Atazanavir and cobicistat: Difference between revisions
(Created page with "{{DrugProjectFormSinglePage |authorTag={{AKT}} |genericName=atazanavir and cobicistat |aOrAn=a |drugClass=combination of a human immunodeficiency virus protease inhibit...") |
No edit summary |
||
| (15 intermediate revisions by the same user not shown) | |||
| Line 3: | Line 3: | ||
|genericName=atazanavir and cobicistat | |genericName=atazanavir and cobicistat | ||
|aOrAn=a | |aOrAn=a | ||
|drugClass=combination of a [[human immunodeficiency virus]] [[protease inhibitor]] and a [[CYP3A]] [[inhibitor]] | |drugClass=combination of a [[HIV|human immunodeficiency virus]] [[protease inhibitor]] and a [[CYP3A]] [[inhibitor]] | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=HIV-1 infection in combination with other [[antiretroviral]] agents | |indication=[[HIV|HIV-1]] infection in combination with other [[antiretroviral]] agents | ||
|adverseReactions=abnormal [[bilirubin]] levels, [[jaundice]], and [[sclera|scleral]] [[icterus]] | |adverseReactions=abnormal [[bilirubin]] levels, [[jaundice]], and [[sclera|scleral]] [[icterus]] | ||
|blackBoxWarningTitle='''<span style="color:#FF0000;"></span>''' | |blackBoxWarningTitle='''<span style="color:#FF0000;"></span>''' | ||
|blackBoxWarningBody=''<span style="color:#FF0000;"></span>'' | |blackBoxWarningBody=''<span style="color:#FF0000;"></span>'' | ||
|fdaLIADAdult= | |fdaLIADAdult=[[Atazanavir]] and [[cobicistat]] is indicated in combination with other [[antiretroviral]] agents for the treatment of [[HIV|human immunodeficiency virus]] ([[HIV|HIV-1]]) infection in adults. | ||
Limitations of Use: Use of | Limitations of Use: Use of [[atazanavir]] and [[cobicistat]] in treatment-experienced patients should be guided by the number of baseline primary [[protease inhibitor]] resistance substitutions. | ||
'''Dosing Information''' | '''Dosing Information''' | ||
* | *[[Atazanavir]] and [[cobicistat]] is a fixed-dose combination product containing 300 mg of [[atazanavir]] and 150 mg of [[cobicistat]]. | ||
*In treatment-naive and -experienced adults, the recommended dosage of | *In treatment-naive and -experienced adults, the recommended dosage of [[atazanavir]] and [[cobicistat]] is one tablet taken once daily orally with food. | ||
*Administer | *Administer [[atazanavir]] and [[cobicistat]] in conjunction with other [[antiretroviral]] agents. | ||
*When coadministered with H2-receptor antagonists or proton-pump inhibitors, dose separation may be required. | *When coadministered with [[H2 antagonist|H2-receptor antagonists]] or [[Proton pump inhibitor|proton-pump inhibitors]], dose separation may be required. | ||
*Dosage in Patients with Renal Impairment | *Dosage in Patients with [[renal impairment|Renal Impairment]] | ||
** | **[[Atazanavir]] and [[cobicistat]] is not recommended in [[HIV|HIV-1]] treatment-experienced patients with [[end-stage renal disease]] managed with [[hemodialysis]]. | ||
** | **[[Atazanavir]] and [[cobicistat]] coadministered with [[tenofovir]] DF is not recommended in patients with estimated [[creatinine clearance]] below 70 mL/min. Coadministration of [[atazanavir]] and [[cobicistat]] and [[tenofovir]] DF in combination with concomitant or recent use of a [[nephrotoxic]] agent is not recommended. | ||
*Not Recommended in Patients with Any Degree of Hepatic Impairment | *Not Recommended in Patients with Any Degree of [[hepatic impairment|Hepatic Impairment]] | ||
** | **[[Atazanavir]] and [[cobicistat]] is not recommended in patients with any degree of [[hepatic impairment]]. | ||
|offLabelAdultGuideSupport=There is limited information regarding ''Off-Label Guideline-Supported Use'' of Atazanavir and cobicistat in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding ''Off-Label Guideline-Supported Use'' of [[Atazanavir]] and [[cobicistat]] in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information regarding ''Off-Label Non–Guideline-Supported Use'' of Atazanavir and cobicistat in adult patients. | |offLabelAdultNoGuideSupport=There is limited information regarding ''Off-Label Non–Guideline-Supported Use'' of [[Atazanavir]] and [[cobicistat]] in adult patients. | ||
|offLabelPedGuideSupport=There is limited information regarding ''Off-Label Guideline-Supported Use'' of Atazanavir and cobicistat in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding ''Off-Label Guideline-Supported Use'' of [[Atazanavir]] and [[cobicistat]] in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding ''Off-Label Non–Guideline-Supported Use'' of Atazanavir and cobicistat in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding ''Off-Label Non–Guideline-Supported Use'' of [[Atazanavir]] and [[cobicistat]] in pediatric patients. | ||
|contraindications= | |contraindications=[[Atazanavir]] and [[cobicistat]] is contraindicated: | ||
*In patients with previously demonstrated clinically significant hypersensitivity (e.g., Stevens-Johnson syndrome, erythema multiforme, or toxic skin eruptions) to any of the components of this product. | *In patients with previously demonstrated clinically significant [[hypersensitivity]] (e.g., [[Stevens-Johnson syndrome]], [[erythema multiforme]], or toxic skin eruptions) to any of the components of this product. | ||
*When coadministered with drugs that are highly dependent on CYP3A or UGT1A1 for clearance, and for which elevated plasma concentrations of the interacting drugs are associated with serious and/or life-threatening events (see Table 1). | *When coadministered with drugs that are highly dependent on [[CYP3A]] or [[UGT1A1]] for clearance, and for which elevated plasma concentrations of the interacting drugs are associated with serious and/or life-threatening events (see Table 1). | ||
*When coadministered with drugs that strongly induce CYP3A and may lead to lower exposure and loss of efficacy of | *When coadministered with drugs that strongly induce [[CYP3A]] and may lead to lower exposure and loss of efficacy of [[atazanavir]] and [[cobicistat]] (see Table 1). | ||
Table 1 displays drugs that are contraindicated with | Table 1 displays drugs that are contraindicated with [[atazanavir]] and [[cobicistat]]. | ||
[[File:Atazanavir and cobicistat T1.JPG|thumb|none|400px|This image is provided by the National Library of Medicine]] | [[File:Atazanavir and cobicistat T1.JPG|thumb|none|400px|This image is provided by the National Library of Medicine]] | ||
|alcohol=Alcohol-Atazanavir and cobicistat interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |warnings=*Cardiac Conduction Abnormalities | ||
**[[Atazanavir]] prolongs the [[PR interval]] of the [[electrocardiogram]] in some patients. | |||
**In healthy volunteers and in patients, abnormalities in [[atrioventricular node|atrioventricular]] (AV) conduction were [[asymptomatic]] and generally limited to first-degree [[AV node|AV]] block. | |||
**There have been reports of second-degree [[AV node|AV]] block and other conduction abnormalities. | |||
**In clinical trials of [[atazanavir]] that included [[electrocardiograms]], [[asymptomatic]] first-degree [[AV node|AV]] block was observed in 6% of [[atazanavir]]-treated patients (n=920) and 5% of patients (n=118) treated with [[atazanavir]] coadministered with [[ritonavir]]. | |||
**Because of limited clinical experience in patients with preexisting conduction system disease (e.g., marked first-degree [[AV node|AV]] block or second- or third-degree [[AV node|AV]] block), consider [[ECG]] monitoring in these patients. | |||
*Severe Skin Reactions | |||
**Cases of [[Stevens-Johnson syndrome]], [[erythema multiforme]], and toxic skin eruptions, including [[drug rash]], [[eosinophilia]] and systemic symptoms ([[DRESS]]) syndrome, have been reported in patients receiving [[atazanavir]]. [[Atazanavir]] and [[cobicistat]] should be discontinued if severe [[rash]] develops. | |||
**Mild-to-moderate [[maculopapular]] skin eruptions have also been reported in [[atazanavir]] clinical trials. These reactions had a median time to onset of 7.3 weeks and median duration of 1.4 weeks and generally did not result in treatment discontinuation. | |||
*Effects on [[serum creatinine|Serum Creatinine]] | |||
**[[Cobicistat]] decreases estimated [[creatinine clearance]] due to inhibition of tubular secretion of [[creatinine]] without affecting actual renal [[glomerular]] function. This effect should be considered when interpreting changes in estimated [[creatinine clearance]] in patients initiating [[atazanavir]] and [[cobicistat]], particularly in patients with medical conditions or receiving drugs needing monitoring with estimated [[creatinine clearance]]. | |||
**Prior to initiating therapy with [[atazanavir]] and [[cobicistat]], assess estimated [[creatinine clearance]]. | |||
**Dosage recommendations are not available for drugs that require dosage adjustments in [[cobicistat]]-treated patients with [[renal impairment]]. Consider alternative medications that do not require dosage adjustments in patients with [[renal impairment]]. | |||
**Although [[cobicistat]] may cause modest increases in [[serum creatinine]] and modest declines in estimated [[creatinine clearance]] without affecting renal [[glomerular]] function, patients who experience a confirmed increase in [[serum creatinine]] of greater than 0.4 mg/dL from baseline should be closely monitored for renal safety. | |||
*New Onset or Worsening [[renal impairment|Renal Impairment]] When Used with [[Tenofovir]] DF | |||
**[[Renal impairment]], including cases of [[acute renal failure]] and [[Fanconi syndrome]], has been reported when [[cobicistat]] was used in an [[antiretroviral]] regimen that contained [[tenofovir]] DF. Therefore, coadministration of [[atazanavir]] and [[cobicistat]] and [[tenofovir]] DF is not recommended in patients who have an estimated [[creatinine clearance]] below 70 mL/min. | |||
**When [[atazanavir]] and [[cobicistat]] is used with [[tenofovir]] DF, document [[urine]] [[glucose]] and [[urine]] [[protein]] at baseline and perform routine monitoring of estimated [[creatinine clearance]], [[urine]] [[glucose]], and [[urine]] [[protein]] during treatment. | |||
**Measure serum [[phosphorus]] in patients at risk for [[renal impairment]]. | |||
**Coadministration of [[atazanavir]] and [[cobicistat]] and [[tenofovir]] DF in combination with concomitant or recent use of a [[nephrotoxic]] agent is not recommended. | |||
**In a clinical trial over 144 weeks (N=692), 10 (2.9%) subjects treated with [[atazanavir]] coadministered with [[cobicistat]] and [[tenofovir]] DF and 11 (3.2%) subjects treated with [[atazanavir]] coadministered with [[ritonavir]] and [[tenofovir]] DF discontinued study drug due to a renal adverse event. | |||
**Seven of the 10 subjects (2.0% overall) in the [[cobicistat]] group had laboratory findings consistent with proximal renal [[tubulopathy]] leading to study drug discontinuation, compared to 7 of 11 subjects (2.0% overall) in the [[ritonavir]] group. One subject in the [[cobicistat]] group had [[renal impairment]] at baseline (e.g., estimated [[creatinine clearance]] less than 70 mL/min). | |||
**The laboratory findings in these 7 subjects with evidence of proximal [[tubulopathy]] improved but did not completely resolve in all subjects upon discontinuation of [[cobicistat]] coadministered with [[atazanavir]] and [[tenofovir]] DF. Renal replacement therapy was not required in any subject. | |||
*[[Nephrolithiasis]] and [[Cholelithiasis]] | |||
**Cases of [[nephrolithiasis]] and/or [[cholelithiasis]] have been reported during postmarketing surveillance in [[HIV]]-[[infection|infected]] patients receiving [[atazanavir]] therapy. | |||
**Some patients required hospitalization for additional management and some had complications. Because these events were reported voluntarily during clinical practice, estimates of frequency cannot be made. | |||
**If signs or symptoms of [[nephrolithiasis]] and/or [[cholelithiasis]] occur, temporary interruption or discontinuation of therapy may be considered. | |||
*[[Hepatotoxicity]] | |||
**Patients with underlying [[hepatitis B]] or [[hepatitis C|C]] viral infections or marked elevations in [[transaminases]] may be at increased risk for developing further [[transaminase]] elevations or hepatic decompensation. | |||
**In these patients, hepatic laboratory testing should be conducted prior to initiating therapy with [[atazanavir]] and [[cobicistat]] and during treatment. | |||
*Risk of Serious Adverse Reactions or Loss of Virologic Response Due to Drug Interactions | |||
**Initiation of [[atazanavir]] and [[cobicistat]], a [[CYP3A]] [[inhibitor]], in patients receiving medications metabolized by [[CYP3A]] or initiation of medications metabolized by [[CYP3A]] in patients already receiving [[atazanavir]] and [[cobicistat]], may increase plasma concentrations of medications metabolized by [[CYP3A]]. | |||
**Initiation of medications that inhibit or induce [[CYP3A]] may increase or decrease concentrations of [[atazanavir]] and [[cobicistat]], respectively. | |||
**Increased concentrations of [[atazanavir]] and [[cobicistat]] may lead to: | |||
***Clinically significant adverse reactions, potentially leading to severe, life threatening, or fatal events from higher exposures of concomitant medications. | |||
***Clinically significant adverse reactions from higher exposures of [[atazanavir]] and [[cobicistat]]. | |||
**Decreased concentrations of [[atazanavir]] and [[cobicistat]] may lead to: | |||
***Loss of therapeutic effect of [[atazanavir]] and [[cobicistat]] and possible development of resistance. | |||
**See Table 5 for steps to prevent or manage these possible and known significant drug interactions, including dosing recommendations. | |||
**Consider the potential for drug interactions prior to and during [[atazanavir]] and [[cobicistat]] therapy; review concomitant medications during [[atazanavir]] and [[cobicistat]] therapy; and monitor for the adverse reactions associated with the concomitant medications. | |||
**When used with concomitant medications, [[atazanavir]] and [[cobicistat]] may result in different drug interactions than those observed or expected with [[atazanavir]] coadministered with [[ritonavir]]. Complex or unknown mechanisms of drug interactions preclude extrapolation of drug interactions with [[atazanavir]] coadministered with [[ritonavir]] to certain [[atazanavir]] and [[cobicistat]] interactions | |||
*[[antiretroviral|Antiretrovirals]] that are Not Recommended | |||
**[[Atazanavir]] and [[cobicistat]] is not recommended in combination with other [[antiretroviral]] drugs that require [[CYP3A]] inhibition to achieve adequate exposures (e.g., other HIV [[protease inhibitors]] or [[elvitegravir]]) because dosing recommendations for such combinations have not been established and coadministration may result in decreased plasma concentrations of the [[antiretroviral]] agents, leading to loss of therapeutic effect and development of resistance. | |||
**[[Aatazanavir]] and [[cobicistat]] is not recommended in combination with [[ritonavir]] or products containing [[ritonavir]] due to similar effects of [[cobicistat]] and [[ritonavir]] on [[CYP3A]]. | |||
*[[Hyperbilirubinemia]] | |||
**Most patients taking [[atazanavir]] experience [[asymptomatic]] elevations in indirect (unconjugated) [[bilirubin]] related to inhibition of UDP-[[glucuronosyltransferase]] (UGT). This [[hyperbilirubinemia]] is reversible upon discontinuation of [[atazanavir]]. | |||
**Hepatic [[transaminase]] elevations that occur with [[hyperbilirubinemia]] should be evaluated for alternative etiologies. | |||
**No long-term safety data are available for patients experiencing persistent elevations in total [[bilirubin]] greater than 5 times the upper limit of normal (ULN). Alternative [[antiretroviral]] therapy to [[atazanavir]] and [[cobicistat]] may be considered if [[jaundice]] or [[sclera|scleral]] [[icterus]] associated with [[bilirubin]] elevations presents cosmetic concerns for patients. | |||
*[[Immune reconstitution syndrome|Immune Reconstitution Syndrome]] | |||
