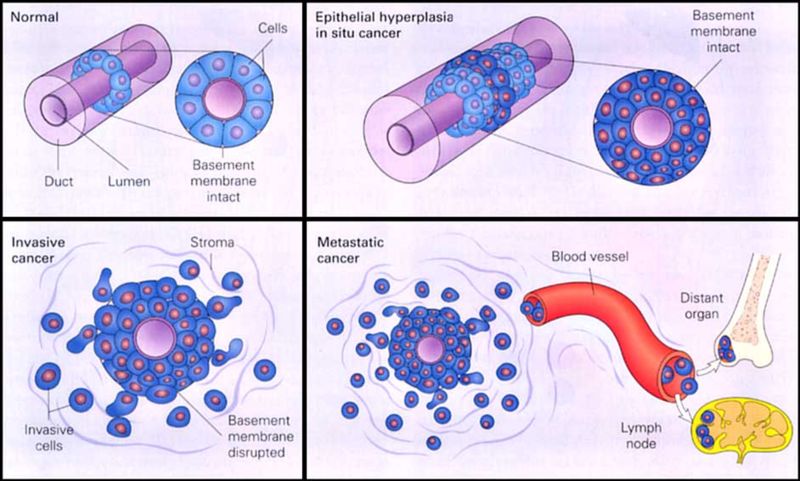
There are numerous ways breast cancer is classified. Like most cancers, breast cancer can be divided into groups based on the tissue of origin, e.g. epithelial (carcinoma) versus stromal (sarcoma). The vast majority of breast cancers arise from epithelial tissue, i.e. they are carcinomas, which can be divided further into subclassifications (e.g. DCIS versus LCIS versus papillary carcinoma).
Other pathologically based classifications:
- Location of the tumour origin - breast duct (i.e. ductal) versus breast lobule (i.e. lobular).
- Histology - see Histologic types section.
- Grade of tumour - well-differentiated (looks almost like normal tissue) versus poorly differentiated (does not look like any normal tissue/mass of proliferating cells) versus moderately differentiated (somewhere between poorly differentiated and well-differentiated).
- Stage of the tumour.
- Immunohistochemical marker status - (ER positive versus ER negative versus HER2/neu positive versus HER2/neu negative), e.g. triple negative breast cancer which is ER negative, PR negative and HER2/neu negative.
- TNM classification -
- Tumour size/invasiveness - presence of invasion (poorer prognosis) versus in situ (better prognosis).
- Nodal status.
- Presence/absence of metastases.
Pathologic types
The latest (2003) World Health Organization (WHO) classification of tumors of the breast[10] recommends the following pathological types:
Invasive breast carcinomas
- Invasive ductal carcinoma
- Most are "not otherwise specified"
- The remainder are given subtypes:
- Mixed type carcinoma
- Pleomorphic carcinoma
- Carcinoma with osteoclastic giant cells
- Carcinoma with choriocarcinomatous features
- Carcinoma with melanotic features
- Invasive lobular carcinoma
- Tubular carcinoma
- Invasive cribriform carcinoma
- Medullary carcinoma
- Mucinous carcinoma and other tumours with abundant mucin
- Mucinous carcinoma
- Cystadenocarcinoma and columnar cell mucinous carcinoma
- Signet ring cell carcinoma
- Neuroendocrine tumours
- Solid neuroendocrine carcinoma (carcinoid of the breast)
- Atypical carcinoid tumour
- Small cell / oat cell carcinoma
- Large cell neuroendocrine carcioma
- Invasive papillary carcinoma
- Invasive micropapillary carcinoma
- Apocrine carcinoma
- Metaplastic carcinomas
- Pure epithelial metaplastic carciomas
- Squamous cell carcinoma
- Adenocarcinoma with spindle cell metaplasia
- Adenosquamous carcinoma
- Mucoepidermoid carcinoma
- Mixed epithelial/mesenchymal metaplastic carcinomas
- Pure epithelial metaplastic carciomas
- Lipid-rich carcinoma
- Secretory carcinoma
- Oncocytic carcinoma
- Adenoid cystic carcinoma
- Acinic cell carcinoma
- Glycogen-rich clear cell carcinoma
- Sebaceous carcinoma
- Inflammatory carcinoma
- Bilateral breast carcinoma
Mesenchymal tumors (including sarcoma)
- Haemangioma
- Angiomatosis
- Haemangiopericytoma
- Pseudoangiomatous stromal hyperplasia
- Myofibroblastoma
- Fibromatosis (aggressive)
- Inflammatory myofibroblastic tumour
- Lipoma
- Angiolipoma
- Granular cell tumour
- Neurofibroma
- Schwannoma
- Angiosarcoma
- Liposarcoma
- Rhabdomyosarcoma
- Osteosarcoma
- Leiomyoma
- Leiomysarcoma
Precursor lesions
- Lobular neoplasia
- lobular carcinoma in situ
- Intraductal proliferative lesions
- Usual ductal hyperplasia
- Flat epithelial hyperplasia
- Atypical ductal hyperplasia
- Ductal carcinoma in situ
- Microinvasive carcinoma
- Intraductal papillary neoplasms
- Central papilloma
- Peripheral papilloma
- Atypical papilloma
- Intraductal papillary carcinoma
- Intracystic papillary carcinoma
Benign epithelial lesions
- Adenosis, includin variants
- Sclerosing adenosis
- Apocrine adenosis
- Blunt duct adenosis
- Microglandular adenosis
- Adenomyoepithelial adenosis
- Radial scar / complex sclerosing lesion
- Adenomas
- Tubular adenoma
- Lactating adenoma
- Apocrine adenoma
- Pleomorphic adenoma
- Ductal adenoma
Myoepithelial lesions
- Myoepitheliosis
- Adenomyoepithelial adenosis
- Adenomyoepithelioma
- Malignant myoepithelioma
Fibroepithelial tumours
- Fibroadenoma
- Phyllodes tumour
- Benign
- Borderline
- Malignant
- Periductal stromal sarcoma, low grade
- Mammary hamartoma
Tumours of the nipple
- Nipple adenoma
- Syringomatous adenoma
- Paget's disease of the nipple
Malignant lymphoma
Metastatic tumours
Tumours of the male breast
- Gynecomastia
- Carcinoma
- In situ
- Invasive
The classifications above show that breast cancer is usually, but not always, classified by its histological appearance. Rare variants are defined on the basis of physical exam findings. For example, Inflammatory breast cancer (IBC), a form of ductal carcinoma or malignant cancer in the ducts, is distinguished from other carcinomas by the inflamed appearance of the affected breast.[11] In the future, some pathologic classifications may be changed. For example, a subset of ductal carcinomas may be re-named basal-like carcinoma (part of the "triple-negative" tumors).
