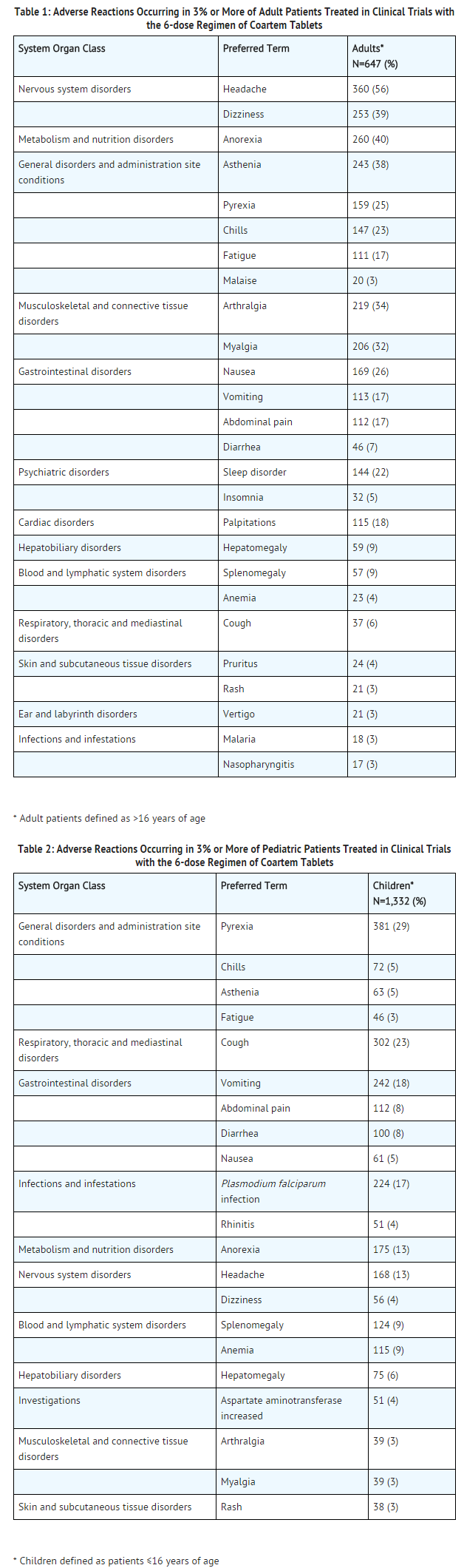
Artemether lumefantrine
{{DrugProjectFormSinglePage |authorTag=Aparna Vuppala, M.B.B.S. [1] |genericName=Artemether and lumefantrine |aOrAn=a |drugClass=antimalarial |indicationType=treatment |indication=acute, uncomplicated malaria infections due to Plasmodium falciparum in patients of 5 kg bodyweight and above |adverseReactions=palpitations, abdominal pain, diarrhea, loss of appetite, nausea, vomiting, arthralgia, myalgia, asthenia, dizziness, headache, Sleep disorder, cough, fatigue, fever, shivering. |blackBoxWarningTitle=Title |blackBoxWarningBody=ConditionName:
- Content
|fdaLIADAdult=*Artemether and lumefantrine (artemether/lumefantrine) Tablets are indicated for treatment of acute, uncomplicated malaria infections due to Plasmodium falciparum in patients of 5 kg bodyweight and above. Artemether and lumefantrine Tablets have been shown to be effective in geographical regions where resistance to chloroquine has been reported
Limitations of Use:
- Artemether and lumefantrine Tablets are not approved for patients with severe or complicated P. falciparum malaria.
- Artemether and lumefantrine Tablets are not approved for the prevention of malaria.
Administration Instructions
- Artemether and lumefantrine Tablets should be taken with food. Patients with acute malaria are frequently averse to food. Patients should be encouraged to resume normal eating as soon as food can be tolerated since this improves absorption of artemether and lumefantrine.
- For patients who are unable to swallow the tablets such as infants and children, artemether and lumefantrine Tablets may be crushed and mixed with a small amount of water (1 to 2 teaspoons) in a clean container for administration immediately prior to use. The container can be rinsed with more water and the contents swallowed by the patient. The crushed tablet preparation should be followed whenever possible by food/drink (e.g., milk, formula, pudding, broth, and porridge).
- In the event of vomiting within 1 to 2 hours of administration, a repeat dose should be taken. If the repeat dose is vomited, the patient should be given an alternative antimalarial for treatment.