**[[Immune reconstitution syndrome]] has been reported in patients treated with combination [[antiretroviral]] therapy, including [[atazanavir]], a component of [[atazanavir]] and [[cobicistat]]. | |||
**During the initial phase of combination [[antiretroviral]] treatment, patients whose [[immune system]] responds may develop an [[inflammatory]] response to indolent or residual [[opportunistic infection|opportunistic infections]] (such as [[Mycobacterium avium]] [[infection]], [[cytomegalovirus]], [[Pneumocystis jiroveci pneumonia]], or [[tuberculosis]]), which may necessitate further evaluation and treatment. | |||
**[[Autoimmune disorders]] (such as [[Graves’ disease]], [[polymyositis]], and [[Guillain-Barré syndrome]]) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of treatment. | |||
*[[diabetes mellitus|Diabetes Mellitus]]/[[Hyperglycemia]] | |||
**New-onset [[diabetes mellitus]], exacerbation of preexisting [[diabetes mellitus]], and [[hyperglycemia]] have been reported during postmarketing surveillance in [[HIV|HIV]]-[[infection|infected]] patients receiving [[protease inhibitor]] therapy. | |||
**Some patients required either initiation or dose adjustments of [[insulin]] or oral [[hypoglycemic]] agents for treatment of these events. In some cases, [[diabetic ketoacidosis]] has occurred. In those patients who discontinued [[protease inhibitor]] therapy, [[hyperglycemia]] persisted in some cases. | |||
**Because these events have been reported voluntarily during clinical practice, estimates of frequency cannot be made and a causal relationship between [[protease inhibitor]] therapy and these events has not been established. | |||
*Fat Redistribution | |||
**Redistribution/accumulation of body fat including central [[obesity]], [[Lipodystrophy|dorsocervical fat enlargement]] (buffalo hump), peripheral [[wasting]], facial [[wasting]], breast enlargement, and “[[cushingoid appearance]]” have been observed in patients receiving [[antiretroviral]] therapy. | |||
**The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established. | |||
*[[Hemophilia]] | |||
**There have been reports of increased bleeding, including spontaneous skin [[hematoma|hematomas]] and [[hemarthrosis]], in patients with [[hemophilia]] type A and B treated with [[protease inhibitors]]. | |||
**In some patients additional factor VIII was given. In more than half of the reported cases, treatment with [[protease inhibitors]] was continued or reintroduced. | |||
**A causal relationship between [[protease inhibitor]] therapy and these events has not been established. | |||
|clinicalTrials=Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | |||
The safety of [[atazanavir]] and [[cobicistat]] coadministered as single agents is based on Week 144 data from a Phase 3 trial, Study 114, in which 692 [[HIV|HIV-1]] infected, [[antiretroviral]] treatment-naive subjects received: | |||
*atazanavir coadministered with [[cobicistat]] and [[emtricitabine]]/[[tenofovir]] DF (N=344) or | |||
*atazanavir coadministered with [[ritonavir]] and [[emtricitabine]]/[[tenofovir]] DF (N=348) | |||
The most common adverse reactions (Grades 2-4) and reported in ≥5% of subjects in the [[atazanavir]] coadministered with [[cobicistat]] group were [[jaundice]] (6%) and [[rash]] (5%). | |||
The proportion of subjects who discontinued study treatment due to adverse events, regardless of severity, was 11% in both the [[atazanavir]] coadministered with [[cobicistat]] and [[atazanavir]] coadministered with [[ritonavir]] groups. Table 2 lists the frequency of adverse reactions (Grades 2-4) occurring in at least 2% of subjects in the [[atazanavir]] coadministered with [[cobicistat]] group in Study 114. | |||
[[File:Atazanavir and cobicistat T2.JPG|thumb|none|400px|This image is provided by the National Library of Medicine]] | |||
Less Common Adverse Reactions: | |||
Selected adverse reactions of at least moderate severity (≥ Grade 2) occurring in less than 2% of subjects receiving [[atazanavir]] coadministered with [[cobicistat]] and [[emtricitabine]]/[[tenofovir]] DF are listed below. These events have been included because of investigator’s assessment of potential causal relationship and were considered serious or have been reported in more than one subject treated with [[atazanavir]] coadministered with [[cobicistat]], and reported with greater frequency compared with the [[atazanavir]] coadministered with [[ritonavir]] group. | |||
*Gastrointestinal Disorders: [[vomiting]], [[upper abdominal pain]] | |||
*General Disorders and Administration Site Conditions: [[fatigue]] | |||
*Musculoskeletal and Connective Tissue Disorders: [[rhabdomyolysis]] | |||
*Psychiatric Disorders: [[depression]], [[abnormal dreams]], [[insomnia]] | |||
*Renal and Urinary Disorders: [[nephropathy]], [[Fanconi syndrome]] acquired, [[nephrolithiasis]] | |||
Laboratory Abnormalities: | |||
The frequency of laboratory abnormalities (Grades 3-4) occurring in at least 2% of subjects in the [[atazanavir]] coadministered with [[cobicistat]] group in Study 114 is presented in Table 3. | |||
[[File:Atazanavir and cobicistat T3.JPG|thumb|none|400px|This image is provided by the National Library of Medicine]] | |||
Increase in [[serum creatinine|Serum Creatinine]]: | |||
[[Cobicistat]], a component of [[atazanavir]] and [[cobicistat]], has been shown to increase [[serum creatinine]] and decrease estimated [[creatinine clearance]] due to inhibition of tubular secretion of [[creatinine]] without affecting actual renal [[glomerular]] function. In Study 114, increases in [[serum creatinine]] and decreases in estimated [[creatinine clearance]] occurred early in treatment in the [[atazanavir]] coadministered with [[cobicistat]] group after which they stabilized. The mean (± SD) change in estimated [[glomerular filtration rate]] (eGFR) by Cockcroft-Gault method after 144 weeks of treatment was −15.1 ± 16.5 mL/min in the [[atazanavir]] coadministered with [[cobicistat]] group and −8.0 ± 16.8 mL/min in the [[atazanavir]] coadministered with [[ritonavir]] group. | |||
Serum [[lipid|Lipids]]: | |||
Changes from baseline in [[total cholesterol]], [[HDL-cholesterol]], [[LDL-cholesterol]], and [[triglycerides]] are presented in Table 4. In both groups, mean values for serum [[lipid|lipids]] remained within the normal range for each laboratory test. The clinical significance of these changes is unknown. | |||
[[File:Atazanavir and cobicistat T4.JPG|thumb|none|400px|This image is provided by the National Library of Medicine]] | |||
|drugInteractions=Potential for [[atazanavir]] and [[cobicistat]] to Affect Other Drugs: | |||
*[[Atazanavir]] is an [[inhibitor]] of [[CYP3A]] and [[UGT1A1]] and a weak inhibitor of [[CYP2C8]]. [[Cobicistat]] is an inhibitor of [[CYP3A]] and CYP2D6. The transporters that [[cobicistat]] inhibits include [[P-glycoprotein]] (P-gp), BCRP, OATP1B1 and OATP1B3. | |||
*Coadministration of [[atazanavir]] and [[cobicistat]] with drugs highly dependent on [[CYP3A]] for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events is contraindicated. | |||
*Coadministration of [[atazanavir]] and [[cobicistat]] and drugs primarily metabolized by [[CYP3A]], [[UGT1A1]] and/or [[CYP2D6]] or drugs that are substrates of [[P-gp]], BCRP, OATP1B1 and/or OATP1B3 may result in increased plasma concentrations of the other drug that could increase or prolong its therapeutic effects and adverse reactions which may require dose adjustments and/or additional monitoring as shown in Table 5. | |||
*Use of [[atazanavir]] and [[cobicistat]] is not recommended when coadministered with drugs highly dependent on [[CYP2C8]] for clearance with narrow therapeutic indices (e.g., [[paclitaxel]], [[repaglinide]]). | |||
Potential for Other Drugs to Affect [[Atazanavir]] and [[Cobicistat]]: | |||
*[[Atazanavir]] and [[cobicistat]] are [[CYP3A4]] substrates; therefore, drugs that induce [[CYP3A4]] may decrease [[atazanavir]] and [[cobicistat]] plasma concentrations and reduce the therapeutic effect of [[atazanavir]] and [[cobicistat]], leading to development of resistance to [[atazanavir]] (see Table 5). [[Cobicistat]] is also metabolized by [[CYP2D6]] to a minor extent. | |||
*Coadministration of [[atazanavir]] and [[cobicistat]] with other drugs that inhibit [[CYP3A4]] may increase the plasma concentrations of [[cobicistat]] and [[atazanavir]] (see Table 5). | |||
*[[Atazanavir]] solubility decreases as pH increases. Reduced plasma concentrations of [[atazanavir]] are expected if [[Proton pump inhibitor|proton-pump inhibitors]], [[antacids]], buffered medications, or [[H2 antagonist|H2-receptor antagonists]] are administered with [[atazanavir]] and [[cobicistat]]. | |||
Established and Other Potentially Significant Drug Interactions: | |||
*Drug interaction trials were not conducted for [[atazanavir]] and [[cobicistat]]. Drug interaction trials were conducted with [[cobicistat]] in combination with [[desipramine]], [[digoxin]], or [[efavirenz]]. | |||
*Table 5 provides dosing recommendations as a result of drug interactions with the components of [[atazanavir]] and [[cobicistat]]. These recommendations are based either on observed drug interactions in studies of [[cobicistat]], [[atazanavir]], or [[atazanavir]] coadministered with [[ritonavir]] or predicted drug interactions based on the expected magnitude of interaction and potential for serious events or loss of therapeutic effect of [[atazanavir]] and [[cobicistat]]. | |||
[[File:Atazanavir and cobicistat T5p1.JPG|none|400px]] | |||
[[File:Atazanavir and cobicistat T5p2.JPG|none|400px]] | |||
[[File:Atazanavir and cobicistat T5p3.JPG|none|400px]] | |||
[[File:Atazanavir and cobicistat T5p4.JPG|none|400px]] | |||
[[File:Atazanavir and cobicistat T5p5.JPG|none|400px]] | |||
[[File:Atazanavir and cobicistat T5p6.JPG|none|400px]] | |||
[[File:Atazanavir and cobicistat T5p7.JPG|none|400px]] | |||
[[File:Atazanavir and cobicistat T5p8.JPG|none|400px]] | |||
[[File:Atazanavir and cobicistat T5p9.JPG|none|400px]] | |||
[[File:Atazanavir and cobicistat T5p10.JPG|none|400px]] | |||
[[File:Atazanavir and cobicistat T5p11.JPG|none|400px]] | |||
<small><small>These images are provided by the National Library of Medicine</small></small> | |||
Drugs with No Observed or Predicted Interactions with the Components of [[Atazanavir]] and [[Cobicistat]]: | |||
*Based on known metabolic profiles, clinically significant drug interactions are not expected between [[atazanavir]] and [[cobicistat]] and [[acetaminophen]], [[atenolol]], [[dapsone]], [[fluconazole]], [[trimethoprim]]/[[sulfamethoxazole]], or [[azithromycin]]. | |||
|useInPregnancyFDA= | |||
Pregnancy Exposure Registry: | |||
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to [[atazanavir]] and [[cobicistat]] during pregnancy. Healthcare providers are encouraged to register patients by calling the [[Antiretroviral]] Pregnancy Registry (APR) at 1-800-258-4263. | |||
Risk Summary: | |||
Prospective pregnancy data from the [[Antiretroviral]] Pregnancy Registry (APR) are not sufficient to adequately assess the risk of [[birth defects]] or [[miscarriage]]. [[Cobicistat]] use in women during pregnancy has not been evaluated; however, [[atazanavir]] use during pregnancy has been evaluated in a limited number of women. The pharmacokinetics of [[atazanavir]] and [[cobicistat]] have not been evaluated in pregnant patients. Available data from the APR show no difference in the risk of overall major birth defects for [[atazanavir]] compared with the background rate for major birth defects of 2.7% in a U.S. reference population of the Metropolitan Atlanta Congenital Defects Program (MACDP). | |||
In animal reproduction studies, no evidence of adverse developmental outcomes was observed following oral administration of the components of [[atazanavir]] and [[cobicistat]] to pregnant rats and rabbits [see Data]. During [[organogenesis]] in the rat and rabbit, [[atazanavir]] exposures (AUC) were similar to those observed at the human clinical dose (300 mg/day [[atazanavir]] boosted with 100 mg/day [[ritonavir]]), while exposures were up to 1.4 (rats) and 3.3 (rabbits) times human exposures at the maximal recommended human dose (MRHD) of 150 mg. | |||
The background risk of major [[birth defects]] and [[miscarriage]] for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major [[birth defects]] and [[miscarriage]] in clinically recognized pregnancies is 2-4% and 15-20%, respectively. | |||
Clinical Considerations: | |||
''Dose Adjustment During Pregnancy and the Postpartum Period'' | |||
Dosing recommendations cannot be made because the pharmacokinetics, safety, and efficacy of [[atazanavir]] and [[cobicistat]] cannot be predicted from studies of other [[atazanavir]]-containing products in pregnant women. | |||
''Maternal Adverse Reactions'' | |||
[[Atazanavir]] | |||
Cases of [[lactic acidosis]] syndrome, sometimes fatal, and symptomatic [[hyperlactatemia]] have occurred in pregnant women using [[atazanavir]] in combination with [[nucleoside]] analogues, which are associated with an increased risk of [[lactic acidosis]] syndrome. | |||
[[Hyperbilirubinemia]] occurs frequently in patients who take [[atazanavir]], including pregnant women. Refer to the [[atazanavir]] prescribing information for use of [[atazanavir]] in pregnancy. | |||
Advise pregnant women of the potential risks of [[lactic acidosis]] syndrome and [[hyperbilirubinemia]]. | |||
''Fetal/Neonatal Adverse Reactions'' | |||
[[Atazanavir]] | |||
All infants, including neonates exposed to [[atazanavir]] in utero, should be monitored for the development of severe [[hyperbilirubinemia]] during the first few days of life. Advise pregnant women of the potential risk to newborn infants. Refer to the [[atazanavir]] prescribing information for use of [[atazanavir]] in pregnancy. | |||
Data: | |||
''Human Data'' | |||
[[Atazanavir]] | |||
Based on prospective reports from the interim APR of approximately 1600 live births following exposure to [[atazanavir]]-containing regimens (including 1037 live births in infants exposed in the first trimester and 569 exposed in second/third trimesters), there was no difference in the overall rate for birth defects for [[atazanavir]] (2.3%) compared with the background birth defect rate of 2.7% in the U.S. reference population of the MACDP. Based on prospective reports to the APR, the prevalence of birth defects in live births was 2.1% following first trimester exposure to [[atazanavir]]-containing regimens. | |||
[[Cobicistat]] | |||
Insufficient numbers of pregnancies with exposure to [[cobicistat]] have been reported to the APR to estimate the rate of birth defects. | |||
Animal Data: | |||
''[[Atazanavir]]'' | |||
[[Atazanavir]] was administered orally to pregnant rats (at 0, 200, 600, and 1920 mg/kg/day) and rabbits (at 0, 4, 15, and 60 mg/kg/day) during [[organogenesis]] (on gestation Days 6 through 15 and 7 through 19, respectively). No significant toxicological effects were observed in embryo-fetal toxicity studies performed with [[atazanavir]] at exposures (AUC) approximately 1.2 times higher (rats) and 0.7 times (rabbits) human exposures at the MRHD. In a rat pre- and postnatal developmental study, [[atazanavir]] was administered orally at doses of 0, 50, 220, and 1000 mg/kg/day from gestation Day 6 to postnatal Day 20. At a maternal toxic dose (1000 mg/kg/day), [[atazanavir]] caused body weight loss or weight gain suppression in the animal offspring at [[atazanavir]] exposures (AUC) of approximately 1.3 times higher than human exposures at the MRHD. | |||
''[[Cobicistat]]'' | |||
[[Cobicistat]] was administered orally to pregnant rats at doses of 0, 25, 50, 125 mg/kg/day on gestation Day 6 to 17. Maternal toxicity was noted at 125 mg/kg/day and was associated with increases in post-implantation loss and decreased fetal weights. No malformations were noted at doses up to 125 mg/kg/day. [[AUC|Systemic exposures]] (AUC) at 50 mg/kg/day in pregnant females were 1.4 times higher than the human exposures at the MRHD. In pregnant rabbits, [[cobicistat]] was administered orally at doses of 0, 20, 50, and 100 mg/kg/day during the gestation Days 7 to 20. No maternal or embryo/fetal effects were noted at the highest dose of 100 mg/kg/day. [[AUC|Systemic exposures]] (AUC) at 100 mg/kg/day were 3.3 times higher than exposures at the MRHD. | |||