Histologic types
Carcinomas
in situ
- Ductal carcinoma (DCIS) 80%
- Lobular carcinoma (LCIS) 20%
Invasive
- Carcinoma NOS (not otherwise specified)
- Lobular carcinoma
- Tubular/cribriform carcinoma
- Mucinous (colloid) carcinoma
- Medullary carcinoma
- Papillary carcinoma
- Metaplastic carcinoma
Sarcomas
Clinical categorizations
Breast cancer is occasionally classified clinically (on physical exam findings, (medical) history). Inflammatory breast cancer (IBC) is an example of a clinically classified breast cancer and can be any histologic type.[12]
Signs and symptoms
Early breast cancer can in some cases present as breast pain (mastodynia) or a painful lump. Since the advent of breast mammography, breast cancer is most frequently discovered as an asymptomatic nodule on a mammogram, before any symptoms are present. A lump under the arm or above the collarbone that does not go away may be present. When breast cancer associates with skin inflammation, this is known as inflammatory breast cancer. In inflammatory breast cancer, the breast tumor itself is causing an inflammatory reaction of the skin, and this can cause pain, swelling, warmth, and redness throughout the breast.
Changes in the appearance or shape of the breast can raise suspicions of breast cancer.
Another reported symptom complex of breast cancer is Paget's disease of the breast. This syndrome presents as eczematoid skin changes at the nipple, and is a late manifestation of an underlying breast cancer.
Most breast symptoms do not turn out to represent underlying breast cancer. Benign breast diseases such as fibrocystic mastopathy], mastitis, functional mastodynia, and fibroadenoma of the breast are more common causes of breast symptoms. The appearance of a new breast symptom should be taken seriously by both patients and their doctors, because of the possibility of an underlying breast cancer at almost any age.
Occasionally, breast cancer presents as metastatic disease, that is, cancer that has spread beyond the original organ. Metastatic breast cancer will cause symptoms that depend on the location of metastasis. More common sites of metastasis include bone, liver, lung, and brain. Unexplained weight loss can occasionally herald an occult breast cancer, as can symptoms of fevers or chills. Bone or joint pains can sometimes be manifestations of metastatic breast cancer, as can jaundice or neurological symptoms. Pleural effusions are not uncommon with metastatic breast cancer. Obviously, these symptoms are "non-specific," meaning they can also be manifestations of many other illnesses.
Epidemiology and etiology
Epidemiological risk factors for a disease can provide important clues as to the etiology of a disease. The first work on breast cancer epidemiology was done by Janet Lane-Claypon, who published a comparative study in 1926 of 500 breast cancer cases and 500 control patients of the same background and lifestyle for the British Ministry of Health.
Today, breast cancer, like other forms of cancer, is considered to be the final outcome of multiple environmental and hereditary factors.
- Lesions to DNA such as genetic mutations. Exposure to estrogen has been experimentally linked to the mutations that cause breast cancer.[13] Beyond the contribution of estrogen, research has implicated viral oncogenesis and the contribution of ionizing radiation.
- Failure of immune surveillance, which usually removes malignancies at early phases of their natural history.
- Abnormal growth factor signaling in the interaction between stromal cells and epithelial cells, for example in the angiogenesis necessary to promote new blood vessel growth near new cancers.
- Inherited defects in DNA repair genes, such as BRCA1, BRCA2 and p53.
Although many epidemiological risk factors have been identified, the cause of any individual breast cancer is often unknowable. In other words, epidemiological research informs the patterns of breast cancer incidence across certain populations, but not in a given individual. Approximately 5% of new breast cancers are attributable to hereditary syndromes, while no etiology is known for the other 95% of cases.[14]
The primary risk factors that have been identified are sex,[15] age,[16] childbearing, hormones,[17] a high-fat diet,[18] alcohol intake,[19] obesity,[20] and environmental factors such as tobacco use and radiation.[21]
Prevention
Phytoestrogens and soy
Phytoestrogens such as found in soybeans have been extensively studied in animal and human in-vitro and epidemiological studies. The literature support the following conclusions:
- Plant estrogen intake, such as from soy products, in early adolescence may protect against breast cancer later in life.[22]
- Plant estrogen intake later in life is not likely to influence breast cancer incidence either positively or negatively.[23]
It seems reasonable to conclude that soybean-based phytoestrogens are not a major contributor to the incidence of breast cancer.
Folic acid (folate)
Studies have found that "folate intake counteracts breast cancer risk associated with alcohol consumption"[24] and "women who drink alcohol and have a high folate intake are not at increased risk of cancer."[25][26][27] A prospective study of over 17,000 women found that those who consume 40 grams of alcohol (about 3-4 drinks) per day have a higher risk of breast cancer. However, in women who take 200 micrograms of folate (folic acid or Vitamin B9) every day, the risk of breast cancer drops below that of alcohol abstainers.[28]
Folate is involved in the synthesis, repair, and functioning of DNA, the body’s genetic map, and a deficiency of folate may result in damage to DNA that may lead to cancer.[29] In addition to breast cancer, studies have also associated diets low in folate with increased risk of pancreatic, and colon cancer.[30][31]
Foods rich in folate include citrus fruits, citrus juices, dark green leafy vegetables (such as spinach), dried beans, and peas. Vitamin B9 can also be taken in a multivitamin pill.