Dosage in Adult Patients (>16 years of age)
- A 3-day treatment schedule with a total of 6 doses is recommended for adult patients with a bodyweight of 35 kg and above:
- tablets as a single initial dose, 4 tablets again after 8 hours and then 4 tablets twice-daily (morning and evening) for the following 2 days (total course of 24 tablets).
- For patients weighing less than 35 kg, see Dosage in Pediatric Patients
Dosage in Patients with Hepatic or Renal Impairment
- No specific pharmacokinetic studies have been carried out in patients with hepatic or renal impairment. Most patients with acute malaria present with some degree of related hepatic and/or renal impairment. In clinical studies, the adverse event profile did not differ in patients with mild or moderate hepatic impairment compared to patients with normal hepatic function. No specific dose adjustments are needed for patients with mild or moderate hepatic impairment.
- In clinical studies, the adverse event profile did not differ in patients with mild or moderate renal impairment compared to patients with normal renal function. There were few patients with severe renal impairment in clinical studies. There is no significant renal excretion of lumefantrine, artemether and dihydroartemisinin (DHA) in healthy volunteers and while clinical experience in this population is limited, no dose adjustment is recommended.
- Caution should be exercised when administering artemether and lumefantrine Tablets in patients with severe hepatic or renal impairment
|offLabelAdultGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Artemether lumefantrine in adult patients.
|offLabelAdultNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Artemether lumefantrine in adult patients.
|fdaLIADPed=*Artemether and lumefantrine (artemether/lumefantrine) Tablets are indicated for treatment of acute, uncomplicated malaria infections due to Plasmodium falciparum in patients of 5 kg bodyweight and above. artemether and lumefantrine Tablets have been shown to be effective in geographical regions where resistance to chloroquine has been reported
Limitations of Use:
- Artemether and lumefantrine Tablets are not approved for patients with severe or complicated P. falciparum malaria.
- Artemether and lumefantrine Tablets are not approved for the prevention of malaria.
Dosage in Pediatric Patients
- A 3-day treatment schedule with a total of 6 doses is recommended as below:
- 5 kg to less than 15 kg bodyweight: One tablet as an initial dose, 1 tablet again after 8 hours and then 1 tablet twice-daily (morning and evening) for the following 2 days (total course of 6 tablets).
- 15 kg to less than 25 kg bodyweight: Two tablets as an initial dose, 2 tablets again after 8 hours and then 2 tablets twice-daily (morning and evening) for the following 2 days (total course of 12 tablets).
- 25 kg to less than 35 kg bodyweight: Three tablets as an initial dose, 3 tablets again after 8 hours and then 3 tablets twice-daily (morning and evening) for the following 2 days (total course of 18 tablets).
- 35 kg bodyweight and above: Four tablets as a single initial dose, 4 tablets again after 8 hours and then 4 tablets twice-daily (morning and evening) for the following 2 days (total course of 24 tablets).
|offLabelPedGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Artemether lumefantrine in pediatric patients.
|offLabelPedNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Artemether lumefantrine in pediatric patients.
|contraindications======Hypersensitivity=====
- Known hypersensitivity to artemether, lumefantrine, or to any of the excipients of artemether and lumefantrine Tablets .
Strong CYP3A4 Inducers
- Coadministration of strong inducers of CYP3A4 such as rifampin, carbamazepine, phenytoin, and St. John’s wort with artemether and lumefantrine Tablets can result in decreased concentrations of artemether and/or lumefantrine and loss of antimalarial efficacy
|warnings======Prolongation of the QT Interval=====
- Some antimalarials (e.g., halofantrine, quinine, quinidine) including artemether and lumefantrine Tablets have been associated with prolongation of the QT interval on the electrocardiogram.
- artemether and lumefantrine Tablets should be avoided in patients:
- with congenital prolongation of the QT interval (e.g., long QT syndrome) or any other clinical condition known to prolong the QTc interval such as patients with a history of symptomatic cardiac arrhythmias, with clinically relevant bradycardia or with severe cardiac disease.
- with a family history of congenital prolongation of the QT interval or sudden death.
- with known disturbances of electrolyte balance, e.g., hypokalemia or hypomagnesemia.
