Lacosamide
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Stefano Giannoni [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Lacosamide is an anticonvulsant that is FDA approved for the treatment of partial-onset seizures. Common adverse reactions include diplopia, headache, dizziness, nausea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Partial seizures
Dosage for lacosamide Tablet and Oral solution
Monotherapy
- Initial: 100 mg twice daily (200 mg per day)
- Every week, dose should be increased by 50 mg twice daily (100 mg per day),up to a recommended maintenance dose of 150 mg twice daily to 200 mg twice daily (300 mg to 400 mg per day).
- Alternatively, lacosamide may be initiated with a single loading dose of 200 mg, followed approximately 12 hours later by 100 mg twice daily (200 mg per day); this dose regimen should be continued for one week.
- Based on individual response and tolerability, the dose can be increased at weekly intervals by 50 mg twice daily (100 mg per day), as needed, up to the recommended maintenance dose of 150 mg twice daily to 200 mg twice daily (300 mg to 400 mg per day).
- The loading dose should be administered with medical supervision because of the increased incidence of CNS adverse reactions.
- For patients who are already on a single antiepileptic and will convert to lacosamide monotherapy, the therapeutic dose of 150 mg twice daily to 200 mg twice daily (300 mg to 400 mg per day) should be maintained for at least 3 days before initiating withdrawal of the concomitant antiepileptic drug.
- A gradual withdrawal of the concomitant antiepileptic drug over at least 6 weeks is recommended.
Adjunctive Therapy
- The initial recommended dose is 50 mg twice daily (100 mg per day). Based on individual patient response and tolerability, the dose can be increased at weekly intervals by 50 mg twice daily (100 mg per day).
- The recommended maintenance dose is 100 mg twice daily to 200 mg twice daily (200 mg to 400 mg per day).
- In clinical trials, the 300 mg twice daily (600 mg per day) dose was not more effective than the 200 mg twice daily dose (400 mg per day), but was associated with a substantially higher rate of adverse reactions.
- Alternatively, lacosamide may be initiated with a single loading dose of 200 mg, followed approximately 12 hours later by a 100 mg twice daily (200 mg per day); this maintenance dose regimen should be continued for one week.
- Based on individual patient response and tolerability, the dose can be increased at weekly intervals by 50 mg twice daily (100 mg per day), as needed, up to the maximum recommended maintenance dose of 200 mg twice daily (400 mg per day).
- The loading dose should be administered with medical supervision because of the increased incidence of CNS adverse reactions.
When discontinuing lacosamide, a gradual withdrawal over at least 1 week is recommended.
Dosage for lacosamide Injection
- Intravenous lacosamide can be administered in the same dosing regimens described for oral dosing, including the loading dose.
- Dosages may be infused intravenously over a period of 15 minutes to 60 minutes.
- Intravenous infusion of 30 to 60 minutes is preferable, and should be used when a 15 minute administration is not required.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Lacosamide in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Lacosamide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Lacosamide FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Lacosamide in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Lacosamide in pediatric patients.
Contraindications
- None
Warnings
Suicidal Behavior and Ideation
- Antiepileptic drugs (AEDs), including lacosamide, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication.
- Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
- Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo.
- In these trials, which had a median treatment duration of 12 weeks, the estimated incidence of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated.
- There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number of events is too small to allow any conclusion about drug effect on suicide.
- The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
- The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed.
- The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication.
- The risk did not vary substantially by age (5-100 years) in the clinical trials analyzed.
Table 1 shows absolute and relative risk by indication for all evaluated AEDs.

- The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar.
- Anyone considering prescribing lacosamide or any other AED must balance this risk with the risk of untreated illness.
- Epilepsy and many other illnesses for which antiepileptics are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior.
- Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
- Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm.
- Behaviors of concern should be reported immediately to healthcare providers.
Dizziness and Ataxia
- Lacosamide may cause dizziness and ataxia.
- In patients with partial-onset seizures taking 1 to 3 concomitant AEDs, dizziness was experienced by 25% of patients randomized to the recommended doses (200 to 400 mg/day) of lacosamide (compared with 8% of placebo patients) and was the adverse event most frequently leading to discontinuation (3%).
- Ataxia was experienced by 6% of patients randomized to the recommended doses (200 to 400 mg/day) of lacosamide (compared to 2% of placebo patients).
- The onset of dizziness and ataxia was most commonly observed during titration.
- There was a substantial increase in these adverse events at doses higher than 400 mg/day.
Cardiac Rhythm and Conduction Abnormalities
PR interval prolongation
- Dose-dependent prolongations in PR interval with lacosamide have been observed in clinical studies in patients and in healthy volunteers.
- In adjunctive clinical trials in patients with partial-onset epilepsy, asymptomatic first-degree atrioventricular (AV) block was observed as an adverse reaction in 0.4% (4/944) of patients randomized to receive lacosamide and 0% (0/364) of patients randomized to receive placebo.
- In clinical trials in patients with diabetic neuropathy, asymptomatic first-degree AV block was observed as an adverse reaction in 0.5% (5/1023) of patients receiving lacosamide and 0% (0/291) of patients receiving placebo.
- Second degree and complete AV block have been reported in patients in pain studies and in patients with seizures.
- When lacosamide is given with other drugs that prolong the PR interval, further PR prolongation is possible.
- Lacosamide should be used with caution in patients with known conduction problems (e.g., marked first-degree AV block, second-degree or higher AV block and sick sinus syndrome without pacemaker), sodium channelopathies (e.g., Brugada Syndrome), on concomitant medications that prolong PR interval, or with severe cardiac disease such as myocardial ischemia or heart failure, or structural heart disease.
- In such patients, obtaining an ECG before beginning lacosamide, and after lacosamide is titrated to steady-state maintenance dose, is recommended.
- In addition, these patients should be closely monitored if they are administered lacosamide through the intravenous route.
- One case of profound bradycardia was observed in a patient during a 15-minute infusion of 150 mg lacosamide.