Oophorectomy and mastectomy
Prophylactic oophorectomy (removal of ovaries), in high-risk individuals, when child-bearing is complete, reduces the risk of developing breast cancer by 60%, as well as reducing the risk of developing ovarian cancer by 96%.[32]
Bilateral prophylactic mastectomies have been shown to prevent breast cancer in high-risk individuals, such as patients with BRCA1 or BRCA2 gene mutations.
Medications
Hormonal therapy has been used for chemoprevention in individuals at high risk for breast cancer. In 2002, a clinical practice guideline by the US Preventive Services Task Force (USPSTF) recommended that "clinicians discuss chemoprevention with women at high risk for breast cancer and at low risk for adverse effects of chemoprevention" with a grade B recommendation.[33][34][35]
Selective estrogen receptor modulators (SERMs)
The guidelines were based on studies of SERMs from the MORE, BCPT P-1, and Italian trials. In the MORE trial, the relative risk reduction for raloxifene was 76%.[36] The P-1 preventative study demonstrated that tamoxifen can prevent breast cancer in high-risk individuals. The relative risk reduction was up to 50% of new breast cancers, though the cancers prevented were more likely estrogen-receptor positive (this is analogous to the effect of finasteride on the prevention of prostate cancer, in which only low-grade prostate cancers were prevented).[37][38] The Italian trial showed benefit from tamoxifen.[39]
Additional randomized controlled trials have been published since the guidelines. The IBIS trial found benefit from tamoxifen.[40] In 2006, the NSABP STAR trial demonstrated that raloxifene had equal efficacy in preventing breast cancer compared with tamoxifen, but that there were fewer side effects with raloxifene.[41] The RUTH Trial concluded that "benefits of raloxifene in reducing the risks of invasive breast cancer and vertebral fracture should be weighed against the increased risks of venous thromboembolism and fatal stroke".[42] On September 14, 2007, Steven Galson, director, US Food and Drug Administration's Center for Drug Evaluation and Research announced approval of the sale of raloxifene to prevent invasive breast cancer in postmenopausal women.[43]
Screening
Breast cancer screening is an attempt to find unsuspected cancers. The most common screening methods are self and clinical breast exams, x-ray mammography, Breast Magnetic resonance imaging (MRI), ultrasound, and genetic testing.
X-ray mammography
Mammography is still the modality of choice for screening of early breast cancer, since it is relatively fast, reasonably accurate, and widely available in developed countries. Breast cancers detected by mammography are usually much smaller (earlier stage) than those detected by patients or doctors as a breast lump.
Due to the high incidence of breast cancer among older women, screening is now recommended in many countries. Recommended screening methods include breast self-examination and mammography. Mammography has been estimated to reduce breast cancer-related mortality by 20-30%.[44] Routine (annual) mammography of women older than age 40 or 50 is recommended by numerous organizations as a screening method to diagnose early breast cancer and has demonstrated a protective effect in multiple clinical trials.[45] The evidence in favor of mammographic screening comes from eight randomized clinical trials from the 1960s through 1980s. Many of these trials have been criticised for methodological errors, and the results were summarized in a review article published in 1993.[46]
Improvements in mortality due to screening are hard to measure; similar difficulty exists in measuring the impact of Pap smear testing on cervical cancer, though worldwide, the impact of that test is likely enormous. Nationwide mortality due to cancer before and after the institution of a screening test is a surrogate indicator about the effectiveness of screening, and results of mammography are favorable.
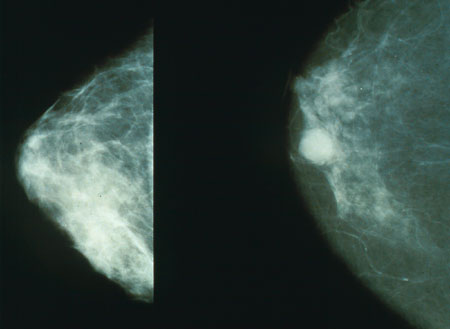
The U.S. National Cancer Institute recommends screening mammography every one to two years beginning at age 40.[47] In the UK, women are invited for screening once every three years beginning at age 50. Women with one or more first-degree relatives (mother, sister, daughter) with premenopausal breast cancer should begin screening at an earlier age. It is usually suggested to start screening at an age that is 10 years less than the age at which the relative was diagnosed with breast cancer.
A clinical practice guideline by the US Preventive Services Task Force recommended "screening mammography, with or without clinical breast examination (CBE), every 1 to 2 years for women aged 40 and older."[48] The Task Force gave a grade B recommendation.[33]
Criticisms of screening mammography
Several scientific groups however have expressed concern about the public's perceptions of the benefits of breast screening.[49] In 2001, a controversial review published in The Lancet claimed that there is no reliable evidence that screening for breast cancer reduces mortality.[50] The results of this study were widely reported in the popular press.[51]
False positives are a major problem of mammographic breast cancer screening. Data reported in the UK Million Woman Study indicates that if 134 mammograms are performed, 20 women will be called back for suspicious findings, and four biopsies will be necessary, to diagnose one cancer. Recall rates are higher in the U.S. than in the UK.[52] The contribution of mammography to the early diagnosis of cancer is controversial, and for those found with benign lesions, mammography can create a high psychological and financial cost.