- receiving other medications that prolong the QT interval, such as class IA (quinidine, procainamide, disopyramide), or class III (amiodarone, sotalol) antiarrhythmic agents; antipsychotics (pimozide, ziprasidone); antidepressants; certain antibiotics (macrolide antibiotics, fluoroquinolone antibiotics, imidazole, and triazole antifungal agents)
- receiving medications that are metabolized by the cytochrome enzyme CYP2D6 which also have cardiac effects (e.g., flecainide, imipramine, amitriptyline, clomipramine)
Use of QT Prolonging Drugs and Other Antimalarials
- Halofantrine and artemether and lumefantrine Tablets should not be administered within 1 month of each other due to the long elimination half-life of lumefantrine (3 to 6 days) and potential additive effects on the QT interval
- Antimalarials should not be given concomitantly with artemether and lumefantrine Tablets, unless there is no other treatment option, due to limited safety data.
- Drugs that prolong the QT interval, including antimalarials such as quinine and quinidine, should be used cautiously following artemether and lumefantrine Tablets, due to the long elimination half-life of lumefantrine (3 to 6 days) and the potential for additive effects on the QT interval; ECG monitoring is advised if use of drugs that prolong the QT interval is medically required
- If mefloquine is administered immediately prior to artemether and lumefantrine Tablets there may be a decreased exposure to lumefantrine, possibly due to a mefloquine-induced decrease in bile production. Therefore, patients should be monitored for decreased efficacy and food consumption should be encouraged while taking artemether and lumefantrine Tablets
Drug Interactions with CYP3A4
- When artemether and lumefantrine Tablets are coadministered with substrates of CYP3A4 it may result in decreased concentrations of the substrate and potential loss of substrate efficacy. When artemether and lumefantrine Tablets are coadministered with an inhibitor of CYP3A4, including grapefruit juice it may result in increased concentrations of artemether and/or lumefantrine and potentiate QT prolongation. When artemether and lumefantrine Tablets are coadministered with inducers of CYP3A4 it may result in decreased concentrations of artemether and/or lumefantrine and loss of antimalarial efficacy.
- Drugs that have a mixed effect on CYP3A4, especially antiretroviral drugs such as HIV protease inhibitors and non-nucleoside reverse transcriptase inhibitors, and those that have an effect on the QT interval should be used with caution in patients taking artemether and lumefantrine Tablets
- Artemether and lumefantrine Tablets may reduce the effectiveness of hormonal contraceptives. Therefore, patients using oral, transdermal patch, or other systemic hormonal contraceptives should be advised to use an additional non-hormonal method of birth control
Drug Interactions with CYP2D6
- Administration of artemether and lumefantrine Tablets with drugs that are metabolized by CYP2D6 may significantly increase plasma concentrations of the coadministered drug and increase the risk of adverse effects. Many of the drugs metabolized by CYP2D6 can prolong the QT interval and should not be administered with artemether and lumefantrine Tablets due to the potential additive effect on the QT interval (e.g., flecainide, imipramine, amitriptyline, clomipramine) .
Recrudescence
- Food enhances absorption of artemether and lumefantrine following administration of artemether and lumefantrine Tablets. Patients who remain averse to food during treatment should be closely monitored as the risk of recrudescence may be greater
- In the event of recrudescent P. falciparum infection after treatment with artemether and lumefantrine Tablets, patients should be treated with a different antimalarial drug.
Hepatic and Renal Impairment
- Artemether and lumefantrine Tablets have not been studied for efficacy and safety in patients with severe hepatic and/or renal impairment .
Plasmodium vivax Infection
- Artemether and lumefantrine Tablets have been shown in limited data (43 patients) to be effective in treating the erythrocytic stage of P. vivax infection. However, relapsing malaria caused by P. vivax requires additional treatment with other antimalarial agents to achieve radical cure i.e., eradicate any hypnozoites forms that may remain dormant in the liver.
|clinicalTrials=*The following serious and otherwise important adverse reactions are discussed in greater detail in other sections of labeling:
Clinical Studies Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rate observed in practice.
- The data described below reflect exposure to a 6-dose regimen of artemether and lumefantrine Tablets in 1,979 patients including 647 adults (older than 16 years) and 1,332 children (16 years and younger). For the 6-dose regimen, artemether and lumefantrine Tablets was studied in active-controlled (366 patients) and non-controlled, open-label trials (1,613 patients). The 6-dose artemether and lumefantrine Tablets population was patients with malaria between ages 2 months and 71 years: 67% (1,332) were 16 years and younger and 33% (647) were older than 16 years. Males represented 73% and 53% of the adult and pediatric populations, respectively. The majority of adult patients were enrolled in studies in Thailand, while the majority of pediatric patients were enrolled in Africa.