- There were two postmarketing reports of third degree AV block in patients with significant cardiac history and also receiving metoprolol and amlodipine during infusion of lacosamide injection at doses higher than recommended.
Atrial fibrillation and Atrial flutter
- In the short-term investigational trials of lacosamide in epilepsy patients, there were no cases of atrial fibrillation or flutter.
- Both atrial fibrillation and atrial flutter have been reported in open label epilepsy trials and in postmarketing experience.
- In patients with diabetic neuropathy, 0.5% of patients treated with lacosamide experienced an adverse reaction of atrial fibrillation or atrial flutter, compared to 0% of placebo-treated patients.
- Lacosamide administration may predispose to atrial arrhythmias (atrial fibrillation or flutter), especially in patients with diabetic neuropathy and/or cardiovascular disease.
Syncope
- In the short-term controlled trials of lacosamide in epilepsy patients with no significant system illnesses, there was no increase in syncope compared to placebo.
- In the short-term controlled trials of lacosamide in patients with diabetic neuropathy, 1.2% of patients who were treated with lacosamide reported an adverse reaction of syncope or loss of consciousness, compared to 0% of placebo-treated patients with diabetic neuropathy.
- Most of the cases of syncope were observed in patients receiving doses above 400 mg/day.
- The cause of syncope was not determined in most cases. However, several were associated with either changes in orthostatic blood pressure, atrial flutter/fibrillation (and associated tachycardia), or bradycardia.
- Cases of syncope have also been observed in open-label clinical epilepsy studies.
- These cases were associated with a history of risk factors for cardiac disease and the use of drugs that slow AV conduction.
Withdrawal of Antiepileptic Drugs (AEDs)
- As with all AEDs, lacosamide should be withdrawn gradually (over a minimum of 1 week) to minimize the potential of increased seizure frequency in patients with seizure disorders.
Multiorgan Hypersensitivity Reactions
- One case of symptomatic hepatitis and nephritis was observed among 4011 subjects exposed to lacosamide during clinical development.
- The event occurred in a healthy volunteer, 10 days after stopping lacosamide treatment.
- The subject was not taking any concomitant medication and potential known viral etiologies for hepatitis were ruled out.
- The subject fully recovered within a month, without specific treatment.
- The case is consistent with a delayed multiorgan hypersensitivity reaction. *Additional potential cases included 2 with rash and elevated liver enzymes and 1 with myocarditis and hepatitis of uncertain etiology.
- Multiorgan hypersensitivity reactions (also known as Drug Reaction with Eosinophilia and Systemic Symptoms, or DRESS) have been reported with other antiepileptics and typically, although not exclusively, present with fever and rash associated with other organ system involvement, that may or may not include eosinophilia, hepatitis, nephritis, lymphadenopathy, and/or myocarditis. Because this disorder is variable in its expression, other organ system signs and symptoms not noted here may occur.
- If this reaction is suspected, lacosamide should be discontinued and alternative treatment started.
Phenylketonurics
- Lacosamide oral solution contains aspartame, a source of phenylalanine.
- A 200 mg dose of lacosamide oral solution (equivalent to 20 mL) contains 0.32 mg of phenylalanine.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- In the premarketing development of adjunctive therapy for partial onset seizures, 1327 patients received lacosamide in controlled and uncontrolled trials, of whom 1000 were treated for longer than 6 months, and 852 for longer than 12 months.
- The monotherapy development program included 425 patients, 310 of whom were treated for longer than 6 months, and 254 for longer than 12 months.
Lacosamide Tablet and Oral solution
Monotherapy Historical-Control Trial (Study 1)
- In the monotherapy trial, 16% of patients randomized to receive lacosamide at the recommended doses of 300 and 400 mg/day discontinued from the trial as a result of an adverse event.
- The adverse reaction most commonly (≥1% on lacosamide) leading to discontinuation was dizziness.
- Adverse reactions observed in this study were generally similar to those observed and attributed to drug in adjunctive placebo-controlled studies.
- One adverse reaction, insomnia, was observed at a rate of ≥2% and was not reported at a similar rate in previous studies.
- This adverse reaction has also been observed in postmarketing experience. Because this study did not include a placebo control group, causality could not be established.
- Dizziness, headache, nausea, somnolence, and fatigue were all reported at lower incidences during the AED Withdrawal Phase and Monotherapy Phase, compared with the Titration Phase.
Adjunctive Therapy Controlled Trials (Studies 2, 3, and 4)
- In adjunctive therapy controlled clinical trials, the rate of discontinuation as a result of an adverse event was 8% and 17% in patients randomized to receive lacosamide at the recommended doses of 200 and 400 mg/day, respectively, 29% at 600 mg/day, and 5% in patients randomized to receive placebo.
- The adverse events most commonly (>1% on lacosamide and greater than placebo) leading to discontinuation were dizziness, ataxia, vomiting, diplopia, nausea, vertigo, and vision blurred.
Table 2 gives the incidence of treatment-emergent adverse events that occurred in ≥2% of adult patients with partial-onset seizures in the lacosamide total group and for which the incidence was greater than placebo.
- The majority of adverse events in the lacosamide patients were reported with a maximum intensity of 'mild' or 'moderate'.

- The overall adverse event rate was similar in male and female patients. *Although there were few non-Caucasian patients, no differences in the incidences of adverse events compared to Caucasian patients were observed.
Laboratory Abnormalities
- Abnormalities in liver function tests have been observed in controlled trials with lacosamide in adult patients with partial-onset seizures who were taking 1 to 3 concomitant anti-epileptic drugs.
- Elevations of ALT to ≥3× ULN occurred in 0.7% (7/935) of lacosamide patients and 0% (0/356) of placebo patients.
- One case of hepatitis with transaminases >20× ULN was observed in one healthy subject 10 days after lacosamide treatment completion, along with nephritis (proteinuria and urine casts).
- Serologic studies were negative for viral hepatitis.