Mammography in women less than 50 years old
Part of the difficulty in interpreting mammograms in younger women stems from the problem of breast density. Radiographically, a dense breast has a preponderance of glandular tissue, and younger age or estrogen hormone replacement therapy contribute to mammographic breast density. After menopause, the breast glandular tissue gradually is replaced by fatty tissue, making mammographic interpretation much more accurate. Some authors speculate that part of the contribution of estrogen hormone replacement therapy to breast cancer mortality arises from the issue of increased mammographic breast density. Breast density is an independent adverse prognostic factor on breast cancer prognosis.
A systematic review by the American College of Physicians concluded "Although few women 50 years of age or older have risks from mammography that outweigh the benefits, the evidence suggests that more women 40 to 49 years of age have such risks".[53]
Enhancements to mammography
In general, digital mammography and computer-aided mammography have increased the sensitivity of mammograms, but at the cost of more numerous false positive results.
Computer-aided diagnosis(CAD) Systems may help radiologists to evaluate X-ray images to detect breast cancer in an early stage. CAD is especially established in US and the Netherlands. It is used in addition to the human evaluation of the diagnostician.
Breast MRI
Magnetic resonance imaging (MRI) has been shown to detect cancers not visible on mammograms, but has long been regarded to have disadvantages. For example, although it is 27-36% more sensitive, it is less specific than mammography.[54] As a result, MRI studies will have more false positives (up to 5%), which may have undesirable financial and psychological costs. It is also a relatively expensive procedure, and one which requires the intravenous injection of a chemical agent to be effective. Proposed indications for using MRI for screening include:[55]
- Strong family history of breast cancer
- Patients with BRCA-1 or BRCA-2 oncogene mutations
- Evaluation of women with breast implants
- History of previous lumpectomy or breast biopsy surgeries
- Axillary metastasis with an unknown primary tumor
- Very dense or scarred breast tissue
However, two studies published in 2007 demonstrated the strengths of MRI-based screening:
- In March 2007, an article published in the New England Journal of Medicine demonstrated that in 3.1% of patients with breast cancer, whose contralateral breast was clinically and mammographically tumor-free, MRI could detect breast cancer. Sensitivity for detection of breast cancer in this study was 91%, specificity 88%.[56]
- In August 2007, an article published in The Lancet compared MRI breast cancer screening to conventional mammographic screening in 7,319 women. MRI screening was highly more sensitive (97% in the MRI group vs. 56% in the mammography group) in recognizing early high-grade Ductal Carcinoma in situ (DCIS), the most important precursor of invasive carcinoma. Despite the high sensitivity, MRI screening had a positive predictive value of 52%, which is totally accepted for cancer screening tests.[57] The author of a comment published in the same issue of The Lancet concludes that "MRI outperforms mammography in tumour detection and diagnosis."[58]
Breast ultrasound
Ultrasound alone is not usually employed as a screening tool but it is a useful additional tool for the characterization of palpable tumours and directing image-guided biopsies. U-Systems is a US-based company that is selling a breast-cancer detection system using ultrasound that is fully-automated. Using an ultrasound allows a look at dense breast tissue which is not possible with digital mammmography. It is closely correlated with the digital mammography. The other significant advantage over digital mammography is that it is a pain-free procedure.
Breast self-exam
Breast self-examination was widely discussed in the 1990s as a useful modality for detecting breast cancer at an earlier stage of presentation. A large clinical trial in China reduced enthusiasm for breast self-exam. In the trial, reported in the Journal of the National Cancer Institute first in 1997 and updated in 2002, 132,979 female Chinese factory workers were taught by nurses at their factories to perform monthly breast self-exam, while 133,085 other workers were not taught self-exam. The women taught self-exam tended to detect more breast nodules, but their breast cancer mortality rate was no different from that of women in the control group. In other words, women taught breast self-exam were mostly likely to detect benign breast disease, but were just as likely to die of breast cancer.[59] An editorial in the Journal of the National Cancer Institute reported in 2002, "Routinely Teaching Breast Self-Examination is Dead. What Does This Mean?"[60]
Genetic testing
A clinical practice guideline by the US Preventive Services Task Force :[48]
- "recommends against routine referral for genetic counseling or routine breast cancer susceptibility gene (BRCA) testing for women whose family history is not associated with an increased risk for deleterious mutations in breast cancer susceptibility gene 1 (BRCA1) or breast cancer susceptibility gene 2 (BRCA2)" The Task Force gave a grade D recommendation.[33]
- "recommends that women whose family history is associated with an increased risk for deleterious mutations in BRCA1 or BRCA2 genes be referred for genetic counseling and evaluation for BRCA testing." The Task Force gave a grade B recommendation.[33]
The Task Force noted that about 2% of women have family histories that indicate increased risk as defined by:
- For non–Ashkenazi Jewish women, any of the following:
- "2 first-degree relatives with breast cancer, 1 of whom received the diagnosis at age 50 years or younger"
- "3 or more first- or second-degree relatives with breast cancer regardless of age at diagnosis"
- "both breast and ovarian cancer among first- and second- degree relatives"
- "a first-degree relative with bilateral breast cancer"
- "a combination of 2 or more first- or second-degree relatives with ovarian cancer regardless of age at diagnosis"
- "a first- or second-degree relative with both breast and ovarian cancer at any age"
- "a history of breast cancer in a male relative."
- "For women of Ashkenazi Jewish heritage, an increased-risk family history includes any first-degree relative (or 2 second-degree relatives on the same side of the family) with breast or ovarian cancer."