- Tables 1 and 2 show the most frequently reported adverse reactions (≥3%) in adults and children respectively who received the 6-dose regimen of artemether and lumefantrine Tablets. Adverse reactions collected in clinical trials included signs and symptoms at baseline but only treatment emergent adverse events, defined as events that appeared or worsened after the start of treatment, are presented below. In adults, the most frequently reported adverse reactions were headache, anorexia, dizziness, and asthenia. In children, the adverse reactions were pyrexia, cough, vomiting, anorexia, and headache. Most adverse reactions were mild, did not lead to discontinuation of study medication, and resolved.
- In limited comparative studies, the adverse reaction profile of artemether and lumefantrine Tablets appeared similar to that of another antimalarial regimen.
- Discontinuation of artemether and lumefantrine Tablets due to adverse drug reactions occurred in 1.1% of patients treated with the 6-dose regimen overall: 0.2% (1/647) in adults and 1.6% (21/1,332) in children.
- Clinically significant adverse reactions reported in adults and/or children treated with the 6-dose regimen of artemether and lumefantrine Tablets which occurred in clinical studies at <3% regardless of causality are listed below:
- Blood and lymphatic system disorders: eosinophilia
- Ear and labyrinth disorders: tinnitus
- Eye disorders: conjunctivitis
- Gastrointestinal disorders: constipation, dyspepsia, dysphagia, peptic ulcer
- General disorders: gait disturbance
- Infections and infestations: abscess, acrodermatitis, bronchitis, ear infection, gastroenteritis, helminthic infection, hookworm infection, impetigo, influenza, lower respiratory tract infection, malaria, nasopharyngitis, oral herpes, pneumonia, respiratory tract infection, subcutaneous abscess, upper respiratory tract infection, urinary tract infection
- Investigations: alanine aminotransferase increased, aspartate aminotransferase increased, hematocrit decreased, lymphocyte morphology abnormal, platelet count decreased, platelet count increased, white blood cell count decreased, white blood cell count increased
- Metabolism and nutrition disorders: hypokalemia
- Musculoskeletal and connective tissue disorders: back pain
- Nervous system disorders: ataxia, clonus, fine motor delay, hyperreflexia, hypoesthesia, nystagmus,tremor
- Psychiatric disorders: agitation, mood swings
- Renal and urinary disorders: hematuria, proteinuria
- Respiratory, thoracic and mediastinal disorders: asthma, pharyngo-laryngeal pain
- Skin and subcutaneous tissue disorders: urticaria
|postmarketing=*The following adverse reactions have been identified during post-approval use of artemether and lumefantrine Tablets. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Hypersensitivity reactions: anaphylaxis, urticaria, angioedema, and serious skin reactions (bullous eruption) have been reported.
|drugInteractions======Rifampin=====
- Oral administration of rifampin, a strong CYP3A4 inducer, with artemether and lumefantrine Tablets resulted in significant decreases in exposure to artemether, dihydroartemisinin (DHA, metabolite of artemether) and lumefantrine by 89%, 85% and 68%, respectively, when compared to exposure values after artemether and lumefantrine Tablets alone. Concomitant use of strong inducers of CYP3A4 such as rifampin, carbamazepine, phenytoin, and St. John’s wort is contraindicated with artemether and lumefantrine Tablets.
Ketoconazole
- Concurrent oral administration of ketoconazole, a potent CYP3A4 inhibitor, with a single dose of artemether and lumefantrine Tablets resulted in a moderate increase in exposure to artemether, DHA, and lumefantrine in a study of 15 healthy subjects. No dose adjustment of artemether and lumefantrine Tablets is necessary when administered with ketoconazole or other potent CYP3A4 inhibitors. However, due to the potential for increased concentrations of lumefantrine which could lead to QT prolongation, artemether and lumefantrine Tablets should be used cautiously with drugs that inhibit CYP3A4
Antiretroviral Drugs
- Both artemether and lumefantrine are metabolized by CYP3A4. Antiretroviral drugs, such as protease inhibitors and non-nucleoside reverse transcriptase inhibitors, are known to have variable patterns of inhibition, induction or competition for CYP3A4. Therefore, the effects of antiretroviral drugs on the exposure to artemether, DHA, and lumefantrine are also variable . Artemether and lumefantrine Tablets should be used cautiously in patients on antiretroviral drugs because decreased artemether, DHA, and/or lumefantrine concentrations may result in a decrease of antimalarial efficacy of artemether and lumefantrine Tablets, and increased lumefantrine concentrations may cause QT prolongation .
