Mammography
|
WikiDoc Resources for Mammography |
|
Articles |
|---|
|
Most recent articles on Mammography Most cited articles on Mammography |
|
Media |
|
Powerpoint slides on Mammography |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Mammography at Clinical Trials.gov Clinical Trials on Mammography at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Mammography
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Mammography Discussion groups on Mammography Patient Handouts on Mammography Directions to Hospitals Treating Mammography Risk calculators and risk factors for Mammography
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Mammography |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Soroush Seifirad, M.D.[2]
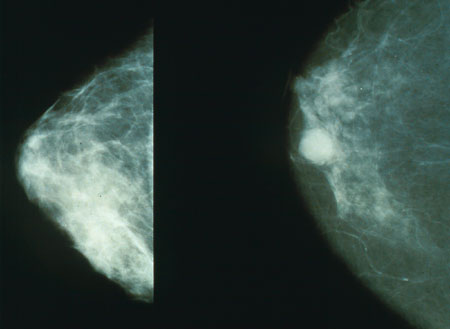
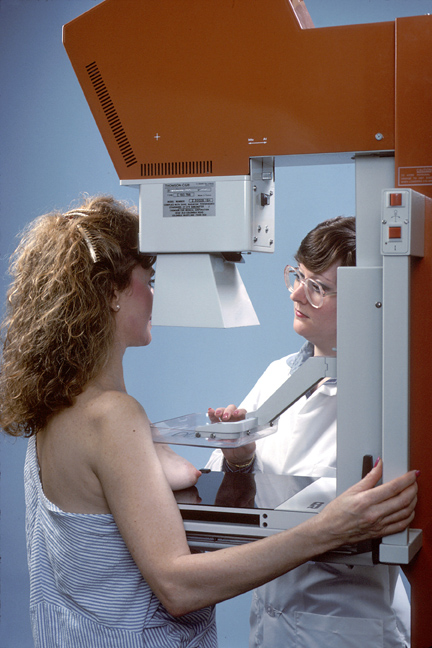
Overview
Mammography has been proven to reduce mortality from breast cancer. No other imaging technique has been shown to reduce risk. In some countries, routine (annual to five-yearly) mammography of older women is encouraged as a screening method to diagnose early breast cancer.
Introduction
Mammography is the process of using low-dose X-rays (usually around 0.7 mSv) to examine the human breast. It is used to look for different types of tumors and cysts. Mammography has been proven to reduce mortality from breast cancer. No other imaging technique has been shown to reduce risk. In some countries routine (annual to five-yearly) mammography of older women is encouraged as a screening method to diagnose early breast cancer. Screening mammograms were first proven to save lives in research published by Sam Shapiro, Philip Strax and Louis Venet in 1966. Like all x-rays, mammograms use doses of ionizing radiation to create this image. Radiologists then analyze the image for any abnormal growths. It is normal to use longer wavelength X-rays (typically Mo-K) than those used for radiography of bones. At this time, mammography is still the modality of choice for screening for early breast cancer. It is the gold-standard which other imaging tests are compared with. CT has no real role in diagnosing breast cancer at the present. Ultrasound, ductography, and magnetic resonance imaging (MRI) are adjuncts to mammography. Ultrasound is typically used for further evaluation of masses found on mammography or palpable masses not seen on mammograms. Ductograms are useful for evaluation of bloody nipple discharge when the mammogram is non-diagnostic. MRI can be useful for further evaluation of questionable findings, or sometimes for pre-surgical evaluation to look for additional lesions. Stereotactic breast biopsies are another common method for further evaluation of suspicious findings. Mammography has a false-negative (missed cancer) rate of at least 10 percent. This is partly due to dense tissues obscuring cancer and the fact that the appearance of cancer on mammograms has a large overlap with the appearance of normal tissues.
Procedure
- During the procedure, the breast is compressed by a dedicated mammography machine to:
- Even out the tissue
- Increase image quality
- Hold the breast still (preventing motion blur).