Diagnosis
Breast cancer is diagnosed by the examination of surgically removed breast tissue. A number of procedures can obtain tissue or cells prior to definitive treatment for histological or cytological examination. Such procedures include fine-needle aspiration, nipple aspirates, ductal lavage, core needle biopsy, and local surgical excision. These diagnostic steps, when coupled with radiographic imaging, are usually accurate in diagnosing a breast lesion as cancer. Occasionally, pre-surgical procedures such as fine needle aspirate may not yield enough tissue to make a diagnosis, or may miss the cancer entirely. Imaging tests are sometimes used to detect metastasis and include chest X-ray, bone scan, Cat scan, MRI, and PET scanning. While imaging studies are useful in determining the presence of metastatic disease, they are not in and of themselves diagnostic of cancer. Only microscopic evaluation of a biopsy specimen can yield a cancer diagnosis. Ca 15.3 (carbohydrate antigen 15.3, epithelial mucin) is a tumor marker determined in blood which can be used to follow disease activity over time after definitive treatment. Blood tumor marker testing is not routinely performed for the screening of breast cancer, and has poor performance characteristics for this purpose.
Staging
Breast cancer is staged according to the TNM system, updated in the American Joint Committee on Cancer (AJCC) Staging Manual, now on its sixth edition. Prognosis is closely linked to results of staging, and staging is also used to allocate patients to treatments both in clinical trials and clinical practice.
Summary of stages:
- Stage 0 - Carcinoma in situ
- Stage I - Tumor (T) does not involve axillary lymph nodes (N).
- Stage IIA – T 2-5 cm, N negative, or T <2 cm and N positive.
- Stage IIB – T > 5 cm, N negative, or T 2-5 cm and N positive (< 4 axillary nodes).
- Stage IIIA – T > 5 cm, N positive, or T 2-5 cm with 4 or more axillary nodes
- Stage IIIB – T has penetrated chest wall or skin, and may have spread to < 10 axillary N
- Stage IIIC – T has > 10 axillary N, 1 or more supraclavicular or infraclavicular N, or internal mammary N.
- Stage IV – Distant metastasis (M)
Breast lesions are examined for certain markers, notably sex steroid hormone receptors. About two thirds of postmenopausal breast cancers are estrogen receptor positive (ER+) and progesterone receptor positive (PR+).[61] Receptor status modifies the treatment as, for instance, only ER-positive tumors, not ER-negative tumors, are sensitive to hormonal therapy.
The breast cancer is also usually tested for the presence of human epidermal growth factor receptor 2, a protein also known as HER2, neu or erbB2. HER2 is a cell-surface protein involved in cell development. In normal cells, HER2 controls aspects of cell growth and division. When activated in cancer cells, HER2 accelerates tumor formation. About 20-30% of breast cancers overexpress HER2. Those patients may be candidates for the drug trastuzumab, both in the postsurgical setting (so-called "adjuvant" therapy), and in the metastatic setting.[62]
Treatment
The mainstay of breast cancer treatment is surgery when the tumor is localized, with possible adjuvant hormonal therapy (with tamoxifen or an aromatase inhibitor), chemotherapy, and/or radiotherapy. At present, the treatment recommendations after surgery (adjuvant therapy) follow a pattern. This pattern is subject to change, as every two years, a worldwide conference takes place in St. Gallen, Switzerland, to discuss the actual results of worldwide multi-center studies. Depending on clinical criteria (age, type of cancer, size, metastasis) patients are roughly divided to high risk and low risk cases, with each risk category following different rules for therapy. Treatment possibilities include radiation therapy, chemotherapy, hormone therapy, and immune therapy.
In planning treatment, doctors can also use PCR tests like Oncotype DX or microarray tests like MammaPrint that predict breast cancer recurrence risk based on gene expression. In February 2007, the MammaPrint test became the first breast cancer predictor to win formal approval from the Food and Drug Administration. This is a new gene test to help predict whether women with early-stage breast cancer will relapse in 5 or 10 years, this could help influence how aggressively the initial tumor is treated.[63]
Prognosis
There are several prognostic factors associated with breast cancer. Stage is the most important, as it takes into consideration local involvement, lymph node status and whether metastatic disease is present. The higher the stage at diagnosis, the worse the prognosis. Breast cancer patients whose lymph nodes are cancer-free have a much better prognosis than those whose lymph nodes are positive for cancer.
The presence of estrogen and progesterone receptors in the cancer cell is another important prognostic factor which may guide treatment. Hormone receptor positive breast cancer is usually associated with much better prognosis compared to hormone negative breast cancer.
HER2/neu status has also been described as a prognostic factor. Patients whose cancer cells are positive for HER2/neu have more aggressive disease and may be treated with trastuzumab, a monoclonal antibody that targets this protein.
Psychological aspects of diagnosis and treatment
The emotional impact of cancer diagnosis, symptoms, treatment, and related issues can be severe. Most larger hospitals are associated with cancer support groups which can help patients cope with the many issues that come up in a supportive environment with other people with experience with similar issues. Online cancer support groups are also very beneficial to cancer patients, especially in dealing with uncertainty and body-image problems inherent in cancer treatment.
Metastasis
Most people understand breast cancer as something that happens in the breast. However it can metastasise (spread) via lymphatics to nearby lymph nodes, usually those under the arm. That is why surgery for breast cancer always involves some type of surgery for the glands under the arm — either axillary clearance, sampling, or sentinel node biopsy.
Breast cancer can also spread to other parts of the body via blood vessels. So it can spread to the lungs, pleura (the lining of the lungs), liver, brain, and most commonly to the bones. Seventy percent of the time that breast cancer spreads to other locations, it spreads to bone, especially the vertebrae and the long bones of the arms, legs, and ribs. Breast cancer cells "set up house" in the bones and form tumors. Usually when breast cancer spreads to bone, it eats away healthy bone, causing weak spots, where the bones can break easily. That is why breast cancer patients are often seen wearing braces or using a wheelchair, and why they complain about aching bones.
When breast cancer is found in bones, it has usually spread to more than one site. At this stage, it is treatable, often for many years, but it is not curable. Like normal breast cells, these tumors in the bone often thrive on female hormones, especially estrogen. Therefore, the doctor often treats the patient with medicines that lower her estrogen levels.