Prior Use of Mefloquine
- Administration of 3 doses of mefloquine followed 12 hours later by a 6-dose regimen of artemether and lumefantrine Tablets in 14 healthy volunteers demonstrated no effect of mefloquine on plasma concentrations of artemether or the artemether/DHA ratio. However, exposure to lumefantrine was reduced, possibly due to lower absorption secondary to a mefloquine-induced decrease in bile production. Patients should be monitored for decreased efficacy and food consumption should be encouraged with administration of artemether and lumefantrine Tablets
Hormonal Contraceptives
- In vitro, the metabolism of ethinyl estradiol and levonorgestrel was not induced by artemether, DHA, or lumefantrine. However, artemether has been reported to weakly induce, in humans, the activity of CYP2C19, CYP2B6, and CYP3A. Therefore, artemether and lumefantrine Tablets may potentially reduce the effectiveness of hormonal contraceptives. Patients using oral, transdermal patch, or other systemic hormonal contraceptives should be advised to use an additional non-hormonal method of birth control
CYP2D6 Substrates
- Lumefantrine inhibits CYP2D6 in vitro. Administration of artemether and lumefantrine Tablets with drugs that are metabolized by CYP2D6 may significantly increase plasma concentrations of the coadministered drug and increase the risk of adverse effects. Many of the drugs metabolized by CYP2D6 can prolong the QT interval and should not be administered with artemether and lumefantrine Tablets due to the potential additive effect on the QT interval (e.g., flecainide, imipramine, amitriptyline, clomipramine)
Sequential Use of Quinine
- single dose of intravenous quinine (10 mg/kg bodyweight) concurrent with the final dose of a 6-dose regimen of artemether and lumefantrine Tablets demonstrated no effect of intravenous quinine on the systemic exposure of DHA or lumefantrine. Quinine exposure was also not altered. Exposure to artemether was decreased. This decrease in artemether exposure is not thought to be clinically significant. However, quinine and other drugs that prolong the QT interval should be used cautiously following treatment with artemether and lumefantrine Tablets due to the long elimination half-life of lumefantrine and the potential for additive QT effects; ECG monitoring is advised if use of drugs that prolong the QT interval is medically required
Interaction with Drugs that are Known to Prolong the QT Interval
- Artemether and lumefantrine Tablets are to be used with caution when coadministered with drugs that may cause prolonged QT interval such as antiarrhythmics of classes IA and III, neuroleptics and antidepressant agents, certain antibiotics including some agents of the following classes: macrolides, fluoroquinolones, imidazole, and triazole antifungal agents
|FDAPregCat=C |useInPregnancyFDA=*Safety data from an observational pregnancy study of approximately 500 pregnant women who were exposed to artemether and lumefantrine Tablets (including a third of patients who were exposed in the first trimester), and published data of over 1,000 pregnant patients who were exposed to artemisinin derivatives, did not show an increase in adverse pregnancy outcomes or teratogenic effects over background rate.
- The efficacy of artemether and lumefantrine Tablets in the treatment of acute, uncomplicated malaria in pregnant women has not been established.
- Artemether and lumefantrine Tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Pregnant rats dosed during the period of organogenesis at or higher than a dose of about half the highest clinical dose of 1120 mg artemether-lumefantrine per day (based on body surface area comparisons), showed increases in fetal loss, early resorptions and post implantation loss. No adverse effects were observed in animals dosed at about one-third the highest clinical dose. Similarly, dosing in pregnant rabbits at about 3 times the clinical dose (based on body surface area comparisons) resulted in abortions, preimplantation loss, post implantation loss and decreases in the number of live fetuses. No adverse reproductive effects were detected in rabbits at 2 times the clinical dose. Embryo-fetal loss is a significant reproductive toxicity. Other artemisinins are known to be embryotoxic in animals. However, because metabolic profiles in animals and humans are dissimilar, artemether exposures in animals may not be predictive of human exposures . These data cannot rule out an increased risk for early pregnancy loss or fetal defects in humans.