In a pre- and postnatal developmental study in rats, [[cobicistat]] was administered orally at doses of 0, 10, 30, and 75 mg/kg from gestation Day 6 to postnatal Day 20, 21, or 22. At doses of 75 mg/kg/day of [[cobicistat]], neither maternal nor developmental toxicity was noted. [[AUC|Systemic exposures]] (AUC) at this dose were 0.9 times lower than exposures at the MRHD. | |||
|useInPregnancyAUS=Data | |||
|useInNursing=Risk Summary: | |||
The Centers for Disease Control and Prevention recommend that [[HIV]]-[[infection|infected]] mothers not breastfeed their infants to avoid risking postnatal transmission of HIV. | |||
There is no information regarding the effects of [[atazanavir]] and [[cobicistat]] on the breastfed infant or on milk production. | |||
[[Atazanavir]] has been detected in human milk. No data are available regarding [[atazanavir]] effects on milk production. [[Cobicistat]] is present in rat milk [see Data]. There is no information regarding the presence of [[cobicistat]] in human milk, the effects on the breastfed infant, or the effects on milk production. Because of the potential for (1) [[HIV]] transmission (in HIV-negative infants), (2) developing viral resistance (in [[HIV]]-positive infants), and (3) adverse reactions in a breastfed infant, instruct women not to breastfeed. | |||
Data: | |||
''Animal Data'' | |||
[[Cobicistat]]: During the prenatal and postnatal development toxicology study at doses up to 75 mg/kg/day, mean [[cobicistat]] milk to plasma ratio of up to 1.9 was measured 2 hours after administration to rats on lactation Day 10. | |||
|useInPed=[[Atazanavir]] is not recommended for use in pediatric patients below the age of 3 months due to the risk of [[kernicterus]]. | |||
The safety and efficacy of [[atazanavir]] and [[cobicistat]] in pediatric patients 3 months to less than 18 years of age have not been established. | |||
|useInGeri=Clinical studies with the components of [[atazanavir]] and [[cobicistat]] did not include sufficient numbers of patients aged 65 and older to determine whether they respond differently from younger patients. In general, appropriate caution should be exercised in the administration and monitoring of [[atazanavir]] and [[cobicistat]] in elderly patients reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. | |||
|useInRenalImpair=[[Atazanavir]] and [[cobicistat]] is not recommended for use in [[HIV]]-treatment-experienced patients with [[end-stage renal disease]] managed with [[hemodialysis]]. | |||
|useInHepaticImpair=[[Atazanavir]] and [[cobicistat]] is not recommended for use in patients with any degree of [[hepatic impairment]]. | |||
|administration=''Renal Testing'': | |||
Prior to starting [[atazanavir]] and [[cobicistat]], assess estimated [[creatinine clearance]] because [[cobicistat]] decreases estimated [[creatinine clearance]] due to inhibition of tubular secretion of [[creatinine]] without affecting actual renal [[glomerular]] function. When coadministering [[atazanavir]] and [[cobicistat]] with [[tenofovir]] disoproxil fumarate ([[tenofovir]] DF), assess estimated [[creatinine clearance]], [[urine]] [[glucose]], and [[urine]] [[protein]] at baseline and routinely monitor during treatment. | |||
''Hepatic Testing'': | |||
In patients with underlying [[hepatitis B]] or [[hepatitis C|C]] viral infections, conduct hepatic laboratory testing prior to initiating therapy and during treatment with [[atazanavir]] and [[cobicistat]]. Administer [[atazanavir]] and [[cobicistat]] in conjunction with other [[antiretroviral]] agents. When coadministered with [[H2 antagonist|H2-receptor antagonists]] or [[Proton pump inhibitor|proton-pump inhibitors]], dose separation may be required. | |||
|overdose=Treatment for overdosage with [[atazanavir]] and [[cobicistat]] should consist of general supportive measures, including monitoring of [[vital signs]] and [[ECG]], and observations of the patient’s clinical status. There is no specific antidote for overdose with [[atazanavir]] and [[cobicistat]]. Since [[atazanavir]] is extensively metabolized by the [[liver]] and both [[atazanavir]] and [[cobicistat]] are highly bound [[plasma proteins]], it is unlikely that [[atazanavir]] and [[cobicistat]] will be significantly removed by [[hemodialysis]] or [[peritoneal dialysis]]. | |||
Atazanavir: Human experience of acute overdose with [[atazanavir]] is limited. A single self-administered overdose of 29.2 g of [[atazanavir]] in an [[HIV]]-[[infection|infected]] patient (73 times the 400-mg recommended dose of [[atazanavir]] administered without a [[CYP3A]] [[inhibitor]]) was associated with [[asymptomatic]] [[bifascicular block]] and [[PR interval]] prolongation. These events resolved spontaneously. At [[atazanavir]] doses resulting in high [[atazanavir]] exposures, [[jaundice]] due to indirect (unconjugated) [[hyperbilirubinemia]] (without associated [[liver]] function test changes) or [[PR interval]] prolongation may be observed. | |||
|drugBox=[[File:Atazanavir drugbox.JPG|thumb|none|400px]] | |||
[[File:Cobicistat drugbox.JPG|thumb|none|400px]] | |||
|mechAction=[[Atazanavir]] and [[cobicistat]] is a fixed-dose combination of the [[HIV|HIV-1]] [[antiretroviral]] drug, [[atazanavir]] and the [[CYP3A]] [[inhibitor]], [[cobicistat]] | |||
[[Atazanavir]] and [[cobicistat]] is a fixed-dose combination of [[atazanavir]] (ATV) and the [[CYP3A]] [[inhibitor]] [[cobicistat]]. ATV is an azapeptide [[HIV|HIV-1]] [[protease inhibitor]] (PI) that selectively inhibits the virus-specific processing of viral Gag and Gag-Pol polyproteins in [[HIV|HIV-1]] infected cells, thus preventing formation of mature virions. [[Cobicistat]] is a mechanism-based inhibitor of [[CYP3A|cytochrome P450 3A]] ([[CYP3A]]). Inhibition of [[CYP3A]]-mediated [[metabolism]] by [[cobicistat]] increases the systemic exposure of the [[CYP3A]] substrate [[atazanavir]]. | |||
|structure=[[Atazanavir]]: [[Atazanavir]] is present as the sulfate salt. The chemical name for [[atazanavir]] sulfate is (3S,8S,9S,12S)-3,12-bis(1,1-dimethylethyl)-8-hydroxy-4,11-dioxo-9-(phenylmethyl)-6-[4-(2-pyridinyl)phenyl]methyl]-2,5,6,10,13-pentaazatetradecanedioic acid dimethyl ester, sulfate (1:1). Its molecular formula is C38H52N6O7•H2SO4, which corresponds to a molecular weight of 802.9 (sulfuric acid salt). The free base molecular weight is 704.9. [[Atazanavir]] sulfate has the following structural formula: | |||
[[File:Atazanavir description.jpg|thumb|none|400px|This image is provided by the National Library of Medicine]] | |||
Atazanavir sulfate is a white to pale-yellow crystalline powder. It is slightly soluble in water (4-5 mg/mL, free base equivalent) with the pH of a saturated solution in water being about 1.9 at 24 ± 3°C. | |||
[[Cobicistat]]: The chemical name for [[cobicistat]] is 1,3-thiazol-5-ylmethyl [(2R,5R)-5-{[(2S)-2-[(methyl{[2-(propan-2-yl)-1,3-thiazol-4-yl]methyl}carbamoyl)amino]-4-(morpholin-4-yl)butanoyl]amino}-1,6-diphenylhexan-2-yl]carbamate. It has a molecular formula of C40H53N7O5S2 and a molecular weight of 776.0. It has the following structural formula: | |||
[[File:Cobicistat structure.png|thumb|none|400px|This image is provided by the National Library of Medicine]] | |||
|PD='''Cardiac Electrophysiology''' | |||
[[Atazanavir]]: In a thorough [[QT]]/[[QTc]] study in 72 healthy subjects, [[atazanavir]] 400 mg and 800 mg ([[Cmax]] was 1.2 times and 2.4 times the [[Cmax]] observed with the recommended dosage of [[atazanavir]] and [[cobicistat]], respectively) without a [[CYP3A]] [[inhibitor]] did not prolong the [[QTc interval]] to any clinically relevant extent. [[Asymptomatic]] prolongation of the [[PR interval]] was noted in subjects receiving [[atazanavir]]. The mean (±SD) maximum change in [[PR interval]] from the predose for [[atazanavir]] 400 mg (n=65), [[atazanavir]] 800 mg (n=66), and placebo (n=67) was 24 (±15) msec, 60 (±25) msec, and 13 (±11) msec, respectively. Steady state [[atazanavir]] exposures ([[Cmax]] and [[AUC]]tau) observed in this healthy volunteer study exceeded those observed in patients treated with [[atazanavir]] coadministered with [[cobicistat]]. There is limited information on the potential for a pharmacodynamic interaction in humans between [[atazanavir]] and other drugs that prolong the [[PR interval]] of the [[electrocardiogram]]. | |||
In 1793 [[HIV]]-[[infection|infected]] patients receiving [[antiretroviral]] regimens, [[QTc]] prolongation was comparable in the [[atazanavir]]-containing and comparator regimens. No [[atazanavir]]-treated healthy subject or [[HIV]]-[[infection|infected]] patient in clinical trials had a [[QTc interval]] >500 msec. | |||
[[Cobicistat]]: In a thorough [[QT]]/[[QTc]] study in 48 healthy subjects, [[cobicistat]] 250 mg (1.7 times the recommended dosage in [[atazanavir]] and [[cobicistat]]) and 400 mg (2.7 times the recommended dosage in [[atazanavir]] and [[cobicistat]]) did not prolong the [[QTc interval]] to any clinically relevant extent. [[Asymptomatic]] prolongation of the [[PR interval]] was noted in subjects receiving [[cobicistat]]. The maximum mean (95% upper confidence bound) difference in [[PR interval|PR]] from placebo after baseline correction was 9.5 (12.1) msec for 250 mg and 20.2 (22.8) msec for 400 mg dose of [[cobicistat]]. | |||
'''Effects on [[serum creatinine|Serum Creatinine]]''' | |||
The effect of [[cobicistat]] on [[serum creatinine]] was investigated in a trial in subjects with normal [[renal function]] ([[glomerular filtration rate|eGFR]] ≥80 mL/min, N=12) and mild-to-moderate [[renal impairment]] ([[glomerular filtration rate|eGFR]] 50-79 mL/min, N=18). A statistically significant change in estimated [[glomerular filtration rate]], calculated by Cockcroft-Gault method (eGFRCG) from baseline, was observed after 7 days of treatment with [[cobicistat]] 150 mg among subjects with normal [[renal function]] (−9.9 ± 13.1 mL/min) and mild-to-moderate [[renal impairment]] (−11.9 ± 7.0 mL/min). No statistically significant changes in eGFRCG were observed compared to baseline for subjects with normal [[renal function]] or mild-to-moderate [[renal impairment]] 7 days after [[cobicistat]] was discontinued. The actual [[glomerular filtration rate]], as determined by the clearance of probe drug [[iohexol]], was not altered from baseline following treatment with [[cobicistat]] among subjects with normal [[renal function]] and mild-to-moderate [[renal impairment]], indicating that [[cobicistat]] inhibits tubular secretion of [[creatinine]], reflected as a reduction in eGFRCG, without affecting the actual [[glomerular filtration rate]]. | |||
|PK='''Absorption, Distribution, [[Metabolism]], and Excretion''' | |||
The pharmacokinetic (PK) properties of the components of [[atazanavir]] and [[cobicistat]] (atazanavir 300 mg and [[cobicistat]] 150 mg) were evaluated in healthy adult volunteers. Results are summarized in Table 6. | |||
[[File:Atazanavir and cobicistat T6.JPG|thumb|none|400px|This image is provided by the National Library of Medicine]] | |||
The pharmacokinetics of [[atazanavir]] was evaluated in [[HIV|HIV-1]] infected subjects who received [[atazanavir]] 300 mg coadministered with [[cobicistat]] 150 mg in combination with [[emtricitabine]]/[[tenofovir]] DF. The steady-state pharmacokinetic parameters of [[atazanavir]] coadministered with [[cobicistat]] are shown in Table 7. | |||
[[File:Atazanavir and cobicistat T7.JPG|thumb|none|400px|This image is provided by the National Library of Medicine]] | |||
'''Specific Populations''' | |||
''[[renal impairment|Renal Impairment]]'' | |||
[[Atazanavir]]: In healthy subjects, the renal elimination of unchanged [[atazanavir]] was approximately 7% of the administered dose. [[Atazanavir]] has been studied in adult subjects with severe [[renal impairment]] (n=20), including those on [[hemodialysis]], at multiple doses of 400 mg once daily. The mean [[atazanavir]] [[Cmax]] was 9% lower, [[AUC]] was 19% higher, and Cmin was 96% higher in subjects with severe [[renal impairment]] not undergoing [[hemodialysis]] (n=10), than in age-, weight-, and gender-matched subjects with normal [[renal function]]. In a 4-hour [[dialysis]] session, 2.1% of the administered dose was removed. When [[atazanavir]] was administered either prior to, or following [[hemodialysis]] (n=10), the geometric means for [[Cmax]], [[AUC]], and Cmin were approximately 25% to 43% lower compared to subjects with normal [[renal function]]. The mechanism of this decrease is unknown. | |||
[[Cobicistat]]: A study of the pharmacokinetics of [[cobicistat]] was performed in non−[[HIV|HIV-1]] infected subjects with severe [[renal impairment]] (estimated [[creatinine clearance]] below 30 mL/min). No clinically relevant differences in [[cobicistat]] pharmacokinetics were observed between subjects with severe [[renal impairment]] and healthy subjects. | |||
''[[hepatic impairment|Hepatic Impairment]]'' | |||
[[Atazanavir]] and [[cobicistat]] has not been studied in patients with [[hepatic impairment]]. | |||
[[Atazanavir]]: [[Atazanavir]] is primarily [[metabolism|metabolized]] and eliminated by the [[liver]]. Increased concentrations of [[atazanavir]] are expected in patients with moderately or severely impaired hepatic function. | |||
[[Cobicistat]]: [[Cobicistat]] is primarily metabolized and eliminated by the liver. No clinically relevant differences in [[cobicistat]] pharmacokinetics were observed between subjects with moderate [[hepatic impairment]] ([[Child-Pugh]] Class B) and healthy subjects. The effect of severe [[hepatic impairment]] ([[Child-Pugh]] Class C) on the pharmacokinetics of [[cobicistat]] has not been studied. | |||
''Gender and Age'' | |||
Atazanavir: There were no clinically important pharmacokinetic differences observed due to age or gender. | |||
Cobicistat: No clinically relevant pharmacokinetic differences have been observed between men and women for cobicistat. | |||
''Assessment of Drug Interactions'' | |||
[[Atazanavir]] has been shown in vivo not to induce its own [[metabolism]], nor to increase the biotransformation of some drugs metabolized by [[CYP3A]]. In a multiple-dose study, [[atazanavir]] decreased the urinary ratio of [[endogenous]] 6β-OH cortisol to [[cortisol]] versus baseline, indicating that [[CYP3A]] production was not induced. | |||
Drug interaction studies were not conducted for [[atazanavir]] and [[cobicistat]] or for [[atazanavir]] coadministered with [[cobicistat]]. Drug interaction studies of [[cobicistat]] were conducted with [[desipramine]], [[digoxin]], and [[efavirenz]]. Drug interaction studies of [[cobicistat]] coadministered with [[elvitegravir]] included [[rosuvastatin]] and [[rifabutin]]. The effects of [[cobicistat]] on the exposure of coadministered drugs are summarized in Table 8. | |||
[[File:Atazanavir and cobicistat T8.JPG|thumb|none|400px|This image is provided by the National Library of Medicine]] | |||
|nonClinToxic='''[[Carcinogenesis]], [[Mutagenesis]], Impairment of [[Fertility]]''' | |||
''[[Carcinogenesis]]'': | |||
[[Atazanavir]]: Long-term [[carcinogenicity]] studies in mice and rats were carried out with [[atazanavir]] for two years. In the mouse study, drug-related increases in hepatocellular [[adenomas]] were found in females at 360 mg/kg/day. The [[AUC|systemic drug exposure]] ([[AUC]]) at the NOAEL in females, (120 mg/kg/day) was 2.8 times and in males (80 mg/kg/day) was 2.9 times higher than those in humans at the clinical dose (300 mg/day [[atazanavir]] coadministered with 100 mg/day [[ritonavir]], nonpregnant patients). In the rat study, no drug-related increases in [[tumor]] incidence were observed at doses up to 1200 mg/kg/day, for which [[AUC]]s were 1.1 (males) or 3.9 (females) times those measured in humans at the clinical dose. | |||