History
Breast cancer may be one of the oldest known forms of cancer tumors in humans. The oldest description of cancer (although the term cancer was not used) was discovered in Egypt and dates back to approximately 1600 BC. The Edwin Smith Papyrus describes 8 cases of tumors or ulcers of the breast that were treated by cauterization.The writing says about the disease, "There is no treatment."[64] For centuries, physicians described similar cases in their practises, with the same sad conclusion. It wasn't until doctors achieved greater understanding of the circulatory system in the 17th century that they could establish a link between breast cancer and the lymph nodes in the armpit. The French surgeon Jean Louis Petit (1674-1750) and later the Scottish surgeon Benjamin Bell (1749-1806) were the first to remove the lymph nodes, breast tissue, and underlying chest muscle. Their successful work was carried on by William Stewart Halsted who started performing mastectomies in 1882. He became known for his Halsted radical mastectomy, a surgical procedure that remained popular up to the 1970s.
Cultural references

In the month of October, breast cancer is recognized by survivors, family and friends of survivors and/or victims of the disease. A pink ribbon is worn to recognize the struggle that sufferers face when battling the cancer.
Pink for October is an initiative started by Matthew Oliphant, which asks that any sites willing to help make people aware of breast cancer, change their template or layout to include the color pink, so that when visitors view the site, they see that the majority of the site is pink. Then after reading a short amount of information about breast cancer, or being redirected to another site, they are aware of the disease itself.
The patron saint of breast cancer is Saint Agatha of Sicily.
See also
- List of breast carcinogenic substances
- Mammary tumor for breast cancer in other animals
- Breast reconstruction
- Alcohol and cancer
- Mammography Quality Standards Act
- National Breast Cancer Coalition
- National Comprehensive Cancer Network
- Breast Cancer Action
- Breakthrough Breast Cancer
- Barron Lerner (Physician)
- William Stewart Halsted (Radical Mastectomy)
- International Agency for Research on Cancer
- The Hormone Foundation
- Susan G. Komen for the Cure
References
- ↑ 1.0 1.1 1.2 World Health Organization (2006). "Fact sheet No. 297: Cancer". Retrieved 2007-04-26. Unknown parameter
|month=ignored (help) - ↑ 2.0 2.1 American Cancer Society (2007). "Cancer Facts & Figures 2007" (PDF). Retrieved 2007-04-26.
- ↑ American Cancer Society (2006). "What Are the Key Statistics for Breast Cancer?". Retrieved 2007-04-26. Unknown parameter
|month=ignored (help) - ↑ "Women's Fear of Heart Disease Has Almost Doubled in Three Years, But Breast Cancer Remains Most Feared Disease" (Press release). Society for Women's Health Research. 2005-07-07. Retrieved 2007-10-15.
- ↑ "Leading Causes of Death for American Women 2004" (PDF). National Heart Lung and Blood Institute. Retrieved 2007-10-15.
- ↑ Laurance, Jeremy (2006-09-29). "Breast cancer cases rise 80% since Seventies". The Independent. Retrieved 2006-10-09.
- ↑ "Breast Cancer: Statistics on Incidence, Survival, and Screening". Imaginis Corporation. 2006. Retrieved 2006-10-09. External link in
|work=(help) - ↑ "Male Breast Cancer Treatment - National Cancer Institute". National Cancer Institute. 2006. Retrieved 2006-10-16. External link in
|work=(help) - ↑ Breast Cancer: Molecular Genetics, Pathogenesis, and Therapeutics (Contemporary Cancer Research) by Anne M. Bowcock (1999)
- ↑ Tumours of the breast and female genital organs, WHO classification of tumours, 2003, ISBN 92 832 2412 4
- ↑ Giordano SH, Hortobagyi GN (2003). "Inflammatory breast cancer: clinical progress and the main problems that must be addressed". Breast Cancer Res. 5 (6): 284–8. doi:10.1186/bcr608. PMID 14580242. Free Full Text.
- ↑ Giordano SH, Hortobagyi GN (2003). "Inflammatory breast cancer: clinical progress and the main problems that must be addressed". Breast Cancer Res. 5 (6): 284–8. doi:10.1186/bcr608. PMID 14580242. Free Full Text.
- ↑ Cavalieri E, Chakravarti D, Guttenplan J; et al. (2006). "Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention". Biochim. Biophys. Acta. 1766 (1): 63–78. doi:10.1016/j.bbcan.2006.03.001. PMID 16675129.
- ↑ Madigan MP, Ziegler RG, Benichou J, Byrne C, Hoover RN (1995). "Proportion of breast cancer cases in the United States explained by well-established risk factors". J. Natl. Cancer Inst. 87 (22): 1681–5. PMID 7473816.
|access-date=requires|url=(help) - ↑ Giordano, Sharon H (May 2004). "Breast carcinoma in men". Cancer. American Cancer Society. 101 (1): 51–57. Unknown parameter
|coauthors=ignored (help) - ↑ "Individual Risk Factors". BreastCancer.org. Retrieved 2007-03-11.
- ↑ Yager JD (2006). "Estrogen carcinogenesis in breast cancer". New Engl J Med. 354 (3): 270–82. PMID 16421368. Unknown parameter
|coauthors=ignored (help) - ↑ Chlebowski RT, Blackburn GL, Thomson CA, Nixon DW, Shapiro A, Hoy MK; et al. "Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women's Intervention Nutrition Study (WINS)". J Natl Cancer Inst. 98 (24): 1767–1776. PMID 17179478.
- ↑ Boffetta, Paolo (2006-03-23). "The burden of cancer attributable to alcohol drinking". International Journal of Cancer. Wiley-Liss, Inc. 119 (4): 884–887. doi:10.1002/ijc.21903. PMID 16557583. Retrieved 2006-10-09. Unknown parameter
|coauthors=ignored (help) - ↑ BBC report Weight link to breast cancer risk
- ↑ ACS (2005). "Breast Cancer Facts & Figures 2005-2006" (PDF). Retrieved 2007-04-26.
