Gemfibrozil
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alejandro Lemor, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Gemfibrozil is a peroxisome proliferator receptor alpha agonist that is FDA approved for the {{{indicationType}}} of types IV and V hyperlipidemia at risk of pancreatitis and who do not respond adequately to a determined dietary effort to control them, and reducing the risk of developing coronary heart disease only in Type IIb patients without history of or symptoms of existing coronary heart disease who have had an inadequate response to weight loss, dietary therapy, exercise, and other pharmacologic agents (such as bile acid sequestrants and nicotinic acid) and who have the following triad of lipid abnormalities: low HDL-cholesterol levels in addition to elevated LDL-cholesterol and elevated triglycerides. Common adverse reactions include dyspepsia, abdominal pain, acute appendicitis, atrial fibrillation, diarrhea, fatigue, eczema, rash, vertigo, constipation, headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Hyperlipidemia type IV and V
- Dosing Information
- 600 mg PO every 12 hours (30 minutes before the morning and evening meals)
Prophylaxis for Disorder of Cardiovascular System
- Dosing Information
- 600 mg PO every 12 hours (30 minutes before the morning and evening meals)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Gemfibrozil in adult patients.
Non–Guideline-Supported Use
Antiviral Drug Adverse Reaction, Antiretroviral - Hyperlipidemia
- Dosing Information
- 600 mg PO every 12 hours
Prophylaxis for Cerebrovascular Accident
- Dosing Information
- 600 mg PO every 12 hours
Hyperlipidemia
- Dosing Information
- 600 mg PO every 12 hours
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Gemfibrozil FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Gemfibrozil in pediatric patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Gemfibrozil in pediatric patients.
Contraindications
- Hepatic or severe renal dysfunction, including primary biliary cirrhosis.
- Preexisting gallbladder disease.
- Hypersensitivity to gemfibrozil.
- Combination therapy of gemfibrozil with repaglinide
- Combination therapy of gemfibrozil with simvastatin
Warnings
1. Because of chemical, pharmacological, and clinical similarities between gemfibrozil and clofibrate, the adverse findings with clofibrate in two large clinical studies may also apply to gemfibrozil. In the first of those studies, the Coronary Drug Project, 1000 subjects with previous myocardial infarction were treated for five years with clofibrate. There was no difference in mortality between the clofibrate-treated subjects and 3000 placebo-treated subjects, but twice as many clofibrate-treated subjects developed cholelithiasis and cholecystitis requiring surgery. In the other study, conducted by the World Health Organization (WHO), 5000 subjects without known coronary heart disease were treated with clofibrate for five years and followed one year beyond. There was a statistically significant (44%) higher age-adjusted total mortality in the clofibrate-treated group than in a comparable placebo-treated control group during the trial period. The excess mortality was due to a 33% increase in non-cardiovascular causes, including malignancy, post-cholecystectomy complications, and pancreatitis. The higher risk of clofibrate-treated subjects for gallbladder disease was confirmed.
Because of the more limited size of the Helsinki Heart Study, the observed difference in mortality from any cause between the gemfibrozil and placebo groups is not statistically significantly different from the 29% excess mortality reported in the clofibrate group in the separate WHO study at the nine year follow-up. Noncoronary heart disease related mortality showed an excess in the group originally randomized to gemfibrozil primarily due to cancer deaths observed during the open-label extension.
During the five year primary prevention component of the Helsinki Heart Study, mortality from any cause was 44 (2.2%) in the gemfibrozil group and 43 (2.1%) in the placebo group; including the 3.5 year follow-up period since the trial was completed, cumulative mortality from any cause was 101 (4.9%) in the gemfibrozil group and 83 (4.1%) in the group originally randomized to placebo (hazard ratio 1:20 in favor of placebo). Because of the more limited size of the Helsinki Heart Study, the observed difference in mortality from any cause between the gemfibrozil and placebo groups at Year-5 or at Year-8.5 is not statistically significantly different from the 29% excess mortality reported in the clofibrate group in the separate WHO study at the nine year follow-up. Noncoronary heart disease related mortality showed an excess in the group originally randomized to gemfibrozil at the 8.5 year follow-up (65 gemfibrozil versus 45 placebo noncoronary deaths).