- Transaminases returned to normal within one month without specific treatment. At the time of this event, bilirubin was normal. The hepatitis/nephritis was interpreted as a delayed hypersensitivity reaction to lacosamide.
Other Adverse Reactions
- The following is a list of treatment-emergent adverse reactions reported by patients treated with lacosamide in all clinical trials in patients with partial-onset seizures, including controlled trials and long-term open-label extension trials.
- Events addressed in other tables or sections are not listed here. Events included in this list from the controlled trials occurred more frequently on drug than on placebo and were based on consideration of lacosamide pharmacology, frequency above that expected in the population, seriousness, and likelihood of a relationship to lacosamide. Events are further classified within system organ class.
Blood and lymphatic system disorders
Cardiac disorders
Ear and labyrinth disorders
Gastrointestinal disorders
- Constipation
- Dyspepsia
- Dry mouth
- Oral hypoaesthesia
General disorders and administration site conditions
- Irritability
- Pyrexia
- Feeling drunk
Injury, poisoning, and procedural complications
- Fall
Musculoskeletal and connective tissue disorders
Nervous system disorders
- Paresthesia
- Cognitive disorder
- Hypoaesthesia
- Dysarthria
- Disturbance in attention
- Cerebellar syndrome
Psychiatric disorders
- Confusional state
- Mood altered
- Depressed mood
Lacosamide Injection
- Adverse reactions with intravenous administration generally were similar to those observed with the oral formulation, although intravenous administration was associated with local adverse events such as injection site pain or discomfort (2.5%), irritation (1%), and erythema (0.5%).
- One case of profound bradycardia (26 bpm: BP 100/60 mmHg) was observed in a patient during a 15-minute infusion of 150 mg lacosamide.
- This patient was on a beta-blocker.
- Infusion was discontinued and the patient experienced a rapid recovery.
- The safety of a 15-minute loading dose administration of lacosamide Injection 200 mg to 400 mg followed by oral administration of lacosamide given twice daily at the same total daily dose as the initial intravenous infusion was assessed in an open-label study in patients with partial onset seizures. *Patients had to have been maintained on a stable dose regimen of 1 to 2 marketed antiepileptics for at least 28 days prior to treatment assignment. Treatment groups were as follows:
- Single dose of intravenous lacosamide Injection 200 mg followed by oral lacosamide 200 mg/day (100 mg every 12 hours).
- Single dose of intravenous lacosamide Injection 300 mg followed by oral lacosamide 300 mg/day (150 mg every 12 hours)
- Single dose of intravenous lacosamide Injection 400 mg followed by oral lacosamide 400 mg/day (200 mg every 12 hours).
Table 3 gives the incidence of adverse events that occurred in ≥5% of adult patients in any lacosamide dosing group.

- Adverse events observed with infusion of lacosamide 200 mg over 15-minutes followed by lacosamide 100 mg administered orally twice day were similar in frequency to those observed in 3-month adjunctive therapy controlled trials. *Considering the difference in period of observations (1 week vs. 3 months), the incidence of CNS adverse reactions, such as dizziness, somnolence, and paresthesia may be higher with 15-minute administration of lacosamide Injection than with administration over a 30-to 60-minute period.
Postmarketing Experience
- The following adverse reactions have been identified during post-approval use of lacosamide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic system disorders
Psychiatric disorders
- Aggression
- Agitation
- Hallucination
- Insomnia
- Psychotic disorder
Skin and subcutaneous tissue disorders
Drug Interactions
Pharmacokinetic Interactions
- Drug-drug interaction studies in healthy subjects showed no pharmacokinetic interactions between lacosamide and carbamazepine, valproate, digoxin, metformin, omeprazole, midazolam, oral contraceptives containing ethinylestradiol and levonorgestrel, or warfarin.
- There was no evidence for any relevant drug-drug interaction of lacosamide with the AEDs used most commonly in the placebo-controlled clinical trials in patients with partial-onset seizures.
- The lack of pharmacokinetic interaction does not rule out the possibility of pharmacodynamic interactions, particularly among drugs that affect the heart conduction system.
Strong CYP3A4 or CYP2C9 Inhibitors
- Patients with renal or hepatic impairment who are taking strong inhibitors of CYP3A4 and CYP2C9 may have a significant increase in exposure to lacosamide.
- Dose reduction may be necessary in these patients.
Concomitant Medications that Prolong PR Interval
- Lacosamide should be used with caution in patients on concomitant medications that prolong PR interval, because of a risk of AV block or bradycardia, e.g., beta-blockers and calcium channel blockers.
- In such patients, obtaining an ECG before beginning lacosamide, and after lacosamide is titrated to steady-state, is recommended.
- In addition, these patients should be closely monitored if they are administered lacosamide through the intravenous route.
Use in Specific Populations
Pregnancy
- Lacosamide produced developmental toxicity (increased embryofetal and perinatal mortality, growth deficit) in rats following administration during pregnancy.
- Developmental neurotoxicity was observed in rats following administration during a period of postnatal development corresponding to the third trimester of human pregnancy.
- These effects were observed at doses associated with clinically relevant plasma exposures.
- Lacosamide has been shown in vitro to interfere with the activity of collapsin response mediator protein-2 (CRMP-2), a protein involved in neuronal differentiation and control of axonal outgrowth.
- Potential related adverse effects on CNS development cannot be ruled out.
- There are no adequate and well-controlled studies in pregnant women. *Lacosamide should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Oral administration of lacosamide to pregnant rats (20, 75, or 200 mg/kg/day) and rabbits (6.25, 12.5, or 25 mg/kg/day) during the period of organogenesis did not produce any teratogenic effects. However, the maximum doses evaluated were limited by maternal toxicity in both species and embryofetal death in rats. These doses were associated with maternal plasma lacosamide exposures [area under the plasma-time concentration curve; (AUC)] ≈2 and 1 times (rat and rabbit, respectively) that in humans at the maximum recommended human dose (MRHD) of 400 mg/day.