- Both front and side images of the breast are taken.Due to imaging limitations, some elements may show up on x-ray as calcium spots. For this reason, women are discouraged from applying the following on the day of the mammogram.
- Until some years ago, mammography was typically performed with screen-film cassettes.
- Now, mammography is undergoing a transition to digital detectors, known as Full Field Digital Mammography (FFDM). This progress is some years later than in general radiology. This is due to several factors:
- The higher resolution demands in mammography
- Significantly increased the expense of the equipment,
- The fact that digital mammography has never been shown to be superior to film-screen mammography for the diagnosis of breast cancer. Computed radiography (CR) may help speed the transition. CR allows facilities to continue to use their existing screen-film units but replace the cassettes with an imaging plate that acts as a digital adapter.
- As of March 1, 2007, 18.3% of facilities in the United States and its territories have at least one FFDM unit.
- After a screening mammogram, some women may have areas of concern which can't be resolved with only the information available from the screening mammogram.
- They would then be called back for a "diagnostic mammogram".
- This phrase is essentially a problem-solving mammogram. During this session, the radiologist will be monitoring each of the additional films as they are taken to determine the cause of the abnormal appearance.
- The outcome of a mammogram may be benign or may require further investigation. If the cause cannot be determined to be benign with sufficient certainty, a biopsy will be recommended.
- The biopsy procedure will be used to obtain actual tissue from the site for the pathologist to examine microscopically to determine the precise cause of the abnormality.
- In the past, biopsies were most frequently done in surgery, under local or general anesthesia. T
- The majority are now done with needles using either ultrasound or mammographic guidance to be sure that the area of concern is the area that is biopsied.
- One study shows that needle biopsies of liver malignancies rarely increase the likelihood that cancer will spread, and has not been found to occur with breast needle biopsies.
Results
- Often women are quite distressed to be called back for a diagnostic mammogram.
- Most of these recalls will be false positive result[1]s.[2]
- It helps to know these approximate statistics:
- Of every 1,000 U.S. women who are screened, about 7% (70) will be called back for a diagnostic session (although some studies estimate the number closer to 10%-15%).
- About 10 of these will be referred for a biopsy; the remaining 60 are found to be of a benign cause.
- Of the 10 referred for biopsy, about 3.5 will have cancer and 6.5 will not. Of the 3.5 who do have cancer, about 2 have a low stage cancer that will be essentially cured after treatment.
- Mammogram results are often expressed in terms of the BI-RADS Assessment Category, often called a "BI-RADS score." The categories range from 0 (Incomplete) to 6 (Known biopsy-proven malignancy).(See below)
- In the UK mammograms are scored on a scale from 1-5 (1 = normal, 2 = benign, 3 = indeterminate, 4 = suspicious of malignancy, 5 = malignant).
- The rates of abnormal and false-positive mammogram results are far lower in countries other than the U.S. that have adopted different quality standards.[3][2]
- For example, in Holland, only about 1% of mammograms yield an abnormal result.
- As a result, false-positives are much less common. Despite the higher rates of false-positives in the U.S., women are about as likely to die from breast cancer in the U.S. as in Holland and elsewhere in Europe.
- While mammography is the only breast cancer screening method that has been shown to save lives, it has its own drawbacks.
- Estimates of the numbers of cancers missed by mammography are usually around 10%-20%.
- This means that of the 350 per 100,000 women who have breast cancer, about 35-70 will not be seen by mammography.
- Reasons for not seeing cancer include observer error, but more frequently it is due to the fact that the cancer is hidden by other dense tissue in the breast and even after retrospective review of the mammogram, cannot be seen.
- Furthermore, one form of breast cancer, lobular cancer, has a growth pattern that produces shadows on the mammogram which are indistinguishable from normal breast tissue.
- Computer-aided diagnosis (CAD) is being tested to decrease the number of cases of cancer that are missed in mammograms. [4][5]
- In one test, a computer identified 71% of the cases of cancer that had been missed by physicians.
- However, the computer also flagged twice as many non-cancerous masses than the physicians did.