The incidence of cancer (excluding basal cell carcinoma) discovered during the trial and in the 3.5 years after the trial was completed was 51 (2.5%) in both originally randomized groups. In addition, there were 16 basal cell carcinomas in the group originally randomized to gemfibrozil and 9 in the group originally randomized to placebo (p=0.22). There were 30 (1.5%) deaths attributed to cancer in the group originally randomized to gemfibrozil and 18 (0.9%) in the group originally randomized to placebo (p=0.11). Adverse outcomes, including coronary events, were higher in gemfibrozil patients in a corresponding study in men with a history of known or suspected coronary heart disease in the secondary prevention component of the Helsinki Heart Study.
A comparative carcinogenicity study was also done in rats comparing three drugs in this class: fenofibrate (10 and 60 mg/kg; 0.3 and 1.6 times the human dose, respectively), clofibrate (400 mg/kg; 1.6 times the human dose), and gemfibrozil (250 mg/kg; 1.7 times the human dose). Pancreatic acinar adenomas were increased in males and females on fenofibrate; hepatocellular carcinoma and pancreatic acinar adenomas were increased in males and hepatic neoplastic nodules in females treated with clofibrate; hepatic neoplastic nodules were increased in males and females treated with clofibrate; hepatic neoplastic nodules were increased in males and females treated with gemfibrozil while testicular interstitial cell (Leydig cell) tumors were increased in males on all three drugs.
2. A gallstone prevalence substudy of 450 Helsinki Heart Study participants showed a trend toward a greater prevalence of gallstones during the study within the gemfibrozil treatment group (7.5% versus 4.9% for the placebo group, a 55% excess for the gemfibrozil group). A trend toward a greater incidence of gallbladder surgery was observed for the gemfibrozil group (17 versus 11 subjects, a 54% excess). This result did not differ statistically from the increased incidence of cholecystectomy observed in the WHO study in the group treated with clofibrate. Both clofibrate and gemfibrozil may increase cholesterol excretion into the bile, leading to cholelithiasis. If cholelithiasis is suspected, gallbladder studies are indicated. gemfibrozil therapy should be discontinued if gallstones are found. Cases of cholelithiasis have been reported with gemfibrozil therapy.
3. Since a reduction of mortality from coronary heart disease has not been demonstrated and because liver and interstitial cell testicular tumors were increased in rats, gemfibrozil should be administered only to those patients described before. If a significant serum lipid response is not obtained, gemfibrozil should be discontinued.
4. Concomitant Anticoagulants – Caution should be exercised when anticoagulants are given in conjunction with gemfibrozil . The dosage of the anticoagulant should be reduced to maintain the prothrombin time at the desired level to prevent bleeding complications. Frequent prothrombin determinations are advisable until it has been definitely determined that the prothrombin level has stabilized.
5. The concomitant administration of gemfibrozil with simvastatin is contraindicated. Concomitant therapy with gemfibrozil and an HMG-CoA reductase inhibitor is associated with an increased risk of skeletal muscle toxicity manifested as rhabdomyolysis, markedly elevated creatine kinase (CPK) levels, and myoglobinuria, leading in a high proportion of cases to acute renal failure and death. IN PATIENTS WHO HAVE HAD AN UNSATISFACTORY LIPID RESPONSE TO EITHER DRUG ALONE, THE BENEFIT OF COMBINED THERAPY WITH GEMFBROZIL AND an HMG-CoA REDUCTASE INHIBITOR DOES NOT OUTWEIGH THE RISKS OF SEVERE MYOPATHY, rhabdomyolysis, AND ACUTE RENAL FAILURE. The use of fibrates alone, including gemfibrozil , may occasionally be associated with myositis. Patients receiving gemfibrozil and complaining of muscle pain, tenderness, or weakness should have prompt medical evaluation for myositis, including serum creatine–kinase level determination. If myositis is suspected or diagnosed, gemfibrozil therapy should be withdrawn.