- When lacosamide (25, 70, or 200 mg/kg/day) was orally administered to rats throughout gestation, parturition, and lactation, increased perinatal mortality and decreased body weights were observed in the offspring at the highest dose. *The no-effect dose for pre- and post-natal developmental toxicity in rats (70 mg/kg/day) was associated with a maternal plasma lacosamide AUC approximately equal to that in humans at the MRHD.
- Oral administration of lacosamide (30, 90, or 180 mg/kg/day) to rats during the neonatal and juvenile periods of postnatal development resulted in decreased brain weights and long-term neurobehavioral changes (altered open field performance, deficits in learning and memory).
- The early postnatal period in rats is generally thought to correspond to late pregnancy in humans in terms of brain development.
- The no-effect dose for developmental neurotoxicity in rats was associated with a plasma lacosamide AUC approximately 0.5 times that in humans at the MRHD.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Lacosamide in women who are pregnant.
Labor and Delivery
- The effects of lacosamide on labor and delivery in pregnant women are unknown. *In a pre- and post-natal study in rats, there was a tendency for prolonged gestation in all lacosamide treated groups at plasma exposures (AUC) at or below the plasma AUC in humans at the maximum recommended human dose of 400 mg/day.
Nursing Mothers
- Studies in lactating rats have shown that lacosamide and/or its metabolites are excreted in milk.
- It is not known whether lacosamide is excreted in human milk. Because many drugs are excreted into human milk, a decision should be made whether to discontinue nursing or to discontinue lacosamide, taking into account the importance of the drug to the mother.
Pediatric Use
- The safety and effectiveness of lacosamide in pediatric patients <17 years have not been established.
- Lacosamide has been shown in vitro to interfere with the activity of collapsin response mediator protein-2 (CRMP-2), a protein involved in neuronal differentiation and control of axonal outgrowth.
- Potential related adverse effects on CNS development cannot be ruled out. *Administration of lacosamide to rats during the neonatal and juvenile periods of postnatal development resulted in decreased brain weights and long-term neurobehavioral changes (altered open field performance, deficits in learning and memory).
- The no-effect dose for developmental neurotoxicity in rats was associated with a plasma lacosamide exposure (AUC) approximately 0.5 times the human plasma AUC at the maximum recommended human dose of 400 mg/day.
Geriatic Use
- There were insufficient numbers of elderly patients enrolled in partial-onset seizure trials (n=18) to adequately assess the effectiveness of lacosamide in this population.
- No lacosamide dose adjustment based on age is necessary.
- In elderly patients, dose titration should be performed with caution
Gender
There is no FDA guidance on the use of Lacosamide with respect to specific gender populations.
Race
- There are no clinically relevant differences in the pharmacokinetics of lacosamide between Asian, Black, and Caucasian subjects.
Renal Impairment
- A maximum dose of 300 mg/day is recommended for patients with severe renal impairment (CLCR≤30 mL/min) and in patients with endstage renal disease. *Lacosamide is effectively removed from plasma by hemodialysis.
- Dosage supplementation of up to 50% following hemodialysis should be considered.
- In all renally impaired patients, dose titration should be performed with caution.
Hepatic Impairment
- Patients with mild to moderate hepatic impairment should be observed closely during dose titration.
- A maximum dose of 300 mg/day is recommended for patients with mild to moderate hepatic impairment. The pharmacokinetics of lacosamide has not been evaluated in severe hepatic impairment.
- Lacosamide use is not recommended in patients with severe hepatic impairment. *Patients with co-existing hepatic and renal impairment should be monitored closely during dose titration.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Lacosamide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Lacosamide in patients who are immunocompromised.
CYP2C19 Polymorphism
There are no clinically relevant differences in the pharmacokinetics of lacosamide between CYP2C19 poor metabolizers and extensive metabolizers. Results from a trial in poor metabolizers (PM) (N=4) and extensive metabolizers (EM) (N=8) of cytochrome P450 (CYP) 2C19 showed that lacosamide plasma concentrations were similar in PMs and EMs, but plasma concentrations and the amount excreted into urine of the O-desmethyl metabolite were about 70% reduced in PMs compared to EMs.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
- Patients with co-existing hepatic and renal impairment should be monitored closely during dose titration.
- Monitor closely patients with known cardiac conduction problems, on concomitant medications that prolong PR interval, or with severe cardiac disease
- Monitor patients for suicidal behavior and ideation
IV Compatibility
Lacosamide injection can be administered intravenously without further dilution or may be mixed with diluents listed below. The diluted solution should not be stored for more than 4 hours at room temperature.
Diluents:
- Sodium Chloride Injection 0.9% (w/v)
- Dextrose Injection 5% (w/v)
- Lactated Ringer's Injection
- Product with particulate matter or discoloration should not be used.
- Any unused portion of lacosamide injection should be discarded.
Overdosage
Signs, Symptoms, and Laboratory Findings of Acute Overdose in Humans
- The types of adverse events experienced by patients exposed to supratherapeutic lacosamide doses during clinical trials were not clinically different from those of patients administered recommended doses of lacosamide.
- The highest reported accidental overdose of lacosamide during clinical development was 1200 mg/day which was non-fatal.
- There has been a single case of intentional overdose in a clinical trial by a patient who self-administered 12,000 mg lacosamide along with large doses of zonisamide, topiramate, and gabapentin.
- The patient presented in a coma with AV block and was hospitalized.
- An EEG revealed epileptic waveforms.
- The patient recovered 2 days later.
- In postmarketing experience, cardiac conduction disorders and fatal cardiac arrest were reported following an acute overdose of 7,000 mg of lacosamide in a patient with cardiovascular risk factors.
Treatment or Management of Overdose
- There is no specific antidote for overdose with lacosamide.
- Standard decontamination procedures should be followed.
- General supportive care of the patient is indicated including monitoring of vital signs and observation of the clinical status of patient.
- A Certified Poison Control Center should be contacted for up to date information on the management of overdose with lacosamide.
- Standard hemodialysis procedures result in significant clearance of lacosamide (reduction of systemic exposure by 50% in 4 hours).