|useInPregnancyAUS=There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Artemether lumefantrine in women who are pregnant. |useInLaborDelivery=There is no FDA guidance on use of Artemether lumefantrine during labor and delivery. |useInNursing=*It is not known whether artemether or lumefantrine is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when artemether and lumefantrine Tablets are administered to a nursing woman. Animal data suggest both artemether and lumefantrine are excreted into breast milk. The benefits of breastfeeding to mother and infant should be weighed against potential risk from infant exposure to artemether and lumefantrine through breast milk. |useInPed=*The safety and effectiveness of artemether and lumefantrine Tablets have been established for the treatment of acute, uncomplicated malaria in studies involving pediatric patients weighing 5 kg or more . The safety and efficacy have not been established in pediatric patients who weigh less than 5 kg. Children from non-endemic countries were not included in clinical trials. |useInGeri=*Clinical studies of artemether and lumefantrine Tablets did not include sufficient numbers of subjects aged 65 years and over to determine they respond differently from younger subjects. In general, the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy in elderly patients should be considered when prescribing artemether and lumefantrine Tablets. |useInGender=There is no FDA guidance on the use of Artemether lumefantrine with respect to specific gender populations. |useInRace=There is no FDA guidance on the use of Artemether lumefantrine with respect to specific racial populations. |useInRenalImpair=*No specific pharmacokinetic studies have been performed in patients with either hepatic or renal impairment. artemether and lumefantrine Tablets have not been studied for efficacy and safety in patients with severe hepatic and/or renal impairment. Based on the pharmacokinetic data in 16 healthy subjects showing no or insignificant renal excretion of lumefantrine, artemether and DHA, no dose adjustment for the use of artemether and lumefantrine Tablets in patients with renal impairment is advised. No dosage adjustment is necessary in patients with mild to moderate hepatic impairment |useInHepaticImpair=*No specific pharmacokinetic studies have been performed in patients with either hepatic or renal impairment. artemether and lumefantrine Tablets have not been studied for efficacy and safety in patients with severe hepatic and/or renal impairment. Based on the pharmacokinetic data in 16 healthy subjects showing no or insignificant renal excretion of lumefantrine, artemether and DHA, no dose adjustment for the use of artemether and lumefantrine Tablets in patients with renal impairment is advised. No dosage adjustment is necessary in patients with mild to moderate hepatic impairment |useInReproPotential=There is no FDA guidance on the use of Artemether lumefantrine in women of reproductive potentials and males. |useInImmunocomp=There is no FDA guidance one the use of Artemether lumefantrine in patients who are immunocompromised.
|administration=* Oral |monitoring=There is limited information regarding Monitoring of Artemether lumefantrine in the drug label.
|IVCompat=There is limited information regarding IV Compatibility of Artemether lumefantrine in the drug label.
|overdose=*There is no information on overdoses of artemether and lumefantrine Tablets higher than the doses recommended for treatment.
- In cases of suspected overdosage, symptomatic and supportive therapy, which would include ECG and blood electrolyte monitoring, should be given as appropriate.
|drugBox=
Artemether lumefantrine
| |
| Combination of | |
| Artemether | Antimalarial |
| Lumefantrine | Antimalarial |
| Identifiers | |
| CAS number | ? |
| ATC code | P01 |
| PubChem | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) |
| Legal status | |
| Routes | Oral |
|mechAction=*Coartem Tablets, a fixed ratio of 1:6 parts of artemether and lumefantrine, respectively, is an antimalarial agent. Artemether is rapidly metabolized into an active metabolite dihydroartemisinin (DHA). The antimalarial activity of artemether and DHA has been attributed to endoperoxide moiety. The exact mechanism by which lumefantrine exerts its antimalarial effect is not well defined. Available data suggest lumefantrine inhibits the formation of β-hematin by forming a complex with hemin. Both artemether and lumefantrine were shown to inhibit nucleic acid and protein synthesis.
Activity In Vitro and In Vivo
- Artemether and lumefantrine are active against the erythrocytic stages of Plasmodium falciparum.
Drug Resistance
- Strains of P. falciparum with a moderate decrease in susceptibility to artemether or lumefantrine alone can be selected in vitro or in vivo, but not maintained in the case of artemether. The clinical relevance of such an effect is not known.