[[Cobicistat]]: In a long-term [[carcinogenicity]] study in mice, no drug-related increases in [[tumor]] incidence were observed at doses up to 50 and 100 mg/kg/day (males and females, respectively). [[Cobicistat]] exposures at these doses were approximately 7 (male) and 16 (females) times, respectively, the human systemic exposure at the therapeutic daily dose. In a long-term [[carcinogenicity]] study of [[cobicistat]] in rats, an increased incidence of follicular cell [[adenomas]] and/or [[carcinomas]] in the [[thyroid gland]] was observed at doses of 25 and 50 mg/kg/day in males, and at 30 mg/kg/day in females. The follicular cell findings are considered to be rat-specific, secondary to hepatic microsomal [[enzyme]] induction and [[thyroid hormone]] imbalance, and are not relevant for humans. At the highest doses tested in the rat [[carcinogenicity]] study, systemic exposures were approximately 2 times the human systemic exposure at the therapeutic daily dose. | |||
''[[Mutagenesis]]'': | |||
[[Atazanavir]]: [[Atazanavir]] tested positive in an in vitro clastogenicity test using primary human [[lymphocytes]], in the absence and presence of [[metabolic]] activation. [[Atazanavir]] tested negative in the in vitro Ames reverse-mutation assay, in vivo [[micronucleus]] and [[DNA]] repair tests in rats, and in vivo [[DNA]] damage test in rat [[duodenum]] (comet assay). | |||
[[Cobicistat]]: [[Cobicistat]] was not genotoxic in the reverse mutation bacterial test (Ames test), mouse lymphoma or rat micronucleus assays. | |||
''Impairment of [[Fertility]]'': | |||
[[Atazanavir]]: At the [[AUC|systemic drug exposure levels]] (AUC) 0.9 (in male rats) or 2.3 (in female rats) times that of the human clinical dose, (300 mg/day [[atazanavir]] coadministered with 100 mg/day [[ritonavir]]) significant effects on mating, [[fertility]], or early embryonic development were not observed. | |||
[[Cobicistat]]: [[Cobicistat]] did not affect [[fertility]] in male or female rats at daily exposures ([[AUC]]) approximately 3-fold higher than human exposures at the recommended 150 mg daily dose. [[Fertility]] was normal in the offspring of rats exposed daily from before birth (in utero) through sexual maturity at daily exposures ([[AUC]]) of approximately similar human exposures at the recommended 150 mg daily dose. | |||
|clinicalStudies=The safety and efficacy of [[atazanavir]] coadministered with [[cobicistat]] were evaluated in a randomized, double-blind, active-controlled trial (Study 114) in [[HIV|HIV-1]] infected treatment-naive subjects with baseline estimated [[creatinine clearance]] above 70 mL/min (N=692). In Study 114, subjects were randomized in a 1:1 ratio to receive either [[atazanavir]] 300 mg coadministered with [[cobicistat]] 150 mg once daily or [[atazanavir]] 300 mg coadministered with [[ritonavir]] 100 mg once daily. All subjects received concomitant treatment with 300 mg of [[tenofovir]] DF and 200 mg of [[emtricitabine]] once a day administered as a single tablet. Randomization was stratified by screening [[HIV|HIV-1]] [[RNA]] level (≤100,000 copies/mL or >100,000 copies/mL). | |||
The mean age of subjects was 37 years (range: 19-70); 83% were male, 60% were White, 18% were Black, and 12% were Asian. The mean baseline plasma [[HIV|HIV-1]] [[RNA]] was 4.8 log10 copies/mL (range: 3.2-6.4). The mean baseline [[CD4+]] cell count was 352 cells/mm3 (range: 1-1455) and 17% had [[CD4+]] cell counts ≤200 cells/mm3. Forty percent (40%) of patients had baseline viral loads >100,000 copies/mL. | |||
Virologic outcomes in Study 114 through Week 144 are presented in Table 9. In Study 114, the mean increase from baseline in CD4+ cell count at Week 144 was 281 cells/mm3 in patients receiving [[atazanavir]] coadministered with [[cobicistat]] and 297 cells/mm3 in patients receiving [[atazanavir]] coadministered with [[ritonavir]]. | |||
[[File:Atazanavir and cobicistat T9.JPG|thumb|none|400px|This image is provided by the National Library of Medicine]] | |||
|howSupplied=[[Atazanavir]] and [[cobicistat]] tablets, 300 mg [[atazanavir]] and 150 mg [[cobicistat]], are oval, biconvex, pink, film-coated, debossed with “3641” on one side and plain on the other side. Each bottle contains 30 tablets (NDC-0003-3641-11), a silica gel desiccant and is closed with a child-resistant closure. | |||
|storage=Store [[atazanavir]] and [[cobicistat]] tablets at 25°C (77°F); excursions permitted between 15°C and 30°C (59°F and 86°F). Keep container tightly closed. | |||
|packLabel=[[File:Atazanavir and cobicistat ingredients.JPG|thumb|none|400px|This image is provided by the National Library of Medicine]] | |||
[[File:Atazanavir and cobicistat label.jpg|thumb|none|400px|This image is provided by the National Library of Medicine]] | |||
|fdaPatientInfo=Advise the patient to read the FDA-approved patient labeling (Patient Information). | |||
*Instructions for Use | |||
**Advise patients to take [[atazanavir]] and [[cobicistat]] with food every day and that [[atazanavir]] and [[cobicistat]] must always be used in combination with other [[antiretroviral]] drugs. | |||
**Inform patients to avoid missing doses as it can result in development of resistance, and not to discontinue therapy without consulting with their healthcare provider. | |||
**Advise patients if a dose of [[atazanavir]] and [[cobicistat]] is missed, they should take the dose as soon as possible and then return to their normal schedule; however, if a dose is skipped, the patient should not double the next dose. | |||
*Drug Interactions | |||
**[[Atazanavir]] and [[cobicistat]] may interact with many drugs; therefore, inform patients of the potential for serious drug interactions with [[atazanavir]] and [[cobicistat]], and that some drugs are contraindicated with [[atazanavir]] and [[cobicistat]] and other drugs require dosage adjustment. Advise patients to report to their healthcare provider the use of any other prescription, nonprescription medication, or herbal products, particularly [[St John's wort]]. | |||
**Instruct patients receiving hormonal contraceptives to use additional or alternative non-hormonal contraceptive measures during therapy with [[atazanavir]] and [[cobicistat]] because no data are available to make recommendations regarding use of hormonal contraceptives and [[atazanavir]] coadministered with [[cobicistat]]. | |||
*Cardiac Conduction Abnormalities | |||
**Inform patients that [[atazanavir]] and [[cobicistat]] may produce changes in the [[electrocardiogram]] (e.g., [[PR interval|PR]] prolongation). Advise patients to consult their healthcare provider if they are experiencing symptoms such as [[dizziness]] or [[lightheadedness]]. | |||
*Severe Skin Reactions | |||
**Inform patients that mild [[rash|rashes]] without other symptoms have been reported with [[atazanavir]] use. These [[rash|rashes]] go away within two weeks with no change in treatment. | |||
**However, inform patients there have been reports of severe skin reactions (e.g., [[Stevens-Johnson syndrome]], [[erythema multiforme]], and toxic skin eruptions) with [[atazanavir]] use. | |||
**Advise patients to seek medical evaluation immediately if signs or symptoms of severe skin reactions or [[hypersensitivity]] reactions develop (including, but not limited to, severe [[rash]] or [[rash]] accompanied by [[fever]], general [[malaise]], [[myalgia|muscle]] or [[joint aches]], [[blisters]], [[oral lesions]], [[conjunctivitis]], or [[facial edema]]). | |||
*[[Nephrolithiasis]] and [[Cholelithiasis]] | |||
**Inform patients that [[kidney stones]] and/or [[gallstones]] have been reported with [[atazanavir]] use. Some patients with [[kidney stones]] and/or [[gallstones]] required hospitalization for additional management and some had complications. | |||
*[[Hyperbilirubinemia]] | |||
**Inform patients that [[asymptomatic]] elevations in indirect [[bilirubin]] have occurred in patients receiving [[atazanavir]], a component of [[atazanavir]] and [[cobicistat]]. | |||
**Tell patients this may be accompanied by yellowing of the skin or whites of the eyes and alternative [[antiretroviral]] therapy may be considered if they have cosmetic concerns. | |||
*Fat Redistribution | |||
**Inform patients that redistribution or accumulation of body fat may occur in patients receiving [[antiretroviral]] therapy including [[protease inhibitors]] and that the cause and long-term health effects of these conditions are not known at this time. | |||
*Pregnancy Registry | |||
**Inform patients that there is a pregnancy exposure registry to monitor fetal outcomes of pregnant women exposed to [[atazanavir]] and [[cobicistat]]. | |||
*Lactation | |||
**Instruct women with [[HIV|HIV-1]] infection not to breastfeed because [[HIV|HIV-1]] can be passed to the baby in breast milk. | |||
|alcohol=Alcohol-[[Atazanavir]] and [[cobicistat]] interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
|brandNames=EVOTAZ | |||
}} | }} | ||
Latest revision as of 16:21, 27 July 2017
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Allison Tu [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Atazanavir and cobicistat is a combination of a human immunodeficiency virus protease inhibitor and a CYP3A inhibitor that is FDA approved for the treatment of HIV-1 infection in combination with other antiretroviral agents. Common adverse reactions include abnormal bilirubin levels, jaundice, and scleral icterus.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Atazanavir and cobicistat is indicated in combination with other antiretroviral agents for the treatment of human immunodeficiency virus (HIV-1) infection in adults.
Limitations of Use: Use of atazanavir and cobicistat in treatment-experienced patients should be guided by the number of baseline primary protease inhibitor resistance substitutions.
Dosing Information
- Atazanavir and cobicistat is a fixed-dose combination product containing 300 mg of atazanavir and 150 mg of cobicistat.
- In treatment-naive and -experienced adults, the recommended dosage of atazanavir and cobicistat is one tablet taken once daily orally with food.
- Administer atazanavir and cobicistat in conjunction with other antiretroviral agents.
- When coadministered with H2-receptor antagonists or proton-pump inhibitors, dose separation may be required.
- Dosage in Patients with Renal Impairment
- Atazanavir and cobicistat is not recommended in HIV-1 treatment-experienced patients with end-stage renal disease managed with hemodialysis.
- Atazanavir and cobicistat coadministered with tenofovir DF is not recommended in patients with estimated creatinine clearance below 70 mL/min. Coadministration of atazanavir and cobicistat and tenofovir DF in combination with concomitant or recent use of a nephrotoxic agent is not recommended.
- Not Recommended in Patients with Any Degree of Hepatic Impairment
- Atazanavir and cobicistat is not recommended in patients with any degree of hepatic impairment.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Atazanavir and cobicistat in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Atazanavir and cobicistat in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Atazanavir and cobicistat FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Atazanavir and cobicistat in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Atazanavir and cobicistat in pediatric patients.
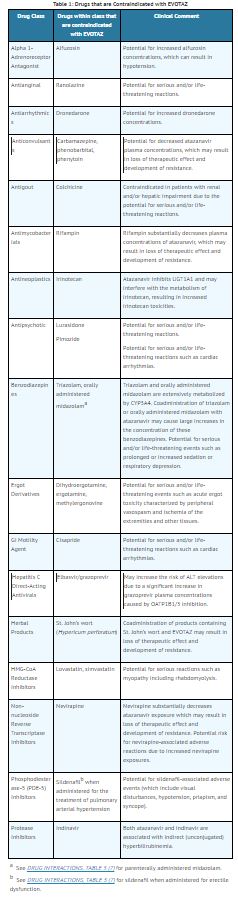
Contraindications
Atazanavir and cobicistat is contraindicated:
- In patients with previously demonstrated clinically significant hypersensitivity (e.g., Stevens-Johnson syndrome, erythema multiforme, or toxic skin eruptions) to any of the components of this product.
- When coadministered with drugs that are highly dependent on CYP3A or UGT1A1 for clearance, and for which elevated plasma concentrations of the interacting drugs are associated with serious and/or life-threatening events (see Table 1).
- When coadministered with drugs that strongly induce CYP3A and may lead to lower exposure and loss of efficacy of atazanavir and cobicistat (see Table 1).
Table 1 displays drugs that are contraindicated with atazanavir and cobicistat.
Warnings
- Cardiac Conduction Abnormalities
- Atazanavir prolongs the PR interval of the electrocardiogram in some patients.
- In healthy volunteers and in patients, abnormalities in atrioventricular (AV) conduction were asymptomatic and generally limited to first-degree AV block.
- There have been reports of second-degree AV block and other conduction abnormalities.
- In clinical trials of atazanavir that included electrocardiograms, asymptomatic first-degree AV block was observed in 6% of atazanavir-treated patients (n=920) and 5% of patients (n=118) treated with atazanavir coadministered with ritonavir.
- Because of limited clinical experience in patients with preexisting conduction system disease (e.g., marked first-degree AV block or second- or third-degree AV block), consider ECG monitoring in these patients.
- Severe Skin Reactions
- Cases of Stevens-Johnson syndrome, erythema multiforme, and toxic skin eruptions, including drug rash, eosinophilia and systemic symptoms (DRESS) syndrome, have been reported in patients receiving atazanavir. Atazanavir and cobicistat should be discontinued if severe rash develops.
- Mild-to-moderate maculopapular skin eruptions have also been reported in atazanavir clinical trials. These reactions had a median time to onset of 7.3 weeks and median duration of 1.4 weeks and generally did not result in treatment discontinuation.
- Effects on Serum Creatinine
- Cobicistat decreases estimated creatinine clearance due to inhibition of tubular secretion of creatinine without affecting actual renal glomerular function. This effect should be considered when interpreting changes in estimated creatinine clearance in patients initiating atazanavir and cobicistat, particularly in patients with medical conditions or receiving drugs needing monitoring with estimated creatinine clearance.
- Prior to initiating therapy with atazanavir and cobicistat, assess estimated creatinine clearance.
- Dosage recommendations are not available for drugs that require dosage adjustments in cobicistat-treated patients with renal impairment. Consider alternative medications that do not require dosage adjustments in patients with renal impairment.
- Although cobicistat may cause modest increases in serum creatinine and modest declines in estimated creatinine clearance without affecting renal glomerular function, patients who experience a confirmed increase in serum creatinine of greater than 0.4 mg/dL from baseline should be closely monitored for renal safety.
- New Onset or Worsening Renal Impairment When Used with Tenofovir DF
- Renal impairment, including cases of acute renal failure and Fanconi syndrome, has been reported when cobicistat was used in an antiretroviral regimen that contained tenofovir DF. Therefore, coadministration of atazanavir and cobicistat and tenofovir DF is not recommended in patients who have an estimated creatinine clearance below 70 mL/min.
- When atazanavir and cobicistat is used with tenofovir DF, document urine glucose and urine protein at baseline and perform routine monitoring of estimated creatinine clearance, urine glucose, and urine protein during treatment.
- Measure serum phosphorus in patients at risk for renal impairment.
- Coadministration of atazanavir and cobicistat and tenofovir DF in combination with concomitant or recent use of a nephrotoxic agent is not recommended.
- In a clinical trial over 144 weeks (N=692), 10 (2.9%) subjects treated with atazanavir coadministered with cobicistat and tenofovir DF and 11 (3.2%) subjects treated with atazanavir coadministered with ritonavir and tenofovir DF discontinued study drug due to a renal adverse event.
- Seven of the 10 subjects (2.0% overall) in the cobicistat group had laboratory findings consistent with proximal renal tubulopathy leading to study drug discontinuation, compared to 7 of 11 subjects (2.0% overall) in the ritonavir group. One subject in the cobicistat group had renal impairment at baseline (e.g., estimated creatinine clearance less than 70 mL/min).
- The laboratory findings in these 7 subjects with evidence of proximal tubulopathy improved but did not completely resolve in all subjects upon discontinuation of cobicistat coadministered with atazanavir and tenofovir DF. Renal replacement therapy was not required in any subject.
- Nephrolithiasis and Cholelithiasis
- Cases of nephrolithiasis and/or cholelithiasis have been reported during postmarketing surveillance in HIV-infected patients receiving atazanavir therapy.