- ↑ Rice S, Whitehead SA (2006). "Phytoestrogens and breast cancer--promoters or protectors?". Endocr. Relat. Cancer. 13 (4): 995–1015. doi:10.1677/erc.1.01159. PMID 17158751.
- ↑ Gikas PD, Mokbel K. (2005Phytoestrogens and the risk of breast cancer: a review of the literature. Int J Fertil Women's Med.
- ↑ Mayo Clinic news release June 26 2001 "Folate Intake Counteracts Breast Cancer Risk Associated with Alcohol Consumption"
- ↑ Boston University,Folate, Alcohol, and Cancer Risk
- ↑ Bailey, L.B. Folate, methyl-related nutrients, alcohol and the MTHFR 677C -> T polymorphous affect cancer risk: intake recommendations. Journal of Nutrition, 2003, 133, 37485-37535
- ↑ Zhang S, Hunter D, Hankinson S, Giovannucci E, Rosner B, Colditz G, Speizer F, Willett W (1999). "A prospective study of folate intake and the risk of breast cancer". JAMA. 281 (17): 1632–7. PMID 10235158.
- ↑ Baglietto, Laura, et al. Does dietary folate intake modify effect of alcohol consumption on breast cancer risk? Prospective cohort study. British Medical Journal, August 8, 2005
- ↑ Jennings E. (1995). "Folic acid as a cancer preventing agent". Medical Hypotheses. 45 (3): 297–303. PMID 8569555.
- ↑ Giovannucci E, Stampfer MJ, Colditz GA, Hunter DJ, Fuchs C, Rosner BA, Speizer FE, Willett WC. (1998). "Multivitamin use, folate, and colon cancer in women in the Nurses' Health Study". Annals of Internal Medicine. 129 (7): 517–524. PMID 9758570.
- ↑ name="Oldref_42">Freudenheim JL, Grahm S, Marshall JR, Haughey BP, Cholewinski S, Wilkinson G (1991). "Folate intake and carcinogenesis of the colon and rectum". International Journal of Epidemiology. 20 (2): 368–374. PMID 1917236.
- ↑ Kauff N, Satagopan J, Robson M, Scheuer L, Hensley M, Hudis C, Ellis N, Boyd J, Borgen P, Barakat R, Norton L, Castiel M, Nafa K, Offit K (2002). "Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation". N Engl J Med. 346 (21): 1609–15. PMID 12023992.
- ↑ 33.0 33.1 33.2 33.3 "Guide to Clinical Preventive Services, Third Edition: Periodic Updates, 2000-2003". Agency for Healthcare Research and Quality. US Preventive Services Task Force. Retrieved 2007-10-07.
- ↑ "Chemoprevention of breast cancer: recommendations and rationale". Ann. Intern. Med. 137 (1): 56–8. 2002. PMID 12093249.
- ↑ Kinsinger LS, Harris R, Woolf SH, Sox HC, Lohr KN (2002). "Chemoprevention of breast cancer: a summary of the evidence for the U.S. Preventive Services Task Force". Ann. Intern. Med. 137 (1): 59–69. PMID 12093250.
- ↑ Cummings SR, Eckert S, Krueger KA; et al. (1999). "The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation". JAMA. 281 (23): 2189–97. PMID 10376571.
- ↑ Fisher B, Costantino JP, Wickerham DL; et al. (2005). "Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study". J. Natl. Cancer Inst. 97 (22): 1652–62. doi:10.1093/jnci/dji372. PMID 16288118.
- ↑ Fisher B, Costantino JP, Wickerham DL; et al. (1998). "Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study". J. Natl. Cancer Inst. 90 (18): 1371–88. PMID 9747868.
- ↑ Veronesi U, Maisonneuve P, Rotmensz N; et al. (2007). "Tamoxifen for the prevention of breast cancer: late results of the Italian Randomized Tamoxifen Prevention Trial among women with hysterectomy". J. Natl. Cancer Inst. 99 (9): 727–37. doi:10.1093/jnci/djk154. PMID 17470740.
- ↑ Cuzick J, Forbes JF, Sestak I; et al. (2007). "Long-term results of tamoxifen prophylaxis for breast cancer--96-month follow-up of the randomized IBIS-I trial". J. Natl. Cancer Inst. 99 (4): 272–82. doi:10.1093/jnci/djk049. PMID 17312304.
- ↑ Vogel VG, Costantino JP, Wickerham DL; et al. (2006). "Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial". JAMA. 295 (23): 2727–41. doi:10.1001/jama.295.23.joc60074. PMID 16754727.
- ↑ Barrett-Connor E, Mosca L, Collins P, et al Raloxifene Use for The Heart (RUTH) Trial Investigators. (2006). "Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women". N. Engl. J. Med. 355 (2): 125–37. doi:10.1056/NEJMoa062462. PMID 16837676.
- ↑ AFP.google.com, US approves Lilly's Evista for breast cancer prevention
- ↑ Elwood J, Cox B, Richardson A. "The effectiveness of breast cancer screening by mammography in younger women". Online J Curr Clin Trials. Doc No 32: [23, 227 words, 195 paragraphs]. PMID 8305999.
- ↑ Fletcher S, Black W, Harris R, Rimer B, Shapiro S (1993). "Report of the International Workshop on Screening for Breast Cancer". J Natl Cancer Inst. 85 (20): 1644–56. PMID 8105098.
- ↑ Fletcher SW, Black W, Harris R, Rimer BK, Shapiro S (1993). "Report of the International Workshop on Screening for Breast Cancer". J. Natl. Cancer Inst. 85 (20): 1644–56. PMID 8105098.
|access-date=requires|url=(help) - ↑ "NCI Statement on Mammography Screening - National Cancer Institute". Retrieved 2007-09-11.