6. Cataracts – Subcapsular bilateral cataracts occurred in 10%, and unilateral in 6.3%, of male rats treated with gemfibrozil at 10 times the human dose.
Adverse Reactions
Clinical Trials Experience
Gastrointestinal
Central Nervous System
- Dizziness
- Somnolence
- Paresthesia
- Peripheral neuritis
- Decreased libido
- Depression
- Headache
- Blurred vision
Genitourinary
Musculoskeletal
Clinical Laboratory
- Increased creatine phosphokinase (CPK)
- Increased bilirubin
- Increased liver transaminases (AST , ALT)
- Increased alkaline phosphatase
Hematopoietic
- Anemia
- Leukopenia
- Bone marrow hypoplasia
- Eosinophilia
Immunologic
- Angioedema
- Laryngeal edema
- Exfoliative dermatitis
Dermatologic
Postmarketing Experience
There is limited information regarding Gemfibrozil Postmarketing Experience in the drug label.
Drug Interactions
HMG-CoA Reductase Inhibitors
The concomitant administration of Gemfibrozil with simvastatin is contraindicated. The risk of myopathy and rhabdomyolysis is increased with combined gemfibrozil and HMG-CoA reductase inhibitor therapy. Myopathy or rhabdomyolysis with or without acute renal failure have been reported as early as three weeks after initiation of combined therapy or after several months. There is no assurance that periodic monitoring of creatine kinase will prevent the occurrence of severe myopathy and kidney damage.
Anticoagulants
Caution should be exercised when anti-coagulants are given in conjunction with gemfibrozil . The dosage of the anticoagulant should be reduced to maintain the prothrombin time at the desired level to prevent bleeding complications. Frequent prothrombin determinations are advisable until it has been definitely determined that the prothrombin level has stabilized.
Repaglinide
In healthy volunteers, co-administration with gemfibrozil (600 mg twice daily for 3 days) resulted in an 8.1-fold (range 5.5- to 15.0- fold) higher repaglinide AUC and a 28.6-fold (range 18.5- to 80.1-fold) higher repaglinide plasma concentration 7 hours after the dose. In the same study, gemfibrozil (600 mg twice daily for 3 days) + itraconazole (200 mg in the morning and 100 mg in the evening at Day 1, then 100 mg twice daily at Day 2–3) resulted in a 19.4- (range 12.9- to 24.7-fold) higher repaglinideAUC and a 70.4-fold (range 42.9- to 119.2-fold) higher repaglinideplasma concentration 7 hours after the dose. In addition, gemfibrozil alone or gemfibrozil + itraconazole prolonged the hypoglycemic effects of repaglinide. Co-administration of gemfibrozil and repaglinideincreases the risk of severe hypoglycemia and is contraindicated.
Bile Acid-Binding Resins
Gemfibrozil AUC was reduced by 30% when gemfibrozil was given (600 mg) simultaneously with resin-granule drugs such as colestipol (5 g). Administration of the drugs two hours or more apart is recommended because gemfibrozil exposure was not significantly affected when it was administered two hours apart from colestipol.