- Hemodialysis has not been performed in the few known cases of overdose, but may be indicated based on the patient's clinical state or in patients with significant renal impairment.
Pharmacology
| none|500px | |
| Clinical data | |
|---|---|
| Trade names | lacosamide |
| Synonyms | (2R)-2-(acetylamino)-N-benzyl-3-methoxypropanamide |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a609028 |
| Pregnancy category |
|
| Routes of administration | Oral, intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | High |
| Elimination half-life | 13 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C13H18N2O3 |
| Molar mass | 250.294 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Mechanism of Action
- The precise mechanism by which lacosamide exerts its antiepileptic effects in humans remains to be fully elucidated.
- In vitro electrophysiological studies have shown that lacosamide selectively enhances slow inactivation of voltage-gated sodium channels, resulting in stabilization of hyperexcitable neuronal membranes and inhibition of repetitive neuronal firing.
Structure
The chemical name of lacosamide, the single (R)-enantiomer, is (R)-2-acetamido-N-benzyl-3-methoxypropionamide (IUPAC). Lacosamide is a functionalized amino acid. Its molecular formula is C13H18N2O3 and its molecular weight is 250.30. The chemical structure is:

Pharmacodynamics
- A pharmacokinetic-pharmacodynamic (efficacy) analysis was performed based on the pooled data from the 3 efficacy trials for partial-onset seizures. *Lacosamide exposure is correlated with the reduction in seizure frequency. However, doses above 400 mg/day do not appear to confer additional benefit in group analyses.
Cardiac Electrophysiology
- Electrocardiographic effects of lacosamide were determined in a double-blind, randomized clinical pharmacology trial of 247 healthy subjects.
- Chronic oral doses of 400 and 800 mg/day were compared with placebo and a positive control (400 mg moxifloxacin).
- Lacosamide did not prolong QTc interval and did not have a dose-related or clinically important effect on QRS duration.
- Lacosamide produced a small, dose-related increase in mean PR interval. At steady-state, the time of the maximum observed mean PR interval corresponded with tmax.
- The placebo-subtracted maximum increase in PR interval (at tmax) was 7.3 ms for the 400 mg/day group and 11.9 ms for the 800 mg/day group. For patients who participated in the controlled trials, the placebo-subtracted mean maximum increase in PR interval for a 400 mg/day lacosamide dose was 3.1 ms in patients with partial-onset seizures and 9.4 ms for patients with diabetic neuropathy.
Pharmacokinetics
- The pharmacokinetics of lacosamide have been studied in healthy adult subjects (age range 18 to 87), adults with partial-onset seizures, adults with diabetic neuropathy, and subjects with renal and hepatic impairment.
- Lacosamide is completely absorbed after oral administration with negligible first-pass effect with a high absolute bioavailability of approximately 100%. *The maximum lacosamide plasma concentrations occur approximately 1 to 4 hour post-dose after oral dosing, and elimination half-life is approximately 13 hours.
- Steady state plasma concentrations are achieved after 3 days of twice daily repeated administration.
- Pharmacokinetics of lacosamide are dose proportional (100-800 mg) and time invariant, with low inter- and intra-subject variability.
- Compared to lacosamide the major metabolite, O-desmethyl metabolite, has a longer Tmax (0.5 to 12 hours) and elimination half-life (15-23 hours).
Absorption and Bioavailability
- Lacosamide is completely absorbed after oral administration.
- The oral bioavailability of lacosamide tablets is approximately 100%.
- Food does not affect the rate and extent of absorption.
- After intravenous administration, Cmax is reached at the end of infusion. The 30- and 60-minute intravenous infusions are bioequivalent to the oral tablet. For the 15-minute intravenous infusion, bioequivalence was met for AUC(0-tz) but not for Cmax.
- The point estimate of Cmax was 20% higher than Cmax for oral tablet and the 90% CI for Cmax exceeded the upper boundary of the bioequivalence range.
- In a trial comparing the oral tablet with an oral solution containing 10 mg/mL lacosamide, bioequivalence between both formulations was shown.
- A single loading dose of 200 mg approximates steady-state concentrations comparable to the 100 mg twice daily oral administration.
Distribution
- The volume of distribution is approximately 0.6 L/kg and thus close to the volume of total body water.
- Lacosamide is less than 15% bound to plasma proteins.
Metabolism and Elimination
- Lacosamide is primarily eliminated from the systemic circulation by renal excretion and biotransformation.
- After oral and intravenous administration of 100 mg [14C]-lacosamide approximately 95% of radioactivity administered was recovered in the urine and less than 0.5% in the feces.
- The major compounds excreted were unchanged lacosamide (approximately 40% of the dose), its O-desmethyl metabolite (approximately 30%), and a structurally unknown polar fraction (~20%).
- The plasma exposure of the major human metabolite, O-desmethyl-lacosamide, is approximately 10% of that of lacosamide. This metabolite has no known pharmacological activity.
- The CYP isoforms mainly responsible for the formation of the major metabolite (O-desmethyl) are CYP3A4, CYP2C9, and CYP2C19.
- The elimination half-life of the unchanged drug is approximately 13 hours and is not altered by different doses, multiple dosing or intravenous administration.
- There is no enantiomeric interconversion of lacosamide.
Special Populations
Renal impairment
- Lacosamide and its major metabolite are eliminated from the systemic circulation primarily by renal excretion.
- The AUC of lacosamide was increased approximately 25% in mildly (CLCR 50-80 mL/min) and moderately (CLCR 30-50 mL/min) and 60% in severely (CLCR≤30 mL/min) renally impaired patients compared to subjects with normal renal function (CLCR>80 mL/min), whereas Cmax was unaffected.
- No dose adjustment is considered necessary in mildly and moderately renal impaired subjects.
- A maximum dose of 300 mg/day is recommended for patients with severe renal impairment (CLCR≤30 mL/min) and in patients with endstage renal disease. *Lacosamide is effectively removed from plasma by hemodialysis. Following a 4-hour hemodialysis treatment, AUC of lacosamide is reduced by approximately 50%. Therefore dosage supplementation of up to 50% following hemodialysis should be considered.