- Some patients required hospitalization for additional management and some had complications. Because these events were reported voluntarily during clinical practice, estimates of frequency cannot be made.
- If signs or symptoms of nephrolithiasis and/or cholelithiasis occur, temporary interruption or discontinuation of therapy may be considered.
- Hepatotoxicity
- Patients with underlying hepatitis B or C viral infections or marked elevations in transaminases may be at increased risk for developing further transaminase elevations or hepatic decompensation.
- In these patients, hepatic laboratory testing should be conducted prior to initiating therapy with atazanavir and cobicistat and during treatment.
- Risk of Serious Adverse Reactions or Loss of Virologic Response Due to Drug Interactions
- Initiation of atazanavir and cobicistat, a CYP3A inhibitor, in patients receiving medications metabolized by CYP3A or initiation of medications metabolized by CYP3A in patients already receiving atazanavir and cobicistat, may increase plasma concentrations of medications metabolized by CYP3A.
- Initiation of medications that inhibit or induce CYP3A may increase or decrease concentrations of atazanavir and cobicistat, respectively.
- Increased concentrations of atazanavir and cobicistat may lead to:
- Clinically significant adverse reactions, potentially leading to severe, life threatening, or fatal events from higher exposures of concomitant medications.
- Clinically significant adverse reactions from higher exposures of atazanavir and cobicistat.
- Decreased concentrations of atazanavir and cobicistat may lead to:
- Loss of therapeutic effect of atazanavir and cobicistat and possible development of resistance.
- See Table 5 for steps to prevent or manage these possible and known significant drug interactions, including dosing recommendations.
- Consider the potential for drug interactions prior to and during atazanavir and cobicistat therapy; review concomitant medications during atazanavir and cobicistat therapy; and monitor for the adverse reactions associated with the concomitant medications.
- When used with concomitant medications, atazanavir and cobicistat may result in different drug interactions than those observed or expected with atazanavir coadministered with ritonavir. Complex or unknown mechanisms of drug interactions preclude extrapolation of drug interactions with atazanavir coadministered with ritonavir to certain atazanavir and cobicistat interactions
- Antiretrovirals that are Not Recommended
- Atazanavir and cobicistat is not recommended in combination with other antiretroviral drugs that require CYP3A inhibition to achieve adequate exposures (e.g., other HIV protease inhibitors or elvitegravir) because dosing recommendations for such combinations have not been established and coadministration may result in decreased plasma concentrations of the antiretroviral agents, leading to loss of therapeutic effect and development of resistance.
- Aatazanavir and cobicistat is not recommended in combination with ritonavir or products containing ritonavir due to similar effects of cobicistat and ritonavir on CYP3A.
- Hyperbilirubinemia
- Most patients taking atazanavir experience asymptomatic elevations in indirect (unconjugated) bilirubin related to inhibition of UDP-glucuronosyltransferase (UGT). This hyperbilirubinemia is reversible upon discontinuation of atazanavir.
- Hepatic transaminase elevations that occur with hyperbilirubinemia should be evaluated for alternative etiologies.
- No long-term safety data are available for patients experiencing persistent elevations in total bilirubin greater than 5 times the upper limit of normal (ULN). Alternative antiretroviral therapy to atazanavir and cobicistat may be considered if jaundice or scleral icterus associated with bilirubin elevations presents cosmetic concerns for patients.
- Immune Reconstitution Syndrome
- Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including atazanavir, a component of atazanavir and cobicistat.
- During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jiroveci pneumonia, or tuberculosis), which may necessitate further evaluation and treatment.
- Autoimmune disorders (such as Graves’ disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of treatment.
- Diabetes Mellitus/Hyperglycemia
- New-onset diabetes mellitus, exacerbation of preexisting diabetes mellitus, and hyperglycemia have been reported during postmarketing surveillance in HIV-infected patients receiving protease inhibitor therapy.
- Some patients required either initiation or dose adjustments of insulin or oral hypoglycemic agents for treatment of these events. In some cases, diabetic ketoacidosis has occurred. In those patients who discontinued protease inhibitor therapy, hyperglycemia persisted in some cases.
- Because these events have been reported voluntarily during clinical practice, estimates of frequency cannot be made and a causal relationship between protease inhibitor therapy and these events has not been established.
- Fat Redistribution
- Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and “cushingoid appearance” have been observed in patients receiving antiretroviral therapy.
- The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
- Hemophilia
- There have been reports of increased bleeding, including spontaneous skin hematomas and hemarthrosis, in patients with hemophilia type A and B treated with protease inhibitors.
- In some patients additional factor VIII was given. In more than half of the reported cases, treatment with protease inhibitors was continued or reintroduced.
- A causal relationship between protease inhibitor therapy and these events has not been established.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The safety of atazanavir and cobicistat coadministered as single agents is based on Week 144 data from a Phase 3 trial, Study 114, in which 692 HIV-1 infected, antiretroviral treatment-naive subjects received:
- atazanavir coadministered with cobicistat and emtricitabine/tenofovir DF (N=344) or
- atazanavir coadministered with ritonavir and emtricitabine/tenofovir DF (N=348)
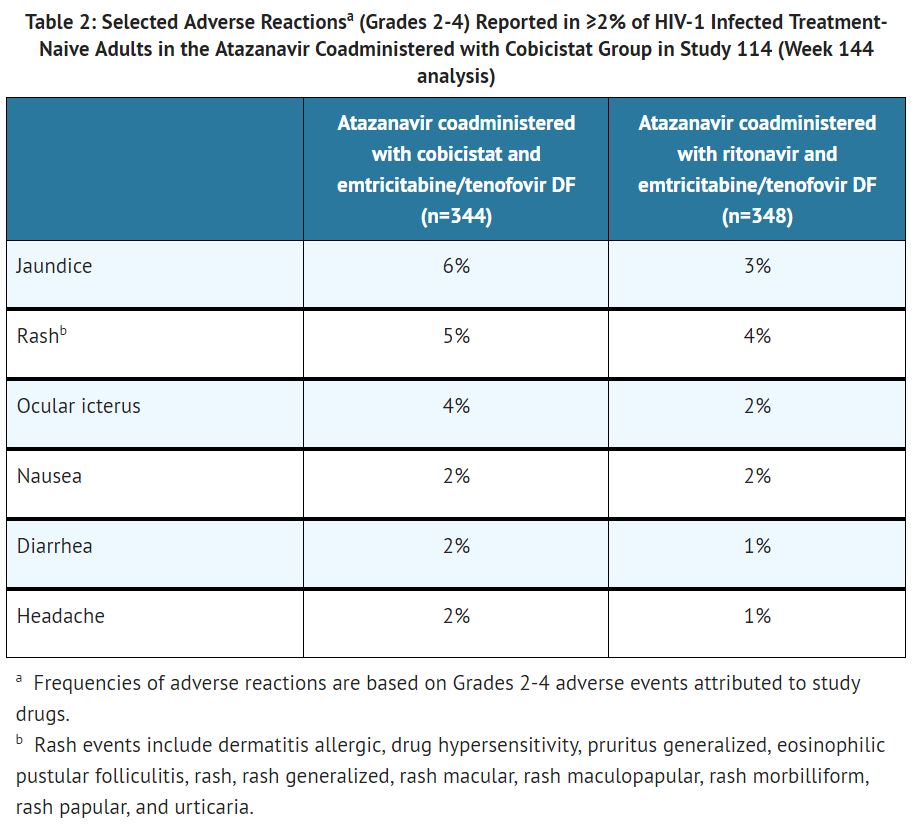
The most common adverse reactions (Grades 2-4) and reported in ≥5% of subjects in the atazanavir coadministered with cobicistat group were jaundice (6%) and rash (5%).
The proportion of subjects who discontinued study treatment due to adverse events, regardless of severity, was 11% in both the atazanavir coadministered with cobicistat and atazanavir coadministered with ritonavir groups. Table 2 lists the frequency of adverse reactions (Grades 2-4) occurring in at least 2% of subjects in the atazanavir coadministered with cobicistat group in Study 114.
Less Common Adverse Reactions: Selected adverse reactions of at least moderate severity (≥ Grade 2) occurring in less than 2% of subjects receiving atazanavir coadministered with cobicistat and emtricitabine/tenofovir DF are listed below. These events have been included because of investigator’s assessment of potential causal relationship and were considered serious or have been reported in more than one subject treated with atazanavir coadministered with cobicistat, and reported with greater frequency compared with the atazanavir coadministered with ritonavir group.
- Gastrointestinal Disorders: vomiting, upper abdominal pain
- General Disorders and Administration Site Conditions: fatigue
- Musculoskeletal and Connective Tissue Disorders: rhabdomyolysis
- Psychiatric Disorders: depression, abnormal dreams, insomnia
- Renal and Urinary Disorders: nephropathy, Fanconi syndrome acquired, nephrolithiasis
Laboratory Abnormalities: The frequency of laboratory abnormalities (Grades 3-4) occurring in at least 2% of subjects in the atazanavir coadministered with cobicistat group in Study 114 is presented in Table 3.
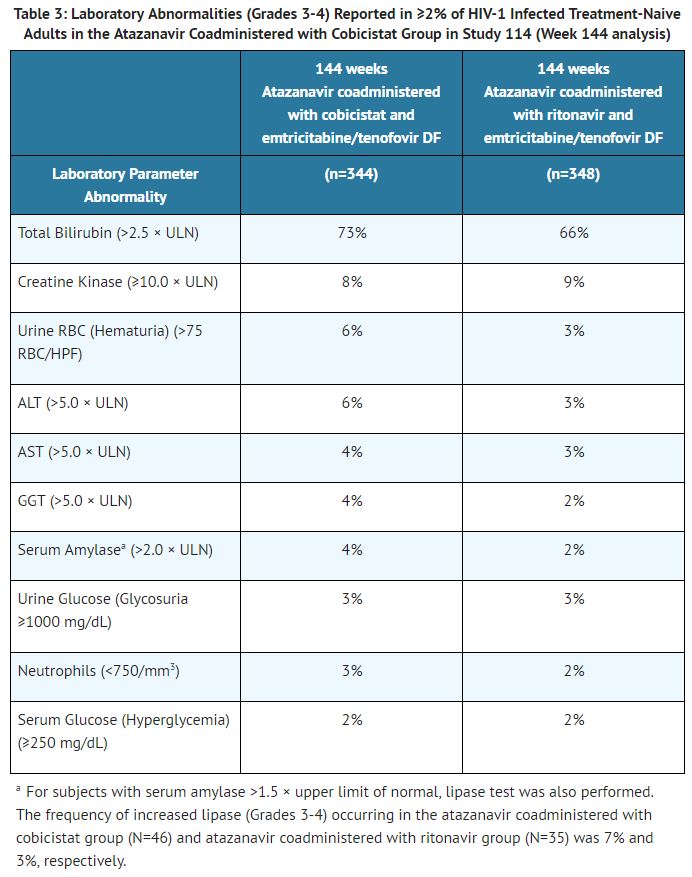
Increase in Serum Creatinine: Cobicistat, a component of atazanavir and cobicistat, has been shown to increase serum creatinine and decrease estimated creatinine clearance due to inhibition of tubular secretion of creatinine without affecting actual renal glomerular function. In Study 114, increases in serum creatinine and decreases in estimated creatinine clearance occurred early in treatment in the atazanavir coadministered with cobicistat group after which they stabilized. The mean (± SD) change in estimated glomerular filtration rate (eGFR) by Cockcroft-Gault method after 144 weeks of treatment was −15.1 ± 16.5 mL/min in the atazanavir coadministered with cobicistat group and −8.0 ± 16.8 mL/min in the atazanavir coadministered with ritonavir group.
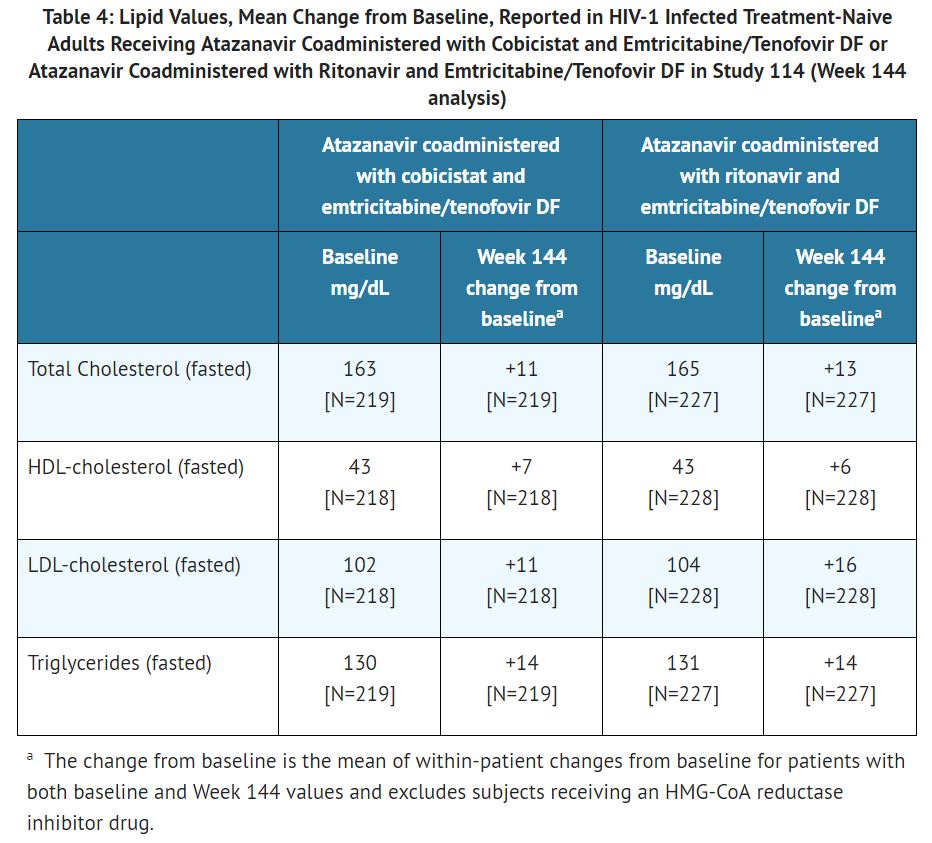
Serum Lipids: Changes from baseline in total cholesterol, HDL-cholesterol, LDL-cholesterol, and triglycerides are presented in Table 4. In both groups, mean values for serum lipids remained within the normal range for each laboratory test. The clinical significance of these changes is unknown.
Postmarketing Experience
There is limited information regarding Atazanavir and cobicistat Postmarketing Experience in the drug label.
Drug Interactions
Potential for atazanavir and cobicistat to Affect Other Drugs:
- Atazanavir is an inhibitor of CYP3A and UGT1A1 and a weak inhibitor of CYP2C8. Cobicistat is an inhibitor of CYP3A and CYP2D6. The transporters that cobicistat inhibits include P-glycoprotein (P-gp), BCRP, OATP1B1 and OATP1B3.
- Coadministration of atazanavir and cobicistat with drugs highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events is contraindicated.
- Coadministration of atazanavir and cobicistat and drugs primarily metabolized by CYP3A, UGT1A1 and/or CYP2D6 or drugs that are substrates of P-gp, BCRP, OATP1B1 and/or OATP1B3 may result in increased plasma concentrations of the other drug that could increase or prolong its therapeutic effects and adverse reactions which may require dose adjustments and/or additional monitoring as shown in Table 5.
- Use of atazanavir and cobicistat is not recommended when coadministered with drugs highly dependent on CYP2C8 for clearance with narrow therapeutic indices (e.g., paclitaxel, repaglinide).
Potential for Other Drugs to Affect Atazanavir and Cobicistat:
- Atazanavir and cobicistat are CYP3A4 substrates; therefore, drugs that induce CYP3A4 may decrease atazanavir and cobicistat plasma concentrations and reduce the therapeutic effect of atazanavir and cobicistat, leading to development of resistance to atazanavir (see Table 5). Cobicistat is also metabolized by CYP2D6 to a minor extent.
- Coadministration of atazanavir and cobicistat with other drugs that inhibit CYP3A4 may increase the plasma concentrations of cobicistat and atazanavir (see Table 5).
- Atazanavir solubility decreases as pH increases. Reduced plasma concentrations of atazanavir are expected if proton-pump inhibitors, antacids, buffered medications, or H2-receptor antagonists are administered with atazanavir and cobicistat.