- In the second study of a larger set of mammograms, a computer recommended six biopsies that physicians did not.
- All six turned out to be cancers that would have been missed.
- Generally, CAD systems in screening mammography have poor specificity and compare poorly to double reading.
- Impact of computer-aided detection prompts on the sensitivity and specificity of screening mammography.
- While data are accumulating suggesting that CAD can find a few additional cancers, this should be put in perspective.
- The additional find rate was 20%, thus in a group of 1,000 women who will have about 4 cancers, CAD may help find an additional 0.8.
- The types of additional cancers that may be found are likely to be early and small.
- As of 2006, there have been no data to show that finding these additional cancers will have any effect on survival rate.
- Some feel that these cancers are likely to be found at the next screening, still at a curable stage, and therefore it remains to be proven whether CAD will be eventually found to have any effect on patient outcome.[6]
BI-RADS
- There is a standard system for reporting the results of a mammogram, which is called the Breast Imaging-Reporting and Data System, or BI-RADS.[7][8]
| Category | Meaning |
| 0 | An unclear result with a need for more tests or comparison with previous mammograms |
| 1 | No abnormalities |
| 2 | No sign of cancer but some abnormalities present, such as benign calcifications |
| 3 | Some abnormalities that are very likely to be benign but need following up |
| 4 | Abnormalities that could be cancerous, possibly requiring a biopsy |
| 5 | Abnormalities very likely to be cancerous, requiring a biopsy |
| 6 | Cancer is present, requiring mammograms to check progress |

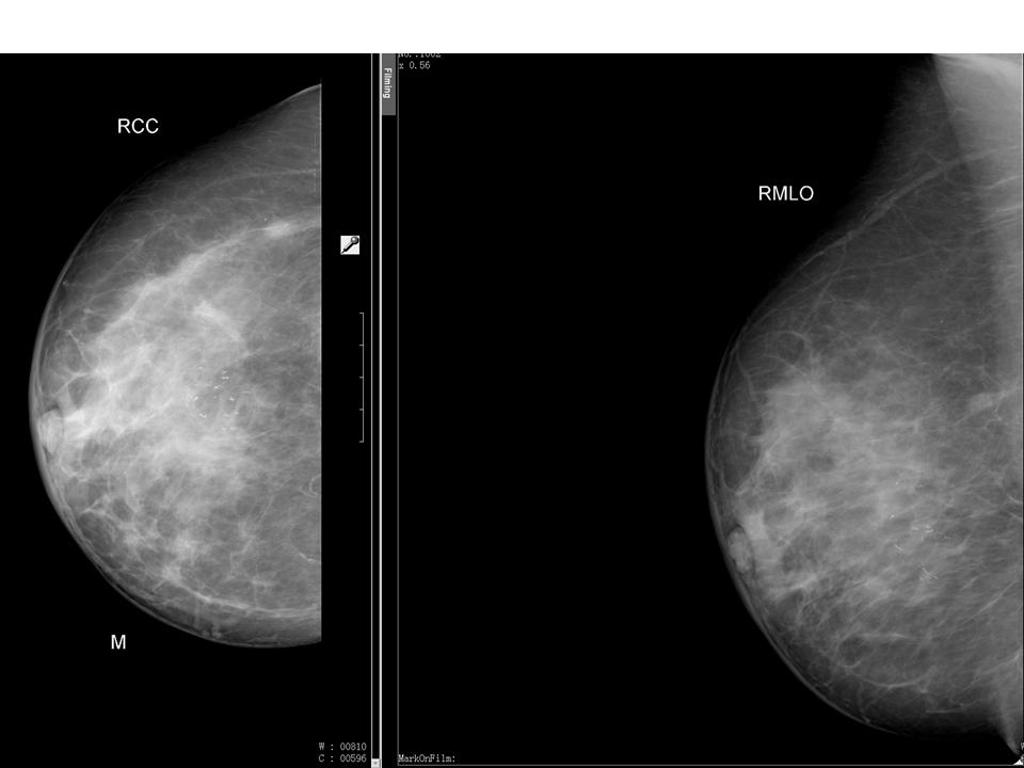
Screening guidelines
- In 2009, the U.S. Preventive Services Task Force (USPSTF) revised their 2002 guideline wherein a mammogram was recommended every 1-2 years for women of 40 years and over.