Colchicine
Myopathy, including rhabdomyolysis, has been reported with chronic administration of colchicine at therapeutic doses. Concomitant use of gemfibrozil may potentiate the development of myopathy. Patients with renal dysfunction and elderly patients are at increased risk. Caution should be exercised when prescribing gemfibrozil with colchicine, especially in elderly patients or patients with renal dysfunction.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C Gemfibrozil has been shown to produce adverse effects in rats and rabbits at doses between 0.5 and 3 times the human dose (based on surface area). There are no adequate and well-controlled studies in pregnant women. Gemfibrozil should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Administration of gemfibrozil to female rats at 2 times the human dose (based on surface area) before and throughout gestation caused a dose-related decrease in conception rate, an increase in stillborns and a slight reduction in pup weight during lactation. There were also dose-related increased skeletal variations. Anophthalmia occurred, but rarely.
Administration of 0.6 and 2 times the human dose (based on surface area) of gemfibrozil to female rats from gestation day 15 through weaning caused dose-related decreases in birth weight and suppressions of pup growth during lactation.
Administration of 1 and 3 times the human dose (based on surface area) of gemfibrozil to female rabbits during organogenesis caused a dose-related decrease in litter size and, at the high dose, an increased incidence of parietal bone variations.
Pregnancy Category (AUS): B3
Drugs which have been taken by only a limited number of pregnant women and women of childbearing age, without an increase in the frequency of malformation or other direct or indirect harmful effects on the human fetus having been observed. Studies in animals have shown evidence of an increased occurrence of fetal damage, the significance of which is considered uncertain in humans.
Labor and Delivery
There is no FDA guidance on use of Gemfibrozil during labor and delivery.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for tumorigenicity shown for gemfibrozil in animal studies, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and efficacy in pediatric patients have not been established.
Geriatic Use
Safety and efficacy in geriatric patients have not been established.
Gender
There is no FDA guidance on the use of Gemfibrozil with respect to specific gender populations.
Race
There is no FDA guidance on the use of Gemfibrozil with respect to specific racial populations.
Renal Impairment
There have been reports of worsening renal insufficiency upon the addition of Gemfibrozil therapy in individuals with baseline plasma creatinine >2.0 mg/dL. In such patients, the use of alternative therapy should be considered against the risks and benefits of a lower dose of Gemfibrozil.
Hepatic Impairment
Abnormal liver function tests have been observed occasionally during Gemfibrozil administration, including elevations of AST,ALT, LDH, [bilirubin]], and alkaline phosphatase. These are usually reversible when Gemfibrozil is discontinued. Therefore, periodic liver function studies are recommended and Gemfibrozil therapy should be terminated if abnormalities persist.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Gemfibrozil in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Gemfibrozil in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
There is limited information regarding Gemfibrozil Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Gemfibrozil and IV administrations.
Overdosage
There have been reported cases of overdosage with gemfibrozil. In one case, a 7-year-old child recovered after ingesting up to 9 grams of gemfibrozil. Symptoms reported with overdosage were abdominal cramps, abnormal liver function tests, diarrhea, increased CPK, joint and muscle pain, nausea and vomiting. Symptomatic supportive measures should be taken, should an overdose occur.
Pharmacology
| |
Gemfibrozil
| |
| Systematic (IUPAC) name | |
| 5-(2,5-dimethylphenoxy)-2,2-dimethyl-pentanoic acid | |
| Identifiers | |
| CAS number | |
| ATC code | C10 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 250.333 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | Close to 100% |
| Protein binding | 95% |
| Metabolism | Hepatic (CYP3A4) |
| Half life | 1.5 hours |
| Excretion | Renal 94% Feces 6% |
| Therapeutic considerations | |
| Pregnancy cat. |
Category C |
| Legal status |
By Prescription |
| Routes | Oral |
Mechanism of Action
The mechanism of action of gemfibrozil has not been definitely established. In man, gemfibrozil has been shown to inhibit peripheral lipolysis and to decrease the hepatic extraction of free fatty acids, thus reducing hepatic triglyceride production. Gemfibrozil inhibits synthesis and increases clearance of VLDL carrier apolipoprotein B, leading to a decrease in VLDL production.