Established and Other Potentially Significant Drug Interactions:
- Drug interaction trials were not conducted for atazanavir and cobicistat. Drug interaction trials were conducted with cobicistat in combination with desipramine, digoxin, or efavirenz.
- Table 5 provides dosing recommendations as a result of drug interactions with the components of atazanavir and cobicistat. These recommendations are based either on observed drug interactions in studies of cobicistat, atazanavir, or atazanavir coadministered with ritonavir or predicted drug interactions based on the expected magnitude of interaction and potential for serious events or loss of therapeutic effect of atazanavir and cobicistat.
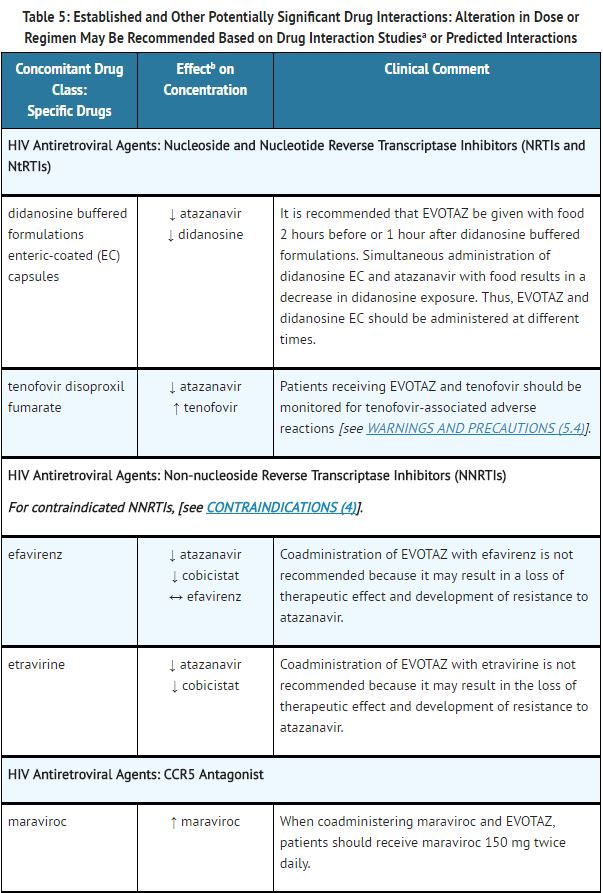
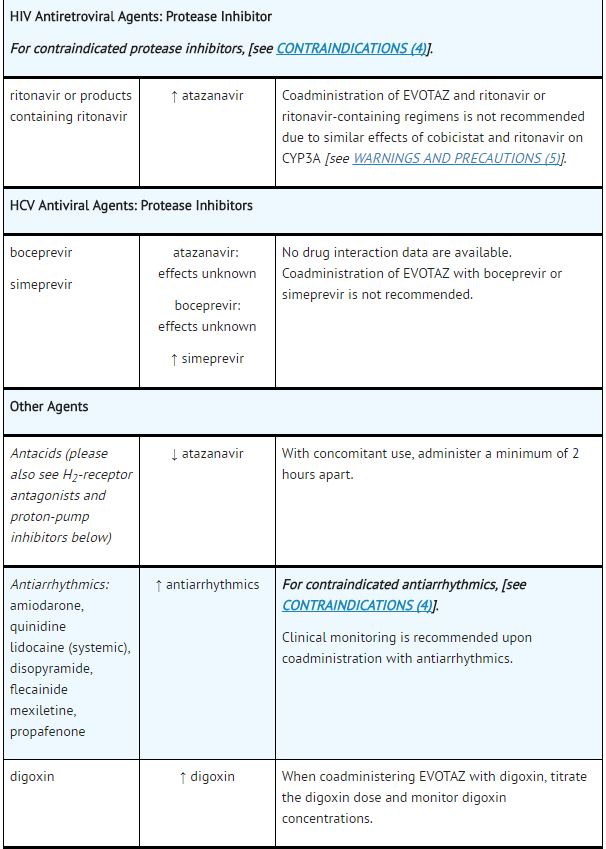
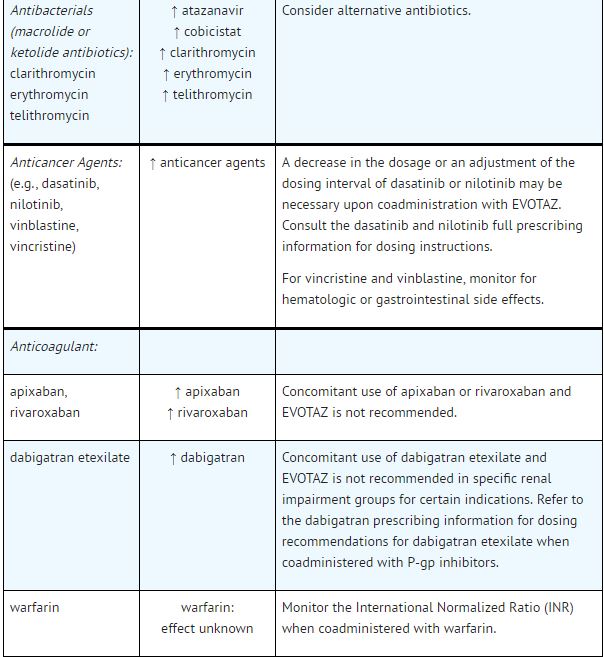
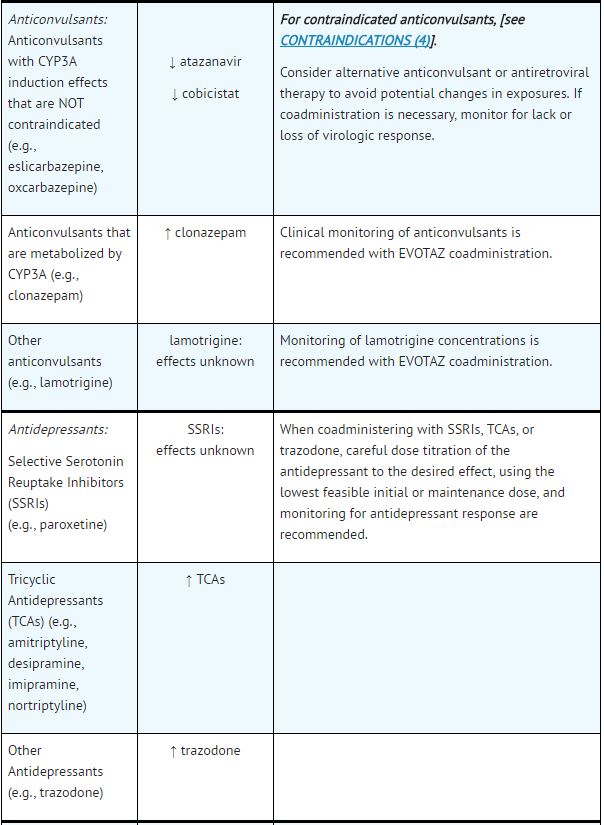
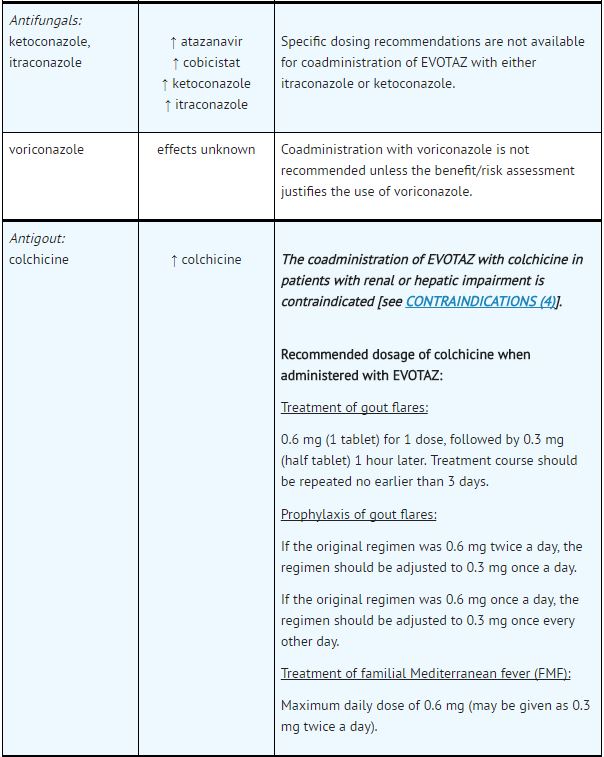
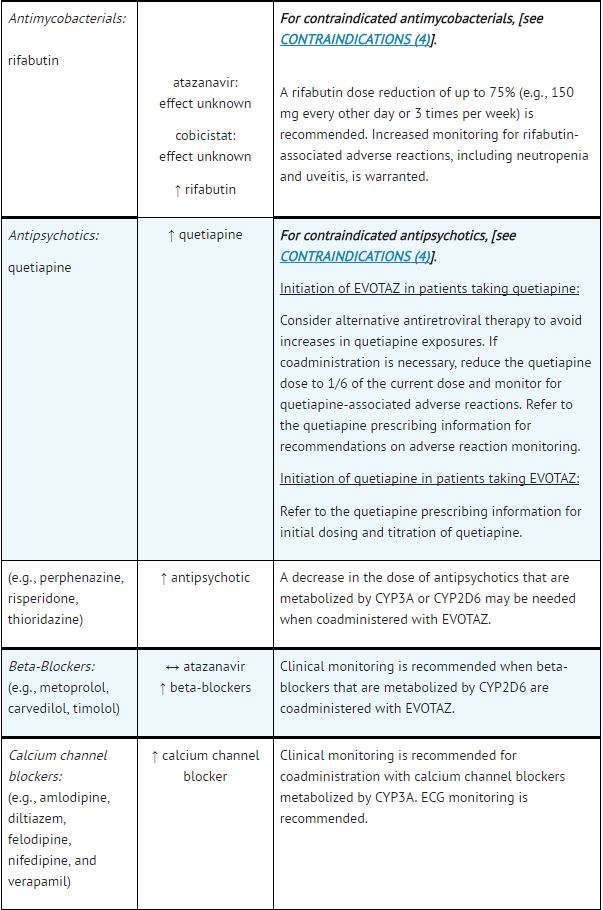
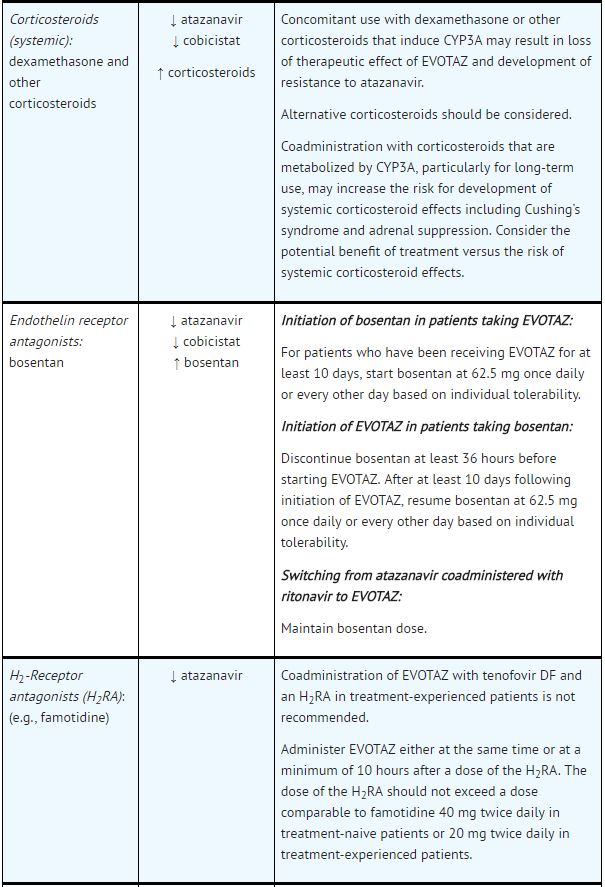
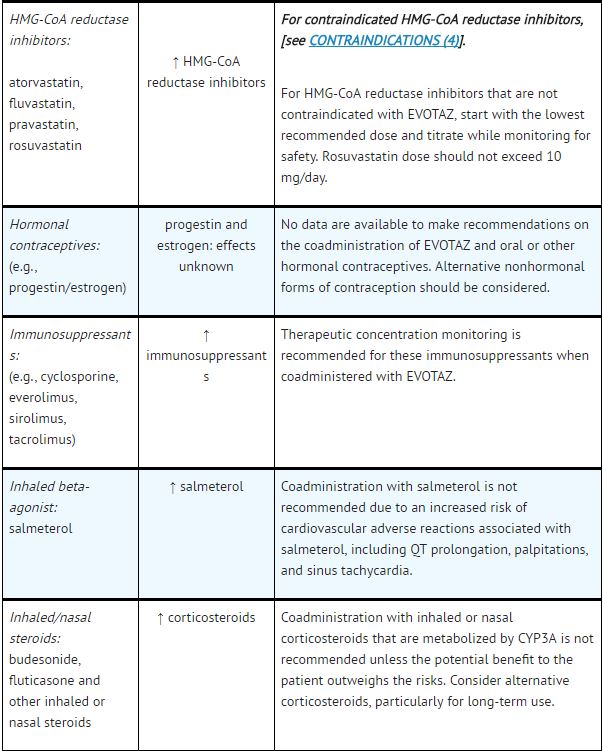
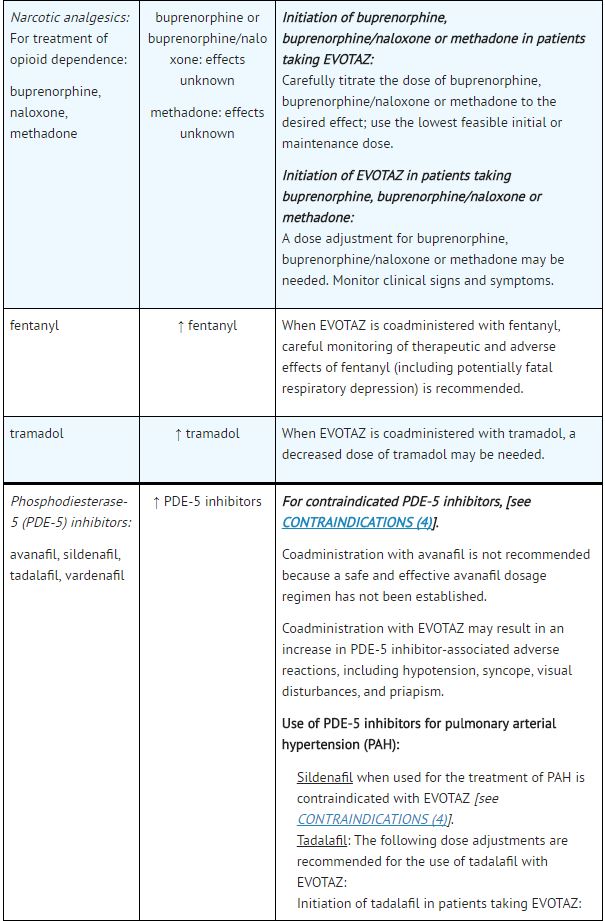
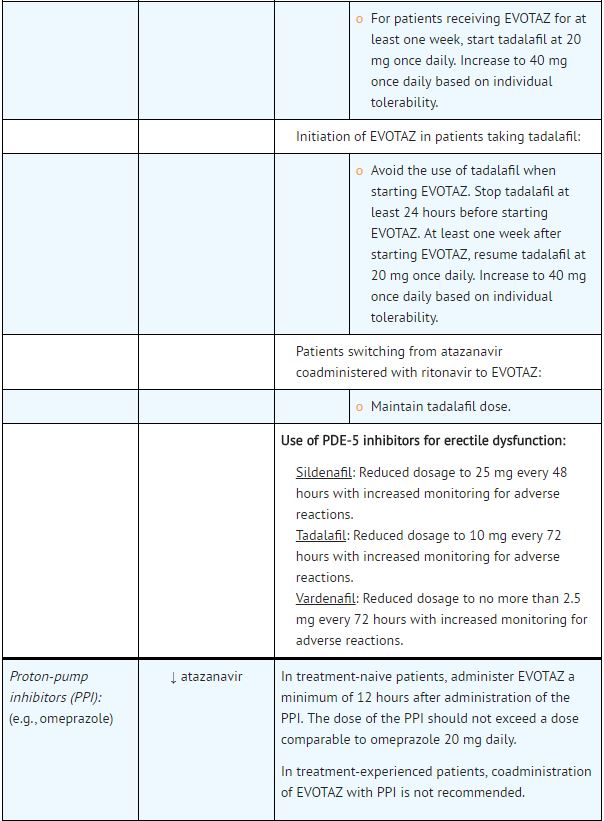
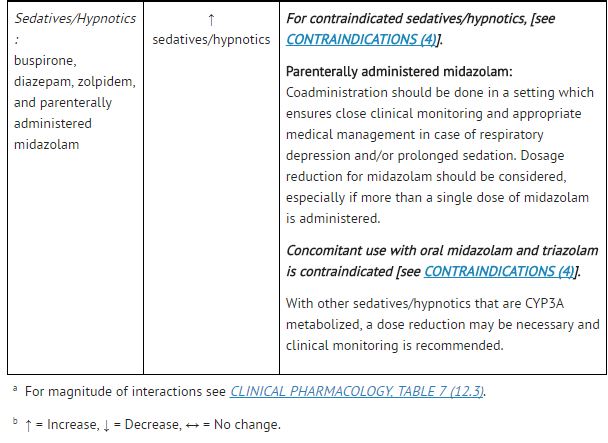
These images are provided by the National Library of Medicine
Drugs with No Observed or Predicted Interactions with the Components of Atazanavir and Cobicistat:
- Based on known metabolic profiles, clinically significant drug interactions are not expected between atazanavir and cobicistat and acetaminophen, atenolol, dapsone, fluconazole, trimethoprim/sulfamethoxazole, or azithromycin.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Pregnancy Exposure Registry: There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to atazanavir and cobicistat during pregnancy. Healthcare providers are encouraged to register patients by calling the Antiretroviral Pregnancy Registry (APR) at 1-800-258-4263.