- One of the reasons for this change *A number of studies demonstrated an increased risk of false-positive results when screening starts at a younger age or takes place every year.
- Currently, USPSTF recommends screening mammography for women aged 50 to 74 years, every 2 years.
- Although this advice is in line with that offered in many European countries, it differs with the recommendations of some other U.S. organizations.
- The American College of Radiology and the Society of Breast Imaging both continue to recommend annual mammograms for women starting at age 40.
- The current guidelines issued by the American Cancer Society advise a mammogram every year for women of 45 to 54 years, and every 2 years for women of 55 years and older.
- In light of the debate, various emerging technologies are now being suggested as alternative options for breast cancer screening.[2]
Clinical decision making
- If BI-RADS 0, 4 or 5
- Further intervention is recommended
- Contact the clinician to discuss the need for biopsy
- Discuss with the patient the need for further imaging.
- If BI-RADS 4c or 5 and the biopsy reported as benign:
- The pathologist should reevaluate the samples since malignant diagnosis is strongly suspected
- May repeat biopsy[7]
Advantages
- Mammography has been estimated to reduce breast cancer-related mortality by 20-30%.[9]
Disadvantages
- Evidence in favor of mammography screening comes from eight randomized controlled clinical trials from the 1960s through 1980s.[2]
- Many of these trials have been criticized for methodological errors, and the results were summarized in a review article published in 1993.[10]
False positive results
- False positive reports are a major problem of mammography breast cancer screening. Approximately 7% of all mammography screenings are false positives. [11]
- Data reported in the UK Million Woman Study indicates that if 134 mammograms are performed, 20 women will be called back for suspicious findings, and four biopsies will be necessary, to diagnose one cancer. Recall rates are higher in the U.S. than in the UK.[12]
- The goal of any screening procedure is to examine a large population of patients and find the small number most likely to have a serious condition.
- These patients are then referred for further, usually more invasive, testing. Thus a screening exam is not intended to be definitive, It is intended to have high sensitivity so as to not miss any cancers.
- The cost of this high sensitivity is a relatively large number of results that would be regarded as suspicious in patients without disease. This is true of mammography.
- The patients called back for further testing from a screening session (about 7%) are sometimes referred to as "false positives", implying an error.
- In fact, it is essential to call back many healthy patients for further testing to capture as many cases of cancer as possible.
- These callbacks should not be regarded as errors.
- Nonetheless, some women who receive false-positive results become anxious, worried and distressed about the possibility of having breast cancer, feelings that can last for many years.
False negative results
- At the same time, mammograms also have a rate of missed tumors, or "false negatives."
- Accurate data regarding the number of false negatives are very difficult to obtain, simply because we cannot perform mastectomies on every woman who has had a mammogram to determine the false negative rate accurately.
- Estimates of the false negative rate depend on the close follow-up of a large number of patients for many years.
- This is difficult in practice because many women do not return for regular mammography making it impossible to know if they ever developed cancer.
- Researchers have found that breast tissue is denser among younger women, making it difficult to detect tumors. For this reason, false negatives are twice as likely to occur in premenopausal mammograms (Prate.)
- This is why the screening program in the UK does not start calling women for screening mammograms until the age of 50.
- The importance of these missed cancers is not clear, particularly if the woman is getting yearly mammograms.
- Research on a closely related situation has shown that small cancers that are not acted upon immediately but are observed over periods of even several years, will have good outcomes.
- A group of 3,184 women had mammograms which were formally classified as "probably benign." [13]
- This classification is for patients who are not clearly normal but have some area of minor concern.
- This results, not in the patient being biopsied, but having early follow up mammography every six months for three years to guarantee no change. Of these 3,184 women, 17 (0.5%) did have cancers.