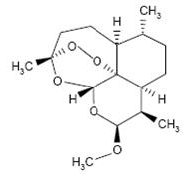
|structure=*Artemether and lumefantrine Tablets contain a fixed combination of 2 antimalarial active ingredients, artemether, an artemisinin derivative, and lumefantrine. Both components are blood schizontocides. The chemical name of artemether is (3R,5aS,6R,8aS,9R,10S,12R,12aR)-10-methoxy-3,6,9-trimethyldecahydro-3,12-epoxypyrano[4,3-j]-1,2-benzodioxepine. Artemether is a white, crystalline powder that is freely soluble in acetone, soluble in methanol and ethanol, and practically insoluble in water. It has the empirical formula C16H26O5 with a molecular weight of 298.4, and the following structural formula:
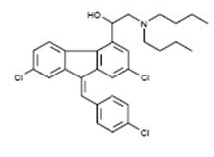
- The chemical name of lumefantrine is (1RS)-2-(dibutylamino)-1-{(9Z)-2,7-dichloro-9-[(4-chlorophenyl)methylene]-9H-fluorene-4-yl}ethanol. Lumefantrine is a yellow, crystalline powder that is freely soluble in N,N-dimethylformamide, chloroform, and ethyl acetate; soluble in dichloromethane; slightly soluble in ethanol and methanol; and insoluble in water. It has the empirical formula C30H32Cl3NO with a molecular weight of 528.9, and the following structural formula:
- artemether and lumefantrine Tablets are for oral administration. Each artemether and lumefantrine Tablet contains 20 mg of artemether and 120 mg lumefantrine. The inactive ingredients are colloidal silicon dioxide, croscarmellose sodium, hypromellose, magnesium stearate, microcrystalline cellulose, and polysorbate 80.
|PD=There is limited information regarding Pharmacodynamics of Artemether lumefantrine in the drug label.
|PK=======Absorption======
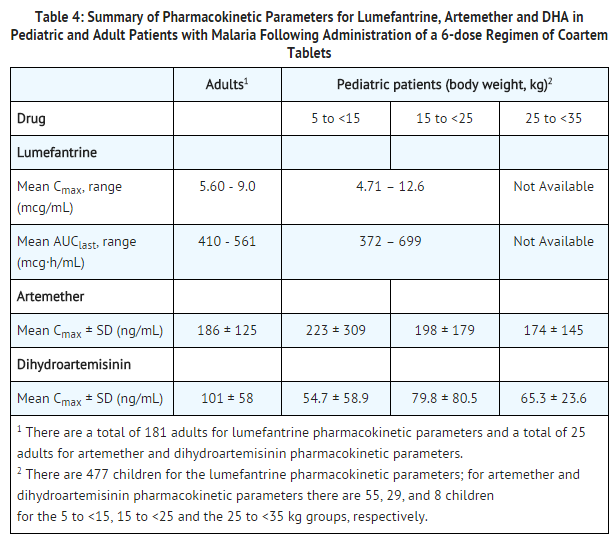
- Following administration of artemether and lumefantrine Tablets to healthy volunteers and patients with malaria, artemether is absorbed with peak plasma concentrations reached about 2 hours after dosing. Absorption of lumefantrine, a highly lipophilic compound, starts after a lag-time of up to 2 hours, with peak plasma concentrations about 6 to 8 hours after administration. The single dose (4 tablets) pharmacokinetic parameters for artemether, dihydroartemisinin (DHA), an active antimalarial metabolite of artemether, and lumefantrine in adult Caucasian healthy volunteers are given in Table 3. Multiple dose data after the 6-dose regimen of artemether and lumefantrine Tablets in adult malaria patients are given in Table 4.
- Food enhances the absorption of both artemether and lumefantrine. In healthy volunteers, the relative bioavailability of artemether was increased between 2- to 3-fold, and that of lumefantrine 16-fold when artemether and lumefantrine Tablets were taken after a high-fat meal compared under fasted conditions. Patients should be encouraged to take artemether and lumefantrine Tablets with a meal as soon as food can be tolerated .
Distribution
- Artemether and lumefantrine are both highly bound to human serum proteins in vitro (95.4% and 99.7%, respectively). Dihydroartemisinin is also bound to human serum proteins (47% to 76%). Protein binding to human plasma proteins is linear.
Biotransformation
- In human liver microsomes and recombinant CYP450 enzymes, the metabolism of artemether was catalyzed predominantly by CYP3A4/5. Dihydroartemisinin (DHA) is an active metabolite of artemether. The metabolism of artemether was also catalyzed to a lesser extent by CYP2B6, CYP2C9 and CYP2C19. In vitro studies with artemether at therapeutic concentrations revealed no significant inhibition of the metabolic activities of CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4/5, and CYP4A9/11. In vitro studies with artemether, DHA, and lumefantrine at therapeutic concentrations revealed no significant induction of the metabolic activities of CYP1A1, CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP3A4, or CYP3A5.
- During repeated administration of artemether and lumefantrine Tablets, systemic exposure of artemether decreased significantly, while concentrations of DHA increased, although not to a statistically significant degree. The artemether/DHA AUC ratio is 1.2 after a single dose and 0.3 after 6 doses given over 3 days. This suggests that there was induction of enzymes responsible for the metabolism of artemether.