Risk Summary: Prospective pregnancy data from the Antiretroviral Pregnancy Registry (APR) are not sufficient to adequately assess the risk of birth defects or miscarriage. Cobicistat use in women during pregnancy has not been evaluated; however, atazanavir use during pregnancy has been evaluated in a limited number of women. The pharmacokinetics of atazanavir and cobicistat have not been evaluated in pregnant patients. Available data from the APR show no difference in the risk of overall major birth defects for atazanavir compared with the background rate for major birth defects of 2.7% in a U.S. reference population of the Metropolitan Atlanta Congenital Defects Program (MACDP).
In animal reproduction studies, no evidence of adverse developmental outcomes was observed following oral administration of the components of atazanavir and cobicistat to pregnant rats and rabbits [see Data]. During organogenesis in the rat and rabbit, atazanavir exposures (AUC) were similar to those observed at the human clinical dose (300 mg/day atazanavir boosted with 100 mg/day ritonavir), while exposures were up to 1.4 (rats) and 3.3 (rabbits) times human exposures at the maximal recommended human dose (MRHD) of 150 mg.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations:
Dose Adjustment During Pregnancy and the Postpartum Period
Dosing recommendations cannot be made because the pharmacokinetics, safety, and efficacy of atazanavir and cobicistat cannot be predicted from studies of other atazanavir-containing products in pregnant women.
Maternal Adverse Reactions
Cases of lactic acidosis syndrome, sometimes fatal, and symptomatic hyperlactatemia have occurred in pregnant women using atazanavir in combination with nucleoside analogues, which are associated with an increased risk of lactic acidosis syndrome. Hyperbilirubinemia occurs frequently in patients who take atazanavir, including pregnant women. Refer to the atazanavir prescribing information for use of atazanavir in pregnancy. Advise pregnant women of the potential risks of lactic acidosis syndrome and hyperbilirubinemia.
Fetal/Neonatal Adverse Reactions
All infants, including neonates exposed to atazanavir in utero, should be monitored for the development of severe hyperbilirubinemia during the first few days of life. Advise pregnant women of the potential risk to newborn infants. Refer to the atazanavir prescribing information for use of atazanavir in pregnancy.
Data:
Human Data
Based on prospective reports from the interim APR of approximately 1600 live births following exposure to atazanavir-containing regimens (including 1037 live births in infants exposed in the first trimester and 569 exposed in second/third trimesters), there was no difference in the overall rate for birth defects for atazanavir (2.3%) compared with the background birth defect rate of 2.7% in the U.S. reference population of the MACDP. Based on prospective reports to the APR, the prevalence of birth defects in live births was 2.1% following first trimester exposure to atazanavir-containing regimens.
Insufficient numbers of pregnancies with exposure to cobicistat have been reported to the APR to estimate the rate of birth defects.
Animal Data:
Atazanavir was administered orally to pregnant rats (at 0, 200, 600, and 1920 mg/kg/day) and rabbits (at 0, 4, 15, and 60 mg/kg/day) during organogenesis (on gestation Days 6 through 15 and 7 through 19, respectively). No significant toxicological effects were observed in embryo-fetal toxicity studies performed with atazanavir at exposures (AUC) approximately 1.2 times higher (rats) and 0.7 times (rabbits) human exposures at the MRHD. In a rat pre- and postnatal developmental study, atazanavir was administered orally at doses of 0, 50, 220, and 1000 mg/kg/day from gestation Day 6 to postnatal Day 20. At a maternal toxic dose (1000 mg/kg/day), atazanavir caused body weight loss or weight gain suppression in the animal offspring at atazanavir exposures (AUC) of approximately 1.3 times higher than human exposures at the MRHD.
Cobicistat was administered orally to pregnant rats at doses of 0, 25, 50, 125 mg/kg/day on gestation Day 6 to 17. Maternal toxicity was noted at 125 mg/kg/day and was associated with increases in post-implantation loss and decreased fetal weights. No malformations were noted at doses up to 125 mg/kg/day. Systemic exposures (AUC) at 50 mg/kg/day in pregnant females were 1.4 times higher than the human exposures at the MRHD. In pregnant rabbits, cobicistat was administered orally at doses of 0, 20, 50, and 100 mg/kg/day during the gestation Days 7 to 20. No maternal or embryo/fetal effects were noted at the highest dose of 100 mg/kg/day. Systemic exposures (AUC) at 100 mg/kg/day were 3.3 times higher than exposures at the MRHD.
In a pre- and postnatal developmental study in rats, cobicistat was administered orally at doses of 0, 10, 30, and 75 mg/kg from gestation Day 6 to postnatal Day 20, 21, or 22. At doses of 75 mg/kg/day of cobicistat, neither maternal nor developmental toxicity was noted. Systemic exposures (AUC) at this dose were 0.9 times lower than exposures at the MRHD.
Pregnancy Category (AUS):
Data
Labor and Delivery
There is no FDA guidance on use of Atazanavir and cobicistat during labor and delivery.
Nursing Mothers
Risk Summary:
The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV.
There is no information regarding the effects of atazanavir and cobicistat on the breastfed infant or on milk production.
Atazanavir has been detected in human milk. No data are available regarding atazanavir effects on milk production. Cobicistat is present in rat milk [see Data]. There is no information regarding the presence of cobicistat in human milk, the effects on the breastfed infant, or the effects on milk production. Because of the potential for (1) HIV transmission (in HIV-negative infants), (2) developing viral resistance (in HIV-positive infants), and (3) adverse reactions in a breastfed infant, instruct women not to breastfeed.
Data:
Animal Data
Cobicistat: During the prenatal and postnatal development toxicology study at doses up to 75 mg/kg/day, mean cobicistat milk to plasma ratio of up to 1.9 was measured 2 hours after administration to rats on lactation Day 10.
Pediatric Use
Atazanavir is not recommended for use in pediatric patients below the age of 3 months due to the risk of kernicterus.
The safety and efficacy of atazanavir and cobicistat in pediatric patients 3 months to less than 18 years of age have not been established.
Geriatic Use
Clinical studies with the components of atazanavir and cobicistat did not include sufficient numbers of patients aged 65 and older to determine whether they respond differently from younger patients. In general, appropriate caution should be exercised in the administration and monitoring of atazanavir and cobicistat in elderly patients reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Atazanavir and cobicistat with respect to specific gender populations.
Race
There is no FDA guidance on the use of Atazanavir and cobicistat with respect to specific racial populations.
Renal Impairment
Atazanavir and cobicistat is not recommended for use in HIV-treatment-experienced patients with end-stage renal disease managed with hemodialysis.
Hepatic Impairment
Atazanavir and cobicistat is not recommended for use in patients with any degree of hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Atazanavir and cobicistat in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Atazanavir and cobicistat in patients who are immunocompromised.
Administration and Monitoring
Administration
Renal Testing:
Prior to starting atazanavir and cobicistat, assess estimated creatinine clearance because cobicistat decreases estimated creatinine clearance due to inhibition of tubular secretion of creatinine without affecting actual renal glomerular function. When coadministering atazanavir and cobicistat with tenofovir disoproxil fumarate (tenofovir DF), assess estimated creatinine clearance, urine glucose, and urine protein at baseline and routinely monitor during treatment.
Hepatic Testing:
In patients with underlying hepatitis B or C viral infections, conduct hepatic laboratory testing prior to initiating therapy and during treatment with atazanavir and cobicistat. Administer atazanavir and cobicistat in conjunction with other antiretroviral agents. When coadministered with H2-receptor antagonists or proton-pump inhibitors, dose separation may be required.
Monitoring
There is limited information regarding Atazanavir and cobicistat Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Atazanavir and cobicistat and IV administrations.
Overdosage
Treatment for overdosage with atazanavir and cobicistat should consist of general supportive measures, including monitoring of vital signs and ECG, and observations of the patient’s clinical status. There is no specific antidote for overdose with atazanavir and cobicistat. Since atazanavir is extensively metabolized by the liver and both atazanavir and cobicistat are highly bound plasma proteins, it is unlikely that atazanavir and cobicistat will be significantly removed by hemodialysis or peritoneal dialysis.
Atazanavir: Human experience of acute overdose with atazanavir is limited. A single self-administered overdose of 29.2 g of atazanavir in an HIV-infected patient (73 times the 400-mg recommended dose of atazanavir administered without a CYP3A inhibitor) was associated with asymptomatic bifascicular block and PR interval prolongation. These events resolved spontaneously. At atazanavir doses resulting in high atazanavir exposures, jaundice due to indirect (unconjugated) hyperbilirubinemia (without associated liver function test changes) or PR interval prolongation may be observed.
Pharmacology

Mechanism of Action
Atazanavir and cobicistat is a fixed-dose combination of the HIV-1 antiretroviral drug, atazanavir and the CYP3A inhibitor, cobicistat
Atazanavir and cobicistat is a fixed-dose combination of atazanavir (ATV) and the CYP3A inhibitor cobicistat. ATV is an azapeptide HIV-1 protease inhibitor (PI) that selectively inhibits the virus-specific processing of viral Gag and Gag-Pol polyproteins in HIV-1 infected cells, thus preventing formation of mature virions. Cobicistat is a mechanism-based inhibitor of cytochrome P450 3A (CYP3A). Inhibition of CYP3A-mediated metabolism by cobicistat increases the systemic exposure of the CYP3A substrate atazanavir.
Structure
Atazanavir: Atazanavir is present as the sulfate salt. The chemical name for atazanavir sulfate is (3S,8S,9S,12S)-3,12-bis(1,1-dimethylethyl)-8-hydroxy-4,11-dioxo-9-(phenylmethyl)-6-[4-(2-pyridinyl)phenyl]methyl]-2,5,6,10,13-pentaazatetradecanedioic acid dimethyl ester, sulfate (1:1). Its molecular formula is C38H52N6O7•H2SO4, which corresponds to a molecular weight of 802.9 (sulfuric acid salt). The free base molecular weight is 704.9. Atazanavir sulfate has the following structural formula:
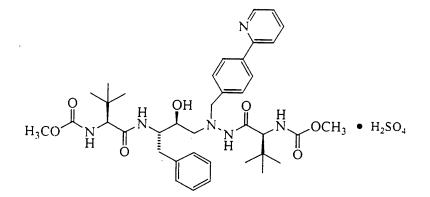
Atazanavir sulfate is a white to pale-yellow crystalline powder. It is slightly soluble in water (4-5 mg/mL, free base equivalent) with the pH of a saturated solution in water being about 1.9 at 24 ± 3°C.
Cobicistat: The chemical name for cobicistat is 1,3-thiazol-5-ylmethyl [(2R,5R)-5-{[(2S)-2-[(methyl{[2-(propan-2-yl)-1,3-thiazol-4-yl]methyl}carbamoyl)amino]-4-(morpholin-4-yl)butanoyl]amino}-1,6-diphenylhexan-2-yl]carbamate. It has a molecular formula of C40H53N7O5S2 and a molecular weight of 776.0. It has the following structural formula:

Pharmacodynamics
Cardiac Electrophysiology
Atazanavir: In a thorough QT/QTc study in 72 healthy subjects, atazanavir 400 mg and 800 mg (Cmax was 1.2 times and 2.4 times the Cmax observed with the recommended dosage of atazanavir and cobicistat, respectively) without a CYP3A inhibitor did not prolong the QTc interval to any clinically relevant extent. Asymptomatic prolongation of the PR interval was noted in subjects receiving atazanavir. The mean (±SD) maximum change in PR interval from the predose for atazanavir 400 mg (n=65), atazanavir 800 mg (n=66), and placebo (n=67) was 24 (±15) msec, 60 (±25) msec, and 13 (±11) msec, respectively. Steady state atazanavir exposures (Cmax and AUCtau) observed in this healthy volunteer study exceeded those observed in patients treated with atazanavir coadministered with cobicistat. There is limited information on the potential for a pharmacodynamic interaction in humans between atazanavir and other drugs that prolong the PR interval of the electrocardiogram.
In 1793 HIV-infected patients receiving antiretroviral regimens, QTc prolongation was comparable in the atazanavir-containing and comparator regimens. No atazanavir-treated healthy subject or HIV-infected patient in clinical trials had a QTc interval >500 msec.
Cobicistat: In a thorough QT/QTc study in 48 healthy subjects, cobicistat 250 mg (1.7 times the recommended dosage in atazanavir and cobicistat) and 400 mg (2.7 times the recommended dosage in atazanavir and cobicistat) did not prolong the QTc interval to any clinically relevant extent. Asymptomatic prolongation of the PR interval was noted in subjects receiving cobicistat. The maximum mean (95% upper confidence bound) difference in PR from placebo after baseline correction was 9.5 (12.1) msec for 250 mg and 20.2 (22.8) msec for 400 mg dose of cobicistat.
Effects on Serum Creatinine
The effect of cobicistat on serum creatinine was investigated in a trial in subjects with normal renal function (eGFR ≥80 mL/min, N=12) and mild-to-moderate renal impairment (eGFR 50-79 mL/min, N=18). A statistically significant change in estimated glomerular filtration rate, calculated by Cockcroft-Gault method (eGFRCG) from baseline, was observed after 7 days of treatment with cobicistat 150 mg among subjects with normal renal function (−9.9 ± 13.1 mL/min) and mild-to-moderate renal impairment (−11.9 ± 7.0 mL/min). No statistically significant changes in eGFRCG were observed compared to baseline for subjects with normal renal function or mild-to-moderate renal impairment 7 days after cobicistat was discontinued. The actual glomerular filtration rate, as determined by the clearance of probe drug iohexol, was not altered from baseline following treatment with cobicistat among subjects with normal renal function and mild-to-moderate renal impairment, indicating that cobicistat inhibits tubular secretion of creatinine, reflected as a reduction in eGFRCG, without affecting the actual glomerular filtration rate.
Pharmacokinetics
Absorption, Distribution, Metabolism, and Excretion
The pharmacokinetic (PK) properties of the components of atazanavir and cobicistat (atazanavir 300 mg and cobicistat 150 mg) were evaluated in healthy adult volunteers. Results are summarized in Table 6.