- Most importantly, when the diagnosis was finally made, they were all still stage 0 or 1, the earliest stages.
- Five years after treatment, none of these 17 women had evidence of recurrence.
- Thus, small early cancers, even though not acted on immediately, were still entirely curable.
- Regardless of the precise number of false negatives, it is very clear that even if some tumors are missed, lives are saved when they are found. Women need to understand that a negative mammogram is not a perfect guarantee that there is no breast cancer present, but it is the best method we have available.
- Breast density
- Mammography in women under 50 years of age can be imprecise due to breast density.
- Breast density is an independent adverse prognostic factor on breast cancer prognosis. [14]
- It could delay diagnosis of breast cancer using mammography, additionally having dense breast is a risk factor for developing breast cancer.
- Body habitus
- It has been shown that mammography might have lower sensitivity in thin women.
- Especially in women with a BMI less than 25 (86 versus 91 percent in the normal population)
- Just like the other radiologic studies the following plays a crucial role in the accurate interpretation of findings:
- Radiologist expertness:
- less experienced radiologists had
- Higher sensitivity to detect breast cancer
- Higher recall rates and lower specificity
- Comparison of the current mammogram with the prior mammograms:
- May increase the specificity of mammography
- Might improve the sensitivity of mammography,
- Have not been shown to improve mortality rates from breast cancer screening.
- Additionally, CAD may increase patient recall rates.
Mammography risks
- The radiation exposure associated with mammography is a potential risk of screening. [15]
- The risk of exposure appears to be greater in younger women.
- The largest study of radiation risk from mammography concluded that for women 40 years of age or older, the risk of radiation-induced breast cancer was minuscule, particularly compared with the potential benefit of mammographic screening, with a benefit-to-risk ratio of 48.5 lives saved for each life lost due to radiation exposure.
- Organizations such as the National Cancer Institute and the United States Preventive Task Force take such risks into account when formulating screening guidelines.
- The majority of health experts agree that the risk of breast cancer for women under 35 is not high enough to warrant the risk of radiation exposure.
- For this reason, and because the radiation sensitivity of the breast in women under 35 is possibly greater than in older women, most radiologists will not perform screening mammography in women under 40.
- However, if there is a significant risk of cancer in a particular patient (BRCA positive, very positive family history, palpable mass), mammography may still be important.
- Often, the radiologist will try to avoid mammography, by using ultrasound, or MRI imaging.
- Similarly, the risk of breast cancer to women over 55 very clearly justifies the risk of mammograms.
- The statistics about mammography and women between the ages of 40 and 55 are the most contentious.
- A 1992 Canadian National Breast Cancer Study showed that mammography had no positive effect on mortality for women between the ages of 40 and 50.
- The study's critics pointed out that there were very serious design flaws in the study that invalidated these results.
- While screening between 40 and 50 is still controversial, the preponderance of the evidence indicates that there is some small benefit in terms of early detection.
- Currently, the American Cancer Society, the National Cancer Institute, and the American College of Radiology encourage mammograms every two years for women ages 40 to 49.[16] [17][18][3]
- In contrast, the American College of Physicians, a large internist group, has recently encouraged individualized screening plans as opposed to wholesale biennial screening of women aged 40 to 49.
- There are some potential risks that are considered to be associated with mammography and mammograms. They include the following:
- They require repeated exposure to radiation, which may cause a very small risk of cancer if used over a lifetime.
- They can lead to non-invasive cancers being diagnosed and treated when treatment is not necessarily required.
- They are not as effective for women with dense breast tissue or breast implants.
- They can lead to women choosing double mastectomies as a preventive measure.
- They have a high rate of false-positive results, which can result in unnecessary biopsies and additional screening. False-positive results are those that suggest that cancer is present when it is not.
- Serial mammography might slightly increase the risk of developing breast cancer in high-risk patients such as patients with a family history of breast cancer and patients with known genetic carcinogenic mutations.