Animal studies suggest that gemfibrozil may, in addition to elevating HDL-cholesterol, reduce incorporation of long-chain fatty acids into newly formed triglycerides, accelerate turnover and removal of cholesterol from the liver, and increase excretion of cholesterol in the feces.
Structure

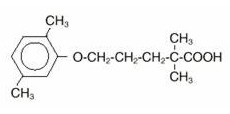
The empirical formula is C15H22O3 and the molecular weight is 250.35; the solubility in water and acid is 0.0019% and in dilute base it is greater than 1%. The melting point is 58°–61° C. Gemfibrozil is a white solid which is stable under ordinary conditions
Pharmacodynamics
Gemfibrozil is well absorbed from the gastrointestinal tract after oral administration. Peak plasma levels occur in 1 to 2 hours with a plasma half-life of 1.5 hours following multiple doses. Gemfibrozil mainly undergoes oxidation of a ring methyl group to successively form a hydroxymethyl and a carboxyl metabolite.
Pharmacokinetics
Gemfibrozil is completely absorbed after oral administration of gemfibrozil tablets, reaching peak plasma concentrations 1 to 2 hours after dosing. Gemfibrozil pharmacokinetics are affected by the timing of meals relative to time of dosing. Approximately seventy percent of the administered human dose is excreted in the urine, mostly as the glucuronide conjugate, with less than 2% excreted as unchanged gemfibrozil. Six percent of the dose is accounted for in the feces. Gemfibrozil is highly bound to plasma proteins and there is potential for displacement interactions with other drugs In one study both the rate and extent of absorption of the drug were significantly increased when administered 0.5 hour before meals. Average AUC (area under the curve) was reduced by 14-44% when gemfibrozil was administered after meals compared to 0.5 hour before meals. In a subsequent study, rate of absorption of gemfibrozil was maximum when administered 0.5 hour before meals with the Cmax 50-60% greater than when given either with meals or fasting. In this study, there were no significant effects on AUC of timing of dose relative to meals
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies have been conducted in rats at 0.2 and 1.3 times the human exposure (based on AUC). The incidence of benign liver nodules and liver carcinomas was significantly increased in high dose male rats. The incidence of liver carcinomas increased also in low dose males, but this increase was not statistically significant (p=0.1). Male rats had a dose-related and statistically significant increase of benign Leydig cell tumors. The higher dose female rats had a significant increase in the combined incidence of benign and malignant liver neoplasms.
Long-term studies have been conducted in mice at 0.1 and 0.7 times the human exposure (based on AUC). There were no statistically significant differences from controls in the incidence of liver tumors, but the doses tested were lower than those shown to be carcinogenic with other fibrates.
Electron microscopy studies have demonstrated a florid hepatic peroxisome proliferation following gemfibrozil administration to the male rat. An adequate study to test for peroxisome proliferation has not been done in humans but changes in peroxisome morphology have been observed. Peroxisome proliferation has been shown to occur in humans with either of two other drugs of the fibrate class when liver biopsies were compared before and after treatment in the same individual.
Administration of approximately 2 times the human dose (based on surface area) to male rats for 10 weeks resulted in a dose-related decrease of fertility. Subsequent studies demonstrated that this effect was reversed after a drug-free period of about eight weeks, and it was not transmitted to the offspring.
Clinical Studies
In the primary prevention component of the Helsinki Heart Study, in which 4081 male patients between the ages of 40 and 55 were studied in a randomized, double-blind, placebo-controlled fashion, gemfibrozil therapy was associated with significant reductions in total plasma triglycerides and a significant increase in high density lipoprotein cholesterol. Moderate reductions in total plasma cholesterol and low density lipoprotein cholesterol were observed for the gemfibrozil treatment group as a whole, but the lipid response was heterogeneous, especially among different Fredrickson types. The study involved subjects with serum non-HDL-cholesterol of over 200 mg/dL and no previous history of coronary heart disease. Over the five-year study period, the gemfibrozil group experienced a 1.4% absolute (34% relative) reduction in the rate of serious coronary events (sudden cardiac deaths plus fatal and nonfatal myocardial infarctions) compared to placebo, p=0.04 (see Table I). There was a 37% relative reduction in the rate of nonfatal myocardial infarction compared to placebo, equivalent to a treatment-related difference of 13.1 events per thousand persons. Deaths from any cause during the double-blind portion of the study totaled 44 (2.2%) in the gemfibrozil randomization group and 43 (2.1%) in the placebo group.