- In all renally impaired patients, the dose titration should be performed with caution.
Hepatic impairment
- Lacosamide undergoes metabolism.
- Subjects with moderate hepatic impairment (Child-Pugh B) showed higher plasma concentrations of lacosamide (approximately 50-60% higher AUC compared to healthy subjects).
- The dose titration should be performed with caution in patients with hepatic impairment.
- A maximum dose of 300 mg/day is recommended for patients with mild or moderate hepatic impairment.
- Patients with mild to moderate hepatic impairment should be observed closely during dose titration.
- A maximum dose of 300 mg/day is recommended for patients with mild to moderate hepatic impairment.
- The pharmacokinetics of lacosamide have not been evaluated in severe hepatic impairment.
- Lacosamide use is not recommended in patients with severe hepatic impairment.
- Patients with co-existing hepatic and renal impairment should be monitored closely during dose titration.
Geriatric
- In the elderly (>65 years), dose and body-weight normalized AUC and Cmax is about 20% increased compared to young subjects (18-64 years).
- This may be related to body weight and decreased renal function in elderly subjects.
- Dose reduction is not considered to be necessary.
Pediatric Patients
- Pharmacokinetics of lacosamide have not been studied in pediatric patients.
Gender
- Lacosamide clinical trials indicate that gender does not have a clinically relevant influence on the pharmacokinetics of lacosamide.
Race
- There are no clinically relevant differences in the pharmacokinetics of lacosamide between Asian, Black, and Caucasian subjects.
CYP2C19 Polymorphism
- There are no clinically relevant differences in the pharmacokinetics of lacosamide between CYP2C19 poor metabolizers and extensive metabolizers. *Results from a trial in poor metabolizers (PM) (N=4) and extensive metabolizers (EM) (N=8) of cytochrome P450 (CYP) 2C19 showed that lacosamide plasma concentrations were similar in PMs and EMs, but plasma concentrations and the amount excreted into urine of the O-desmethyl metabolite were about 70% reduced in PMs compared to EMs.
Drug interactions
In Vitro Assessment of Drug Interactions
- In vitro metabolism studies indicate that lacosamide does not induce the enzyme activity of drug metabolizing cytochrome P450 isoforms CYP1A2, 2B6, 2C9, 2C19 and 3A4.
- Lacosamide did not inhibit CYP 1A1, 1A2, 2A6, 2B6, 2C8, 2C9, 2D6, 2E1, 3A4/5 at plasma concentrations observed in clinical studies.
- In vitro data suggest that lacosamide has the potential to inhibit CYP2C19 at therapeutic concentrations. However, an in vivo study with omeprazole did not show an inhibitory effect on omeprazole pharmacokinetics.
Lacosamide was not a substrate or inhibitor for P-glycoprotein
- Lacosamide is a substrate of CYP3A4, CYP2C9, and CYP2C19.
- Patients with renal or hepatic impairment who are taking strong inhibitors of CYP3A4 and CYP2C9 may have increased exposure to lacosamide.
- Since <15% of lacosamide is bound to plasma proteins, a clinically relevant interaction with other drugs through competition for protein binding sites is unlikely.
In Vivo Assessment of Drug Interactions
Drug interaction studies with AEDs
Effect of lacosamide on concomitant AEDs
- Lacosamide 400 mg/day had no influence on the pharmacokinetics of 600 mg/day valproic acid and 400 mg/day carbamazepine in healthy subjects.
- The placebo-controlled clinical studies in patients with partial-onset seizures showed that steady-state plasma concentrations of levetiracetam, carbamazepine, carbamazepine epoxide, lamotrigine, topiramate, oxcarbazepine monohydroxy derivative (MHD), phenytoin, valproic acid, phenobarbital, gabapentin, clonazepam, and zonisamide were not affected by concomitant intake of lacosamide at any dose.
Effect of concomitant AEDs on lacosamide
- Drug-drug interaction studies in healthy subjects showed that 600 mg/day valproic acid had no influence on the pharmacokinetics of 400 mg/day lacosamide. Likewise, 400 mg/day carbamazepine had no influence on the pharmacokinetics of lacosamide in a healthy subject study.
- Population pharmacokinetics results in patients with partial-onset seizures showed small reductions (15% to 20% lower) in lacosamide plasma concentrations when lacosamide was coadministered with carbamazepine, phenobarbital or phenytoin.
Drug-drug interaction studies with other drugs
Digoxin
- There was no effect of lacosamide (400 mg/day) on the pharmacokinetics of digoxin (0.5 mg once daily) in a study in healthy subjects.
Metformin
- There were no clinically relevant changes in metformin levels following coadministration of lacosamide (400 mg/day).
- Metformin (500 mg three times a day) had no effect on the pharmacokinetics of lacosamide (400 mg/day).
Omeprazole
- Omeprazole is a CYP2C19 substrate and inhibitor.
- There was no effect of lacosamide (600 mg/day) on the pharmacokinetics of omeprazole (40 mg single dose) in healthy subjects. The data indicated that lacosamide had little in vivo inhibitory or inducing effect on CYP2C19.
Omeprazole at a dose of 40 mg once daily had no effect on the pharmacokinetics of lacosamide (300 mg single dose). However, plasma levels of the O-desmethyl metabolite were reduced about 60% in the presence of omeprazole.
Midazolam
- Midazolam is a 3A4 substrate.
- There was no effect of lacosamide (200 mg single dose or repeat doses of 400 mg/day given as 200 mg BID) on the pharmacokinetics of midazolam (single dose, 7.5 mg), indicating no inhibitory or inducing effects on CYP3A4.
Oral Contraceptives
- There was no influence of lacosamide (400 mg/day) on the pharmacodynamics and pharmacokinetics of an oral contraceptive containing 0.03 mg ethinylestradiol and 0.15 mg levonorgestrel in healthy subjects, except that a 20% increase in ethinylestradiol Cmax was observed.