- ↑ 48.0 48.1 "Screening for breast cancer: recommendations and rationale". Ann. Intern. Med. 137 (5 Part 1): 344–6. 2002. PMID 12204019.
- ↑ "Women 'misjudge screening benefits'". BBC. Monday, 15 October, 2001. Retrieved 2007-04-04. Check date values in:
|date=(help) - ↑ Olsen O, Gøtzsche P (2001). "Cochrane review on screening for breast cancer with mammography". Lancet. 358 (9290): 1340–2. PMID 11684218.
- ↑ "New concerns over breast screening". BBC. Thursday, 18 October, 2001. Retrieved 2007-04-04. Check date values in:
|date=(help) - ↑ Smith-Bindman R, Ballard-Barbash R, Miglioretti DL, Patnick J, Kerlikowske K (2005). "Comparing the performance of mammography screening in the USA and the UK". Journal of medical screening. 12 (1): 50–4. doi:10.1258/0969141053279130. PMID 15814020.
- ↑ Armstrong K, Moye E, Williams S, Berlin JA, Reynolds EE (2007). "Screening mammography in women 40 to 49 years of age: a systematic review for the American College of Physicians". Ann. Intern. Med. 146 (7): 516–26. PMID 17404354.
- ↑ Hrung J, Sonnad S, Schwartz J, Langlotz C (1999). "Accuracy of MR imaging in the work-up of suspicious breast lesions: a diagnostic meta-analysis". Acad Radiol. 6 (7): 387–97. PMID 10410164.
- ↑ Morrow M (2004). "Magnetic resonance imaging in breast cancer: one step forward, two steps back?". JAMA. 292 (22): 2779–80. PMID 15585740.
- ↑ Lehman CD, Gatsonis C, Kuhl CK, Hendrick RE, Pisano ED, Hanna L, Peacock S, Smazal SF, Maki DD, Julian TB, DePeri ER, Bluemke DA, Schnall MD (2007). "MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer". N Engl J Med. 356 (13): 1295–1303. PMID 17392300.
- ↑ Kuhl CK, Schrading S, Bieling HB, Wardelmann E, Leutner CC, Koenig R, Kuhn W, Schild HH (2007). "MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study". The Lancet. 370 (9586): 485–492. PMID.
- ↑ Boetes C, Mann RM (2007). "Ductal carcinoma in situ and breast MRI". The Lancet. 370 (9586): 459–460. PMID.
- ↑ Thomas DB, Gao DL, Ray RM; et al. (2002). "Randomized trial of breast self-examination in Shanghai: final results". J. Natl. Cancer Inst. 94 (19): 1445–57. PMID 12359854.
- ↑ Harris R, Kinsinger LS (2002). "Routinely teaching breast self-examination is dead. What does this mean?". J. Natl. Cancer Inst. 94 (19): 1420–1. PMID 12359843.
- ↑ Rusiecki JA, Holford TR, Zahm SH, Zheng T. Breast cancer risk factors according to joint estrogen receptor and progesterone receptor status. Cancer Detect Prev 2005;29:419-26
- ↑ accessed 1/30/07 cancer.gov
- ↑ "FDA Approves New Breast Cancer Test". Associated Press, February 6, 2007.
- ↑ "The History of Cancer". American Cancer Society. 2002-03-25. Retrieved 2006-10-09.
External links
- Template:Dmoz
- Breast localisation and excision: Operation Script on Wikisurgery.
- Breast localisation and excision : Information for patients on Wikisurgery.
- Breast subareolar excision : Operation Script on Wikisurgery.
- Breast subareolar excision daycase : Information for patients on Wikisurgery.
- Breast wide excision: Operation Script on Wikisurgery.
- Breast wide excision: Information for patients on Wikisurgery.
- Fine needle aspiration: Operation Script on Wikisurgery.
- Mastectomy: Operation Script on Wikisurgery.
- Mastectomy: Information for patients on Wikisurgery.
- Mastectomy subcutaneous male daycase: Information for patients on Wikisurgery.
- Trucut needle biopsy: Operation Script on Wikisurgery.
General
- American Cancer Society - Learn About Breast Cancer Page
- History of breast cancer treatment
- The Breast: Neoplasms of the Breast
- Abortion and Breast Cancer
- National Cancer Institute: Breast Cancer
- Imaginis -Award winning Breast Cancer site
Research and statistics
- A better diagnosis and prognosis for overweight women with breast cancer
- Breast cancer incidence by country
- eMaxHealth Breast Cancer Publishes Research News on Breast Cancer from Research Institutions and Universities
Clinical
- RadiologyInfo - The radiology information resource for patients: Breast Cancer
- Surgery Choices for Women with Early-Stage Breast Cancer, National Cancer Institute
- Mastectomy vs.n Lumpectomy: Who Decides?, National Research Center for Women & Families
- Australia: Cancer Control Bulletin Alcohol and cancer risk
- Cornell University Alcohol and the Risk of Breast Cancer
Videos
- Health Video: Breast Cancer - Overview, Prevention and Treatment
- Early-onset Breast Cancer among Black Women
Other
- Mayo Clinic: How the grading system for cancerous tumors was developed.
- Interviews with Breast Cancer Experts
|
Breast Cancer Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Breast cancer On the Web |
|
American Roentgen Ray Society Images of Breast cancer |
ar:سرطان الثدي bs:Tumori dojke bg:Рак на гърдата da:Brystkræft de:Brustkrebs fa:سرطان پستان hr:Rak dojke id:Kanker payudara it:Carcinoma mammario he:סרטן השד ka:მკერდის კიბო mk:Рак на дојка ms:Penyakit barah payudara nl:Borstkanker no:Brystkreft simple:Breast cancer sk:Karcinóm prsníka fi:Rintasyöpä sv:Bröstcancer th:มะเร็งเต้านม ur:سرطان پستان