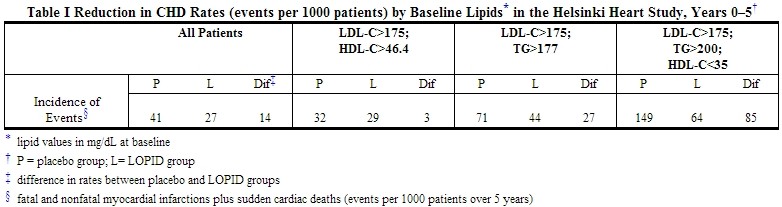
Among Fredrickson types, during the 5-year double-blind portion of the primary prevention component of the Helsinki Heart Study, the greatest reduction in the incidence of serious coronary events occurred in Type IIb patients who had elevations of both LDL-cholesterol and total plasma triglycerides. This subgroup of Type IIb gemfibrozil group patients had a lower mean HDL-cholesterol level at baseline than the Type IIa subgroup that had elevations of LDL-cholesterol and normal plasma triglycerides. The mean increase in HDL-cholesterol among the Type IIb patients in this study was 12.6% compared to placebo. The mean change in LDL-cholesterol among Type IIb patients was –4.1% with gemfibrozil compared to a rise of 3.9% in the placebo subgroup. The Type IIb subjects in the Helsinki Heart Study had 26 fewer coronary events per thousand persons over five years in the gemfibrozil group compared to placebo. The difference in coronary events was substantially greater between gemfibrozil and placebo for that subgroup of patients with the triad of LDL-cholesterol >175 mg/dL (>4.5 mmol), triglycerides >200 mg/dL (>2.2 mmol), and HDL-cholesterol <35 mg/dL (<0.90 mmol) (see Table I).
Further information is available from a 3.5 year (8.5 year cumulative) follow-up of all subjects who had participated in the Helsinki Heart Study. At the completion of the Helsinki Heart Study, subjects could choose to start, stop, or continue to receive gemfibrozil; without knowledge of their own lipid values or double-blind treatment, 60% of patients originally randomized to placebo began therapy with gemfibrozil and 60% of patients originally randomized to gemfibrozil continued medication. After approximately 6.5 years following randomization, all patients were informed of their original treatment group and lipid values during the five years of the double-blind treatment. After further elective changes in gemfibrozil treatment status, 61% of patients in the group originally randomized to gemfibrozil were taking drug; in the group originally randomized to placebo, 65% were taking gemfibrozil. The event rate per 1000 occurring during the open-label follow-up period is detailed in Table II.
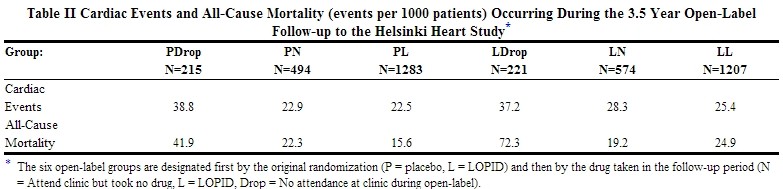
Cumulative mortality through 8.5 years showed a 20% relative excess of deaths in the group originally randomized to gemfibrozil versus the originally randomized placebo group and a 20% relative decrease in cardiac events in the group originally randomized to gemfibrozil versus the originally randomized placebo group (see Table III). This analysis of the originally randomized "intent-to-treat population neglects the possible complicating effects of treatment switching during the open-label phase. Adjustment of hazard ratios taking into account open-label treatment status from years 6.5 to 8.5, could change the reported hazard ratios for mortality toward unity.