Warfarin
- Co-administration of lacosamide (400 mg/day) with warfarin (25 mg single dose) did not result in a clinically relevant change in the pharmacokinetic and pharmacodynamic effects of warfarin in a study in healthy male subjects.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- There was no evidence of drug related carcinogenicity in mice or rats.
- Mice and rats received lacosamide once daily by oral administration for 104 weeks at doses producing plasma exposures (AUC) up to approximately 1 and 3 times, respectively, the plasma AUC in humans at the maximum recommended human dose (MRHD) of 400 mg/day.
- Lacosamide was negative in an in vitro Ames test and an in vivo mouse micronucleus assay.
- Lacosamide induced a positive response in the in vitro mouse lymphoma assay.
- No adverse effects on male or female fertility or reproduction were observed in rats at doses producing plasma exposures (AUC) up to approximately 2 times the plasma AUC in humans at the MRHD.
Clinical Studies
Monotherapy in Patients with Partial Onset Seizures
- The efficacy of lacosamide in monotherapy was established in a historical-control, multicenter, randomized trial that included 425 patients, age 16 to 70 years, with partial-onset seizures (Study 1).
- To be included in Study 1, patients were required to be taking stable doses of 1 or 2 marketed antiepileptic drugs.
- This treatment continued into the 8 week baseline period.
- To remain in the study, patients were required to have at least 2 partial onset seizures per 28 days during the 8 week baseline period.
- The baseline period was followed by a 3 week titration period, during which lacosamide was added to the ongoing antiepileptic regimen.
- This was followed by a 16-week maintenance period (i.e., a 6-week withdrawal period for background antiepileptic drugs, followed by a 10-week monotherapy period).
- Patients were randomized 3 to 1 to receive lacosamide 400 mg/day or lacosamide 300 mg/day.
- Treatment assignments were blinded.
- Response to treatment was based upon a comparison of the number of patients who met exit criteria during the maintenance phase, compared to historical controls.
- The historical control consisted of a pooled analysis of the control groups from 8 studies of similar design, which utilized a sub-therapeutic dose of an antiepileptic drug.
- Statistical superiority to the historical control was considered to be demonstrated if the upper limit from a 2-sided 95% confidence interval for the percentage of patients meeting exit criteria in patients receiving lacosamide remained below the lower 95% prediction limit of 65% derived from the historical control data.
- The exit criteria were one or more of the following:
- (1) Doubling of average monthly seizure frequency during any 28 consecutive days.
- (2) Doubling of highest consecutive 2-day seizure frequency.
- (3) Occurrence of a single generalized tonic-clonic seizure
- (4) Clinically significant prolongation or worsening of overall seizure duration, frequency, type or pattern considered by the investigator to require trial discontinuation.
- (5) Status epilepticus or new onset of serial/cluster seizures. The study population profile appeared comparable to that of the historical control population.
- For the lacosamide 400 mg/day group, the estimate of the percentage of patients meeting at least 1 exit criterion was 30% (95% CI: 25%, 36%).
- The upper limit of the 2-sided 95% CI (36%) was below the threshold of 65% derived from the historical control data, meeting the pre-specified criteria for efficacy. Lacosamide 300 mg/day also met the pre-specified criteria for efficacy.
Adjunctive Therapy in Patients with Partial Onset Seizures
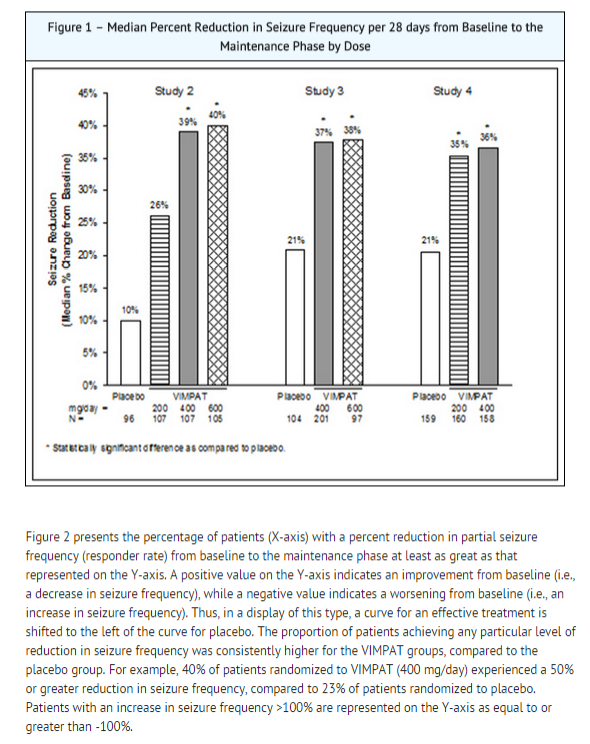
- The efficacy of lacosamide as adjunctive therapy in partial-onset seizures was established in three 12-week, randomized, double-blind, placebo-controlled, multicenter trials in adult patients (Study 2, Study 3, and Study 4).
- Enrolled patients had partial-onset seizures with or without secondary generalization, and were not adequately controlled with 1 to 3 concomitant AEDs.
- During an 8-week baseline period, patients were required to have an average of ≥4 partial-onset seizures per 28 days with no seizure-free period exceeding 21 days. In these 3 trials, patients had a mean duration of epilepsy of 24 years and a median baseline seizure frequency ranging from 10 to 17 per 28 days. 84% of patients were taking 2 to 3 concomitant AEDs with or without concurrent vagal nerve stimulation.
- Study 2 compared doses of lacosamide 200, 400, and 600 mg/day with placebo. *Study 3 compared doses of lacosamide 400 and 600 mg/day with placebo.
- Study 4 compared doses of lacosamide 200 and 400 mg/day with placebo.
- In all three trials, following an 8-week baseline phase to establish baseline seizure frequency prior to randomization, subjects were randomized and titrated to the randomized dose (a 1-step back-titration of lacosamide 100 mg/day or placebo was allowed in the case of intolerable adverse events at the end of the titration phase).