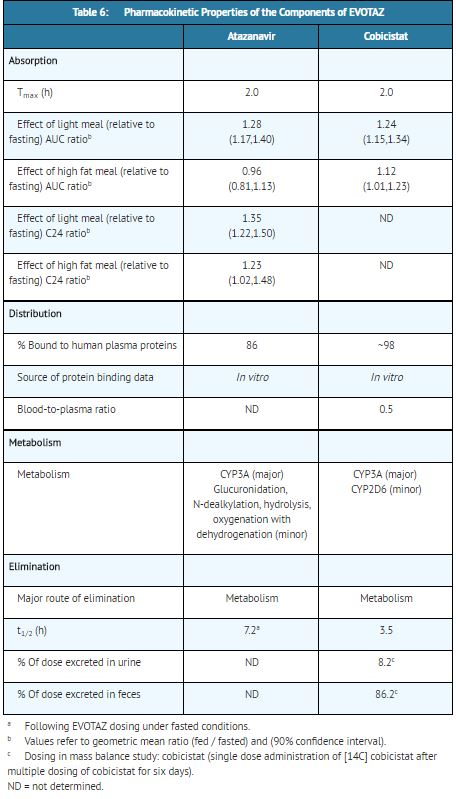
The pharmacokinetics of atazanavir was evaluated in HIV-1 infected subjects who received atazanavir 300 mg coadministered with cobicistat 150 mg in combination with emtricitabine/tenofovir DF. The steady-state pharmacokinetic parameters of atazanavir coadministered with cobicistat are shown in Table 7.
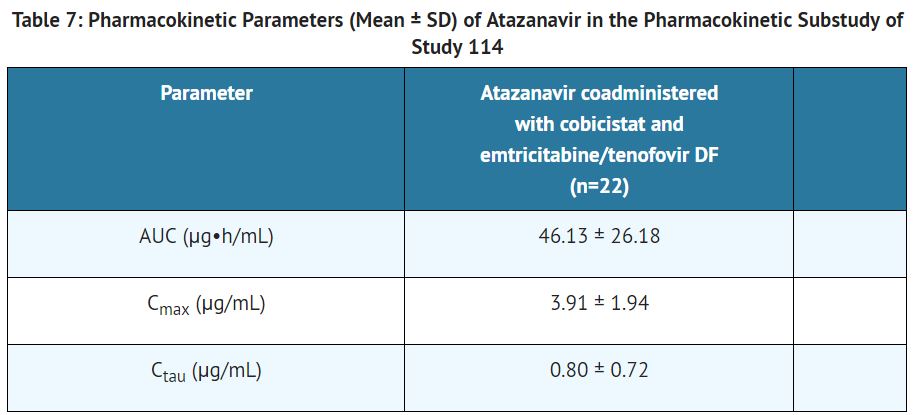
Specific Populations
Atazanavir: In healthy subjects, the renal elimination of unchanged atazanavir was approximately 7% of the administered dose. Atazanavir has been studied in adult subjects with severe renal impairment (n=20), including those on hemodialysis, at multiple doses of 400 mg once daily. The mean atazanavir Cmax was 9% lower, AUC was 19% higher, and Cmin was 96% higher in subjects with severe renal impairment not undergoing hemodialysis (n=10), than in age-, weight-, and gender-matched subjects with normal renal function. In a 4-hour dialysis session, 2.1% of the administered dose was removed. When atazanavir was administered either prior to, or following hemodialysis (n=10), the geometric means for Cmax, AUC, and Cmin were approximately 25% to 43% lower compared to subjects with normal renal function. The mechanism of this decrease is unknown.
Cobicistat: A study of the pharmacokinetics of cobicistat was performed in non−HIV-1 infected subjects with severe renal impairment (estimated creatinine clearance below 30 mL/min). No clinically relevant differences in cobicistat pharmacokinetics were observed between subjects with severe renal impairment and healthy subjects.
Atazanavir and cobicistat has not been studied in patients with hepatic impairment.
Atazanavir: Atazanavir is primarily metabolized and eliminated by the liver. Increased concentrations of atazanavir are expected in patients with moderately or severely impaired hepatic function.
Cobicistat: Cobicistat is primarily metabolized and eliminated by the liver. No clinically relevant differences in cobicistat pharmacokinetics were observed between subjects with moderate hepatic impairment (Child-Pugh Class B) and healthy subjects. The effect of severe hepatic impairment (Child-Pugh Class C) on the pharmacokinetics of cobicistat has not been studied.
Gender and Age
Atazanavir: There were no clinically important pharmacokinetic differences observed due to age or gender.
Cobicistat: No clinically relevant pharmacokinetic differences have been observed between men and women for cobicistat.
Assessment of Drug Interactions
Atazanavir has been shown in vivo not to induce its own metabolism, nor to increase the biotransformation of some drugs metabolized by CYP3A. In a multiple-dose study, atazanavir decreased the urinary ratio of endogenous 6β-OH cortisol to cortisol versus baseline, indicating that CYP3A production was not induced.
Drug interaction studies were not conducted for atazanavir and cobicistat or for atazanavir coadministered with cobicistat. Drug interaction studies of cobicistat were conducted with desipramine, digoxin, and efavirenz. Drug interaction studies of cobicistat coadministered with elvitegravir included rosuvastatin and rifabutin. The effects of cobicistat on the exposure of coadministered drugs are summarized in Table 8.
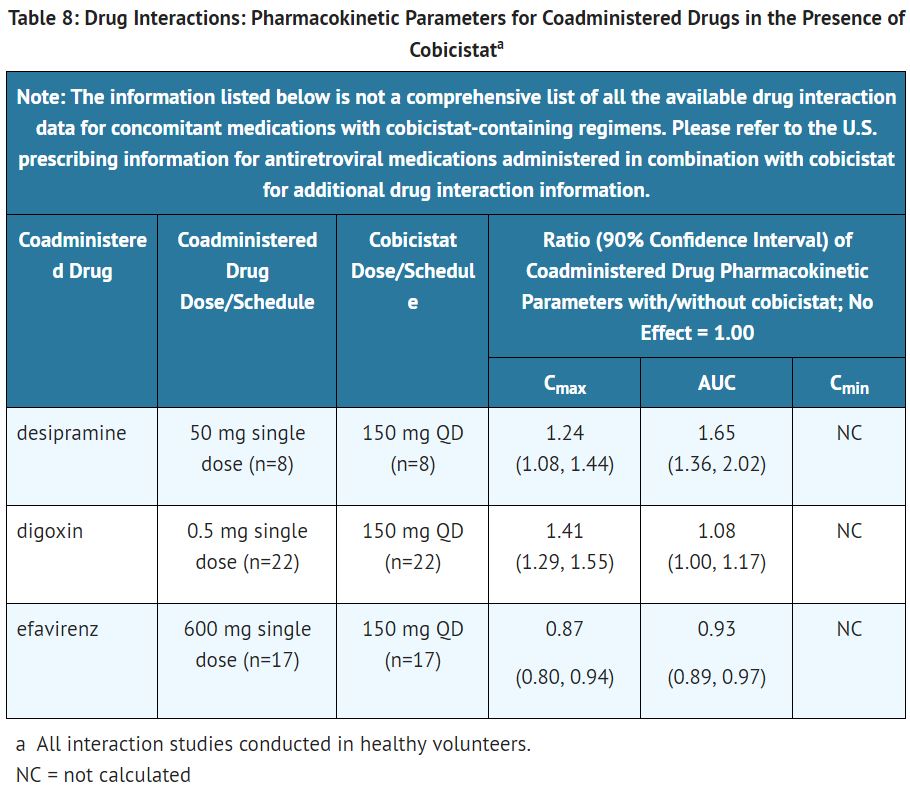
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Atazanavir: Long-term carcinogenicity studies in mice and rats were carried out with atazanavir for two years. In the mouse study, drug-related increases in hepatocellular adenomas were found in females at 360 mg/kg/day. The systemic drug exposure (AUC) at the NOAEL in females, (120 mg/kg/day) was 2.8 times and in males (80 mg/kg/day) was 2.9 times higher than those in humans at the clinical dose (300 mg/day atazanavir coadministered with 100 mg/day ritonavir, nonpregnant patients). In the rat study, no drug-related increases in tumor incidence were observed at doses up to 1200 mg/kg/day, for which AUCs were 1.1 (males) or 3.9 (females) times those measured in humans at the clinical dose.
Cobicistat: In a long-term carcinogenicity study in mice, no drug-related increases in tumor incidence were observed at doses up to 50 and 100 mg/kg/day (males and females, respectively). Cobicistat exposures at these doses were approximately 7 (male) and 16 (females) times, respectively, the human systemic exposure at the therapeutic daily dose. In a long-term carcinogenicity study of cobicistat in rats, an increased incidence of follicular cell adenomas and/or carcinomas in the thyroid gland was observed at doses of 25 and 50 mg/kg/day in males, and at 30 mg/kg/day in females. The follicular cell findings are considered to be rat-specific, secondary to hepatic microsomal enzyme induction and thyroid hormone imbalance, and are not relevant for humans. At the highest doses tested in the rat carcinogenicity study, systemic exposures were approximately 2 times the human systemic exposure at the therapeutic daily dose.
Atazanavir: Atazanavir tested positive in an in vitro clastogenicity test using primary human lymphocytes, in the absence and presence of metabolic activation. Atazanavir tested negative in the in vitro Ames reverse-mutation assay, in vivo micronucleus and DNA repair tests in rats, and in vivo DNA damage test in rat duodenum (comet assay).
Cobicistat: Cobicistat was not genotoxic in the reverse mutation bacterial test (Ames test), mouse lymphoma or rat micronucleus assays.
Impairment of Fertility:
Atazanavir: At the systemic drug exposure levels (AUC) 0.9 (in male rats) or 2.3 (in female rats) times that of the human clinical dose, (300 mg/day atazanavir coadministered with 100 mg/day ritonavir) significant effects on mating, fertility, or early embryonic development were not observed.
Cobicistat: Cobicistat did not affect fertility in male or female rats at daily exposures (AUC) approximately 3-fold higher than human exposures at the recommended 150 mg daily dose. Fertility was normal in the offspring of rats exposed daily from before birth (in utero) through sexual maturity at daily exposures (AUC) of approximately similar human exposures at the recommended 150 mg daily dose.
Clinical Studies
The safety and efficacy of atazanavir coadministered with cobicistat were evaluated in a randomized, double-blind, active-controlled trial (Study 114) in HIV-1 infected treatment-naive subjects with baseline estimated creatinine clearance above 70 mL/min (N=692). In Study 114, subjects were randomized in a 1:1 ratio to receive either atazanavir 300 mg coadministered with cobicistat 150 mg once daily or atazanavir 300 mg coadministered with ritonavir 100 mg once daily. All subjects received concomitant treatment with 300 mg of tenofovir DF and 200 mg of emtricitabine once a day administered as a single tablet. Randomization was stratified by screening HIV-1 RNA level (≤100,000 copies/mL or >100,000 copies/mL).
The mean age of subjects was 37 years (range: 19-70); 83% were male, 60% were White, 18% were Black, and 12% were Asian. The mean baseline plasma HIV-1 RNA was 4.8 log10 copies/mL (range: 3.2-6.4). The mean baseline CD4+ cell count was 352 cells/mm3 (range: 1-1455) and 17% had CD4+ cell counts ≤200 cells/mm3. Forty percent (40%) of patients had baseline viral loads >100,000 copies/mL.
Virologic outcomes in Study 114 through Week 144 are presented in Table 9. In Study 114, the mean increase from baseline in CD4+ cell count at Week 144 was 281 cells/mm3 in patients receiving atazanavir coadministered with cobicistat and 297 cells/mm3 in patients receiving atazanavir coadministered with ritonavir.
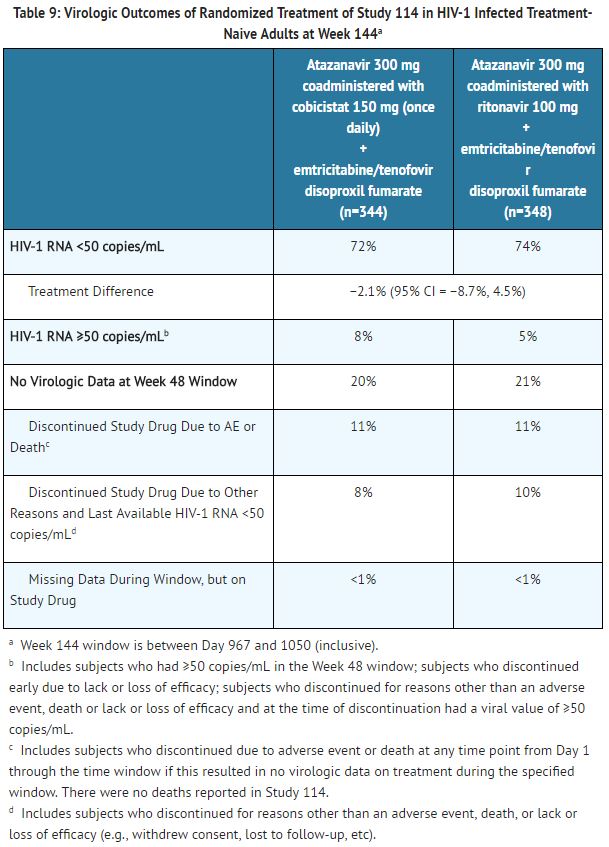
How Supplied
Atazanavir and cobicistat tablets, 300 mg atazanavir and 150 mg cobicistat, are oval, biconvex, pink, film-coated, debossed with “3641” on one side and plain on the other side. Each bottle contains 30 tablets (NDC-0003-3641-11), a silica gel desiccant and is closed with a child-resistant closure.
Storage
Store atazanavir and cobicistat tablets at 25°C (77°F); excursions permitted between 15°C and 30°C (59°F and 86°F). Keep container tightly closed.
Images
Drug Images
{{#ask: Page Name::Atazanavir and cobicistat |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Atazanavir and cobicistat |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
- Instructions for Use
- Advise patients to take atazanavir and cobicistat with food every day and that atazanavir and cobicistat must always be used in combination with other antiretroviral drugs.
- Inform patients to avoid missing doses as it can result in development of resistance, and not to discontinue therapy without consulting with their healthcare provider.
- Advise patients if a dose of atazanavir and cobicistat is missed, they should take the dose as soon as possible and then return to their normal schedule; however, if a dose is skipped, the patient should not double the next dose.
- Drug Interactions
- Atazanavir and cobicistat may interact with many drugs; therefore, inform patients of the potential for serious drug interactions with atazanavir and cobicistat, and that some drugs are contraindicated with atazanavir and cobicistat and other drugs require dosage adjustment. Advise patients to report to their healthcare provider the use of any other prescription, nonprescription medication, or herbal products, particularly St John's wort.
- Instruct patients receiving hormonal contraceptives to use additional or alternative non-hormonal contraceptive measures during therapy with atazanavir and cobicistat because no data are available to make recommendations regarding use of hormonal contraceptives and atazanavir coadministered with cobicistat.
- Cardiac Conduction Abnormalities
- Inform patients that atazanavir and cobicistat may produce changes in the electrocardiogram (e.g., PR prolongation). Advise patients to consult their healthcare provider if they are experiencing symptoms such as dizziness or lightheadedness.
- Severe Skin Reactions
- Inform patients that mild rashes without other symptoms have been reported with atazanavir use. These rashes go away within two weeks with no change in treatment.
- However, inform patients there have been reports of severe skin reactions (e.g., Stevens-Johnson syndrome, erythema multiforme, and toxic skin eruptions) with atazanavir use.
- Advise patients to seek medical evaluation immediately if signs or symptoms of severe skin reactions or hypersensitivity reactions develop (including, but not limited to, severe rash or rash accompanied by fever, general malaise, muscle or joint aches, blisters, oral lesions, conjunctivitis, or facial edema).
- Nephrolithiasis and Cholelithiasis
- Inform patients that kidney stones and/or gallstones have been reported with atazanavir use. Some patients with kidney stones and/or gallstones required hospitalization for additional management and some had complications.
- Hyperbilirubinemia
- Inform patients that asymptomatic elevations in indirect bilirubin have occurred in patients receiving atazanavir, a component of atazanavir and cobicistat.
- Tell patients this may be accompanied by yellowing of the skin or whites of the eyes and alternative antiretroviral therapy may be considered if they have cosmetic concerns.
- Fat Redistribution
- Inform patients that redistribution or accumulation of body fat may occur in patients receiving antiretroviral therapy including protease inhibitors and that the cause and long-term health effects of these conditions are not known at this time.
- Pregnancy Registry
- Inform patients that there is a pregnancy exposure registry to monitor fetal outcomes of pregnant women exposed to atazanavir and cobicistat.
- Lactation
Precautions with Alcohol
Alcohol-Atazanavir and cobicistat interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
EVOTAZ
Look-Alike Drug Names
There is limited information regarding Atazanavir and cobicistat Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.