It is not clear to what extent the findings of the primary prevention component of the Helsinki Heart Study can be extrapolated to other segments of the dyslipidemic population not studied (such as women, younger or older males, or those with lipid abnormalities limited solely to HDL-cholesterol) or to other lipid-altering drugs.
The secondary prevention component of the Helsinki Heart Study was conducted over five years in parallel and at the same centers in Finland in 628 middle-aged males excluded from the primary prevention component of the Helsinki Heart Study because of a history of angina, myocardial infarction, or unexplained ECG changes. The primary efficacy endpoint of the study was cardiac events (the sum of fatal and non-fatal myocardial infarctions and sudden cardiac deaths). The hazard ratio (gemfibrozil:placebo) for cardiac events was 1.47 (95% confidence limits 0.88–2.48, p=0.14). Of the 35 patients in the gemfibrozil group who experienced cardiac events, 12 patients suffered events after discontinuation from the study. Of the 24 patients in the placebo group with cardiac events, 4 patients suffered events after discontinuation from the study. There were 17 cardiac deaths in the gemfibrozil group and 8 in the placebo group (hazard ratio 2.18; 95% confidence limits 0.94–5.05, p=0.06). Ten of these deaths in the gemfibrozil group and 3 in the placebo group occurred after discontinuation from therapy. In this study of patients with known or suspected coronary heart disease, no benefit from gemfibrozil treatment was observed in reducing cardiac events or cardiac deaths. Thus, gemfibrozil has shown benefit only in selected dyslipidemic patients without suspected or established coronary heart disease. Even in patients with coronary heart disease and the triad of elevated LDL-cholesterol, elevated triglycerides, plus low HDL-cholesterol, the possible effect of gemfibrozil on coronary events has not been adequately studied.
No efficacy in the patients with established coronary heart disease was observed during the Coronary Drug Project with the chemically and pharmacologically related drug, clofibrate. The Coronary Drug Project was a 6-year randomized, double-blind study involving 1000 clofibrate, 1000 nicotinic acid, and 3000 placebo patients with known coronary heart disease. A clinically and statistically significant reduction in myocardial infarctions was seen in the concurrent nicotinic acid group compared to placebo; no reduction was seen with clofibrate.
How Supplied
Gemfibrozil Tablets USP, 600 mg, white, capsule-shaped tablets with the logo "B260" debossed on one side and bisected on the other side of the tablet, each containing 600 mg gemfibrozil, are available as follows:
NDC 24658-260-30: Bottles of 30
NDC 24658-260-60: Bottles of 60
NDC 24658-260-90: Bottles of 90
NDC 24658-260-18: Bottles of 180
NDC 24658-260-05: Bottles of 500
Storage
Store at 20° - 25°C (68° - 77°F). Protect from light and humidity.
Images
Drug Images
{{#ask: Page Name::Gemfibrozil |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Gemfibrozil |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Why this medication is prescribed
Gemfibrozil is used with diet changes (restriction of cholesterol and fat intake) to reduce the amount of cholesterol and certain fatty substances in your blood. Accumulation of cholesterol and fats along the walls of your arteries (a process known as atherosclerosis) decreases blood flow and, therefore, the oxygen supply to your heart, brain, and other parts of your body. Lowering your blood level of cholesterol and fats may help to prevent heart disease, angina (chest pain), strokes, and heart attacks.
This medication is sometimes prescribed for other uses; ask your doctor or pharmacist for more information.
How this medication should be used
Gemfibrozil comes in tablets and capsules to take by mouth. It usually is taken twice a day, 30 minutes before the morning and evening meals. Follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand. Take gemfibrozil exactly as directed. Do not take more or less of it or take it more often than prescribed by your doctor.