- According to a recently published study by Jansen-van der Weide et.al. average increased the risk of breast cancer because of low-dose radiation exposure was (OR between 1.3 and 2 with respect to the patients' risk and exposure) observed compared to that of high-risk women not exposed to low-dose radiation.
- Pooled OR revealed an increased risk of breast cancer among high-risk women due to low-dose radiation exposure (OR = 1.3, 95% CI: 0.9- 1.8).
- Exposure before age 20 (OR = 2.0, 95% CI: 1.3-3.1)
- A mean of ≥5 exposures (OR = 1.8, 95% CI: 1.1-3.0)
- When using low-dose radiation among high-risk women, a careful approach is needed, by means of
- They recommended a careful approach in these subgroup of patients as follows:
- Reducing repeated exposure,
- Avoidance of exposure at a younger age
- Using non-ionising screening techniques.
- According to another study by Diana L. Miglioretti et.al. radiation-induced breast cancer incidence and mortality from digital mammography screening are impacted by:[19]
- Dose variability from screening and resultant diagnostic work-up,
- Initiation age
- Screening frequency.
- Women with large breasts may be at higher risk of radiation-induced breast cancer;
- However, we should keep in mind that "the benefits of screening outweigh these risks".
Screening Vs diagnostic mammography
- Screening mammography
- Performed in a woman with no clinical sign and symptoms
- To decrease morbidity and mortality by detection of early, treatable breast cancer.
- Screening mammography is the best modality currently available to detect clinically occult breast cancer.
- Application of the screening mammography is the only modality that has been shown a significant decrease in mortality and morbidity of breast cancer.
- Generally standard views are obtained for each breast.
- Standard views are as follows:
- Cranial-Caudal (CC)
- Mediolateral-oblique (MLO)
- In certain cases, additional views may be obtained to maximize imaging of all breast tissue.
- Additonal views are as follows:
- Anterior compression
- Cleavage view
- Exaggerated craniocaudal (XCCL) view
- Diagnostic mammography
- Diagnostic mammography is indicated in the following patients:
- Patients who present with the abnormal sign and symptoms
- Women with abnormal screening mammography.
- Patients with:
- Palpable lump (Particularly if raised clinical concern)
- Nipple discharge
- Focal pain
- The views obtained are tailored to evaluate a specific abnormality
- Ultrasounography might be added to increase the accuracy of studies
- Additional views that might be recommended are as follows:
- Focal spot compression view
- Magnification spot compression view
- 90 degree lateral view (true lateral view )
- Tangential views
- Rolled views
- Surveillance mammography
- Performed in women with a history of breast cancer.
- Generally recommended for the five years following the diagnosis of breast cancer
- Procedure is similar to diagnostic mammography (Presence of an onsite radiologist is recommended)
- After having negative results for five years, the patient may return to routine screening
Future (investigational) modern mammography utilities
- 3D-Tomosynthesis
- While traditional mammograms are 2-D and provide a flat image, tomosynthesis creates a 3-D image.
- Standard mammograms and tomosynthesis both use X-rays.
- This screening procedure is similar to a mammogram, but it produces a 3-D image rather than a flat one.
- As a result, it may provide more accurate information about whether or not there are any changes in the breast.
- The addition of 3D-Tomosynthesis to conventional digital mammography increases sensitivity (cancer detection rates) and may increase the positive predictive value of the screening exam.
- According to a recently published study on more than 35,000 women by Manisha Bahl et.al., in women 65 Y/O and older, digital breast tomosynthesis (DBT) achieved a higher specificity for detecting breast cancer and identified the disease at an earlier stage compared to traditional 2-D mammography. (Published on 2 April 2019 in Radiology)
- Stereoscopic digital mammography
- Currently under investigation
- Full-field digital mammogram images taken at a slight angle from each other using a stereoscopic viewer
- It has been shown in recent studies that
- Stereoscopic digital mammography has a higher specificity of for cancer detection compared to the standard imaging
- The sensitivity was the same,
- The recall rate for stereoscopic imaging was lower (9.6 versus 12.9 percent for standard imaging)
- In its current format, the radiation dose was twice compared to that of a standard imaging
Alternatives to mammography
- While the cost of mammography is relatively low, its sensitivity is not ideal, with reports listing the range from 45% to about 90% depending on factors such as the density of the breast.