- During the titration phase, in all 3 adjunctive therapy trials, treatment was initiated at 100 mg/day (50 mg twice daily), and increased in weekly increments of 100 mg/day to the target dose.
- The titration phase lasted 6 weeks in Study 2 and Study 3, and 4 weeks in Study 4. In all three trials, the titration phase was followed by a maintenance phase that lasted 12 weeks, during which patients were to remain on a stable dose of lacosamide.
- A reduction in 28 day seizure frequency (baseline to maintenance phase), as compared to the placebo group, was the primary variable in all three adjunctive therapy trials.
- A statistically significant effect was observed with lacosamide treatment (Figure 1) at doses of 200 mg/day (Study 4), 400 mg/day (Studies 2, 3, and 4), and 600 mg/day (Studies 2 and 3).
- Subset evaluations of lacosamide demonstrate no important differences in seizure control as a function of gender or race, although data on race was limited (about 10% of patients were non-Caucasian).

How Supplied
Lacosamide Tablets 50 mg
- Are pink, oval, film-coated tablets debossed with "SP" on one side and "50" on the other. They are supplied as follows:
- Bottles of 60 NDC 0131-2477-35
- Unit Dose Carton of 60 tablets [6 cards, each card contains 10 tablets] NDC 0131-2477-60
Lacosamide Tablets 100 mg
- Are dark yellow, oval, film-coated tablets debossed with "SP" on one side and "100" on the other. They are supplied as follows:
- Bottles of 60 NDC 0131-2478-35
- Unit Dose Carton of 60 tablets [6 cards, each card contains 10 tablets] NDC 0131-2478-60
Lacosamide Tablets 150 mg
- Are salmon, oval, film-coated tablets debossed with "SP" on one side and "150" on the other. They are supplied as follows:
- Bottles of 60 NDC 0131-2479-35
- Unit Dose Carton of 60 tablets [6 cards, each card contains 10 tablets] NDC 0131-2479-60
Lacosamide Tablets 200 mg
- Are blue, oval, film-coated tablets debossed with "SP" on one side and "200" on the other. They are supplied as follows:
- Bottles of 60 NDC 0131-2480-35
- Unit Dose Carton of 60 tablets [6 cards, each card contains 10 tablets] NDC 0131-2480-60
Lacosamide injection 200 mg/20 m
- Is a clear, colorless sterile solution supplied in 20 mL colorless single-use glass vials.
- 200 mg/20 mL vial in cartons of 10 vials NDC 0131-1810-67
Lacosamide oral solution 10 mg/mL
- Is a clear, colorless to yellow or yellow-brown, strawberry-flavored liquid. It is supplied in PET bottles as follows:
- 200 mL bottles NDC 0131-5410-71
- 465 mL bottles NDC 0131-5410-70
Storage
- Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F).
- Do not freeze lacosamide injection or oral solution. Discard any unused lacosamide oral solution remaining after seven (7) weeks of first opening the bottle.
Images
Drug Images
{{#ask: Page Name::Lacosamide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel






{{#ask: Label Page::Lacosamide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Suicidal Thinking and Behavior
- Patients, their caregivers, and families should be counseled that AEDs, including lacosamide, may increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. *Behaviors of concern should be reported immediately to healthcare providers.
Dizziness and Ataxia
- Patients should be counseled that lacosamide use may cause dizziness, double vision, abnormal coordination and balance, and somnolence. *Patients taking lacosamide should be advised not to drive, operate complex machinery, or engage in other hazardous activities until they have become accustomed to any such effects associated with lacosamide.
Cardiac Rhythm and Conduction Abnormalities
- Patients should be counseled that lacosamide is associated with electrocardiographic changes that may predispose to irregular beat and syncope, particularly in patients with underlying cardiovascular disease, with heart conduction problems or who are taking other medications that affect the heart.
- Patients who develop syncope should lay down with raised legs and contact their health care provider.
Multiorgan Hypersensitivity Reactions
- Patients should be aware that lacosamide may cause serious hypersensitivity reactions affecting multiple organs such as the liver and kidney. Lacosamide should be discontinued if a serious hypersensitivity reaction is suspected. *Patients should also be instructed to report promptly to their physicians any symptoms of liver toxicity (e.g. fatigue, jaundice, dark urine).
Pregnancy Registry
- Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during lacosamide therapy.
- Encourage patients to enroll in the North American Antiepileptic Drug (NAAED) pregnancy registry if they become pregnant.
- This registry is collecting information about the safety of AEDs during pregnancy.
Precautions with Alcohol
Alcohol-Lacosamide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- VIMPAT[1]
Look-Alike Drug Names
There is limited information regarding Lacosamide Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Lacosamide |Label Name=Lacosamide Package 1.png
}}
{{#subobject:
|Label Page=Lacosamide |Label Name=Lacosamide Package 2.png
}}
{{#subobject:
|Label Page=Lacosamide |Label Name=Lacosamide Package 3.png
}}
{{#subobject:
|Label Page=Lacosamide |Label Name=Lacosamide package 4.png
}}
{{#subobject:
|Label Page=Lacosamide |Label Name=Lacosamide package 5.png
}}
{{#subobject:
|Label Page=Lacosamide |Label Name=Lacosamide package 6.png
}}
{{#subobject:
|Label Page=Lacosamide |Label Name=Lacosamide package 7.png
}}
| File:Lacosamide.svg | |
| Clinical data | |
|---|---|
| Trade names | lacosamide |
| Synonyms | (2R)-2-(acetylamino)-N-benzyl-3-methoxypropanamide |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a609028 |
| Routes of administration | Oral, intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | High |
| Elimination half-life | 13 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C13H18N2O3 |
| Molar mass | 250.294 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
- Pages with script errors
- Pages using deprecated image syntax
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- Articles with changed CASNo identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Drugboxes which contain changes to verified fields
- Pages with broken file links