Continue to take gemfibrozil even if you feel well. Do not stop taking gemfibrozil without talking to your doctor.
Special precautions
Before taking gemfibrozil:
- tell your doctor and pharmacist if you are allergic to gemfibrozil or any other drugs.
- tell your doctor and pharmacist what prescription and nonprescription medications you are taking, especially anticoagulants ('blood thinners') such as warfarin (Coumadin) and vitamins. If you take insulin or oral diabetes medications, your dose may need to be changed because gemfibrozil can increase the amount of sugar in your blood. Talk to your doctor before changing your dose.
- tell your doctor if you have or have ever had ulcers; diabetes; or gallbladder, kidney, or liver disease.
- tell your doctor if you are pregnant, plan to become pregnant, or are breast-feeding. If you become pregnant while taking gemfibrozil, call your doctor.
- if you are having surgery, including dental surgery, tell the doctor or dentist that you are taking gemfibrozil.
Special dietary instructions
Eat a low-cholesterol, low-fat diet. This kind of diet includes cottage cheese, fat-free milk, fish (not canned in oil), vegetables, poultry, egg whites, and polyunsaturated oils and margarines (corn, safflower, canola, and soybean oils). Avoid foods with excess fat in them such as meat (especially liver and fatty meat), egg yolks, whole milk, cream, butter, shortening, lard, pastries, cakes, cookies, gravy, peanut butter, chocolate, olives, potato chips, coconut, cheese (other than cottage cheese), coconut oil, palm oil, and fried foods.
What to do if you forget a dose
Take the missed dose as soon as you remember it. However, if it is almost time for the next dose, skip the missed dose and continue your regular dosing schedule. Do not take a double dose to make up for a missed one.
Side Effects
Minor Side Effects
Gemfibrozil may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
- stomach pain
- diarrhea
- constipation
- vomiting
- gas
- headache
- dizziness
- blurred vision
- flushing
Severe Side Effects
If you experience either of the following symptoms, call your doctor immediately:
- muscle pain
- weakness
If you experience a serious side effect, you or your doctor may send a report to the Food and Drug Administration's (FDA) MedWatch Adverse Event Reporting program online [at http://www.fda.gov/MedWatch/report.htm] or by phone [1-800-332-1088].
Storage conditions needed for this medication
Keep this medication in the container it came in, tightly closed, and out of reach of children. Store it at room temperature and away from excess heat and moisture (not in the bathroom). Throw away any medication that is outdated or no longer needed. Talk to your pharmacist about the proper disposal of your medication.
In case of emergency/overdose
In case of overdose, call your local poison control center at 1-800-222-1222. If the victim has collapsed or is not breathing, call local emergency services at 911.
Other information
Keep all appointments with your doctor and the laboratory. Your doctor will order certain lab tests to check your response to gemfibrozil.
Do not let anyone else take your medication. Ask your pharmacist any questions you have about refilling your prescription.
Brand names
- Lopid®
Precautions with Alcohol
Alcohol-Gemfibrozil interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Lopid
Look-Alike Drug Names
There is limited information regarding Gemfibrozil Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Gemfibrozil |Pill Name=Gemfibrozil pill.jpg |Drug Name=Gemfibrozil |Pill Ingred=calcium stearate, carnauba wax, colloidal silicon dioxide , croscarmellose sodium, hydroxypropyl cellulose, microcrystalline, polyethylene glycol 3350, polyvinyl alcohol, starch, (pregelatinized corn), sodium lauryl sulfate, talc, titanium dioxide|+sep=; |Pill Imprint=B260 |Pill Dosage=600 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=20.00 |Pill Scoring=2 |Pill Image= |Drug Author=Aphena Pharma Solutions - Tennessee, Inc. |NDC=24658-260-30
}}
{{#subobject:
|Label Page=Gemfibrozil |Label Name=Gemfibrozil.jpg
}}