- Neither is the X-ray based technology completely benign, as noted above.
- Therefore there is considerable ongoing research into the use of alternative technologies.
- One approach, contrast-enhanced magnetic resonance imaging (MRI), has shown substantial progress.
- In this method, the breast is scanned in an MRI device before and after the intravascular injection of a contrast agent (Gadolinium DTPA).
- The pre-contrast images are "subtracted" from the post-contrast images, and any areas that have increased blood flow are seen as bright spots on a dark background.
- Since breast cancers generally have an increased blood supply, the contrast agent causes these lesions to "light up" on the images.
- The available literature suggests that the sensitivity of contrast-enhanced breast MRI is considerably higher than that of either radiographic mammography or ultrasound and is generally reported to be in excess of 95% (though not all reported studies have been as encouraging).
- The specificity (the confidence that a lesion is cancerous and not a false positive) is only fair, thus a positive finding by MRI should not be interpreted as a definitive diagnosis.
- The reports of 4,271 breast MRIs from eight large scale clinical trials were reviewed recently by CD Lehman.
- Overall the sensitivity ranged from 71% to 100% in these reports, however, the call-back rates were low at 10% and the risk of having a benign biopsy was reported at 5%, a significant improvement over mammography.
- Several medical instrument vendors have entered this arena with breast MRI solutions.
- One company, Aurora Systems, stands out as being the only manufacturer to make a breast-dedicated unit and as the exclusive patent holder of certain solutions to fat signal suppression that appears to be more or less essential.
- Siemens, General Electric, and Philips Medical, the leading manufacturers of MRI instruments, offer breast MRI products or add-ons, and several third-party companies (e.g., MRI Devices/IGC) offer aftermarket products to enable breast MRI on conventional MRI instruments.
Regulation
- Mammography facilities in the United States and its territories (including military bases) are subject to the Mammography Quality Standards Act (MQSA).
- The act requires annual inspections and accreditation every 3 years through an FDA-approved body.
- Facilities found deficient during the inspection or accreditation process can be barred from performing mammograms until corrective action has been verified or, in extreme cases, can be required to notify past patients that their exams were sub-standard and should not be trusted.
- Despite the passage of the MQSA by Congress in 1992 and the nearly 1 billion dollar cost, the aggregate sensitivity of mammography in the USA is similar to what it was in the 1970s.
- At this time MQSA applies only to traditional mammography and not related scans such as breast ultrasound, stereotactic breast biopsy, or breast MRI.
References
- ↑ Linver MN, Rosenberg RD (1998) Callback rate after screening mammography. AJR Am J Roentgenol 171 (1):262-3. DOI:10.2214/ajr.171.1.9648803 PMID: 9648803
- ↑ 2.0 2.1 2.2 2.3 Fuller MS, Lee CI, Elmore JG (2015) Breast cancer screening: an evidence-based update. Med Clin North Am 99 (3):451-68. DOI:10.1016/j.mcna.2015.01.002 PMID: 25841594
- ↑ 3.0 3.1 Tsuchida J, Nagahashi M, Rashid OM, Takabe K, Wakai T (2015) At what age should screening mammography be recommended for Asian women? Cancer Med 4 (7):1136-44. DOI:10.1002/cam4.468 PMID: 25914223
- ↑ 4.0 4.1 Katzen J, Dodelzon K (2018) A review of computer aided detection in mammography. Clin Imaging 52 ():305-309. DOI:10.1016/j.clinimag.2018.08.014 PMID: 30216858
- ↑ 5.0 5.1 Henriksen EL, Carlsen JF, Vejborg IM, Nielsen MB, Lauridsen CA (2019) The efficacy of using computer-aided detection (CAD) for detection of breast cancer in mammography screening: a systematic review. Acta Radiol 60 (1):13-18. DOI:10.1177/0284185118770917 PMID: 29665706
- ↑ Kwong A, Hancock SL, Bloom JR, Pal S, Birdwell RL, Mariscal C et al. (2008) Mammographic screening in women at increased risk of breast cancer after treatment of Hodgkin's disease. Breast J 14 (1):39-48. DOI:10.1111/j.1524-4741.2007.00524.x PMID: 18186864
- ↑ 7.0 7.1 Balleyguier C, Ayadi S, Van Nguyen K, Vanel D, Dromain C, Sigal R (2007) BIRADS classification in mammography. Eur J Radiol 61 (2):192-4. DOI:10.1016/j.ejrad.2006.08.033 PMID: 17164080
- ↑ Hu S, Szymanski J, Khairy Z, Lo Y, Wang Y (2018) Breast pathology and mammography BI-RADS category correlation study - A single institute experience. Ann Diagn Pathol 35 ():11-15. DOI:10.1016/j.anndiagpath.2018.02.002 PMID: 30072013
- ↑ Elwood J, Cox B, Richardson A. "The effectiveness of breast cancer screening by mammography in younger women". Online J Curr Clin Trials. Doc No 32: [23, 227 words, 195 paragraphs]. PMID 8305999.
- ↑ Fletcher SW, Black W, Harris R, Rimer BK, Shapiro S (1993). "Report of the International Workshop on Screening for Breast Cancer". J. Natl. Cancer Inst. 85 (20): 1644–56. PMID 8105098.
|access-date=requires|url=(help) - ↑ Brewer NT, Salz T, Lillie SE (2007). "Systematic review: the long-term effects of false-positive mammograms". Ann Intern Med. 146 (7): 502–10. PMID 17404352.
- ↑ Smith-Bindman R, Ballard-Barbash R, Miglioretti DL, Patnick J, Kerlikowske K (2005). "Comparing the performance of mammography screening in the USA and the UK". Journal of medical screening. 12 (1): 50–4. doi:10.1258/0969141053279130. PMID 15814020.
- ↑ Lee KA, Talati N, Oudsema R, Steinberger S, Margolies LR (2018) BI-RADS 3: Current and Future Use of Probably Benign. Curr Radiol Rep 6 (2):5. DOI:10.1007/s40134-018-0266-8 PMID: 29399419
- ↑ Armstrong K, Moye E, Williams S, Berlin JA, Reynolds EE (2007). "Screening mammography in women 40 to 49 years of age: a systematic review for the American College of Physicians". Ann. Intern. Med. 146 (7): 516–26. PMID 17404354.
- ↑ Stout NK, Lee SJ, Schechter CB, Kerlikowske K, Alagoz O, Berry D et al. (2014) Benefits, harms, and costs for breast cancer screening after US implementation of digital mammography. J Natl Cancer Inst 106 (6):dju092. DOI:10.1093/jnci/dju092 PMID: 24872543
- ↑ Fancher CE, Scott A, Allen A, Dale P (2017) Mammographic Screening at Age 40 or 45? What Difference Does It Make? The Potential Impact of American Cancer Society Mammography Screening Guidelines. Am Surg 83 (8):847-849. PMID: 28822389
- ↑ Estrada SS (2016) Review of the New American Cancer Society Guidelines for Breast Cancer Screening for Women at Average Risk. J Adv Pract Oncol 7 (5):563-566. PMID: 29282431
- ↑ Seely JM, Alhassan T (2018) Screening for breast cancer in 2018-what should we be doing today? Curr Oncol 25 (Suppl 1):S115-S124. DOI:10.3747/co.25.3770 PMID: 29910654
- ↑ Miglioretti DL, Lange J, van den Broek JJ, Lee CI, van Ravesteyn NT, Ritley D et al. (2016) Radiation-Induced Breast Cancer Incidence and Mortality From Digital Mammography Screening: A Modeling Study. Ann Intern Med 164 (4):205-14. DOI:10.7326/M15-1241 PMID: 26756460