- In human liver microsomes and in recombinant CYP450 enzymes, lumefantrine was metabolized mainly by CYP3A4 to desbutyl-lumefantrine. The systemic exposure to the metabolite desbutyl-lumefantrine was less than 1% of the exposure to the parent compound. In vitro, lumefantrine significantly inhibits the activity of CYP2D6 at therapeutic plasma concentrations.
- Caution is recommended when combining artemether and lumefantrine Tablets with substrates, inhibitors, or inducers of CYP3A4, especially antiretroviral drugs and those that prolong the QT interval (e.g., macrolide antibiotics, pimozide)
- Coadministration of artemether and lumefantrine Tablets with CYP2D6 substrates may result in increased plasma concentrations of the CYP2D6 substrate and increase the risk of adverse reactions. In addition, many of the drugs metabolized by CYP2D6 can prolong the QT interval and should not be administered with artemether and lumefantrine Tablets due to the potential additive effect on the QT interval (e.g., flecainide, imipramine, amitriptyline, clomipramine)
Elimination
- Artemether and DHA are cleared from plasma with an elimination half-life of about 2 hours. Lumefantrine is eliminated more slowly, with an elimination half-life of 3 to 6 days in healthy volunteers and in patients with falciparum malaria. Demographic characteristics such as sex and weight appear to have no clinically relevant effects on the pharmacokinetics of artemether and lumefantrine.
- In 16 healthy volunteers, neither lumefantrine nor artemether was found in the urine after administration of artemether and lumefantrine Tablets, and urinary excretion of DHA amounted to less than 0.01% of the artemether dose.
Hepatic and Renal Impairment
- No specific pharmacokinetic studies have been performed in patients with either hepatic or renal impairment. There is no significant renal excretion of lumefantrine, artemether and DHA in healthy volunteers and while clinical experience in this population is limited, no dose adjustment in renal impairment is recommended.
Pediatric Patients
- The PK of artemether, DHA, and lumefantrine were obtained in 2 pediatric studies by sparse sampling using a population-based approach. PK estimates derived from a composite plasma concentration profile for artemether, DHA, and lumefantrine are provided in Table 4.
- Systemic exposure to artemether, DHA, and lumefantrine, when dosed on a mg/kg body weight basis in pediatric patients (≥5 to <35 kg body weight), is comparable to that of the recommended dosing regimen in adult patients.
Geriatric Patients
- No specific pharmacokinetic studies have been performed in patients older than 65 years of age.
Drug Interactions
Rifampin (strong CYP3A4 inducer)
- Oral administration of rifampin (600 mg daily), a strong CYP3A4 inducer, with artemether and lumefantrine Tablets (6-dose regimen over 3 days) in 6 HIV-1 and tuberculosis co-infected adults without malaria resulted in significant decreases in exposure, in terms of AUC, to artemether, DHA and lumefantrine by 89%, 85% and 68%, respectively, when compared to exposure values after artemether and lumefantrine Tablets alone. Concomitant use of strong inducers of CYP3A4 such as rifampin, carbamazepine, phenytoin, and St. John’s wort is contraindicated with artemether and lumefantrine Tablets .
Ketoconazole (potent CYP3A4 inhibitor)
- Concurrent oral administration of ketoconazole (400 mg on day 1 followed by 200 mg on days 2, 3, 4 and 5) with artemether and lumefantrine Tablets (single dose of 4 tablets of 20 mg artemether/120 mg lumefantrine per tablet) with a meal led to an increase in exposure, in terms of area under the curve (AUC), of artemether (2.3-fold), DHA (1.5-fold), and lumefantrine (1.6-fold) in 13 healthy subjects. The pharmacokinetics of ketoconazole was not evaluated. Based on this study, dose adjustment of artemether and lumefantrine Tablets is considered unnecessary when administered with ketoconazole or other CYP3A4 inhibitors. However, due to the potential for increased concentrations of lumefantrine which could lead to QT prolongation, artemether and lumefantrine Tablets should be used cautiously with other drugs that inhibit CYP3A4 (e.g., antiretroviral drugs, macrolide antibiotics, antidepressants, imidazole antifungal agents).
Antimalarials
- The oral administration of mefloquine in 14 healthy volunteers administered as 3 doses of 500 mg, 250 mg and 250 mg, followed 12 hours later by artemether and lumefantrine Tablets (6 doses of 4 tablets of 20 mg artemether/120 mg lumefantrine per tablet), had no effect on plasma concentrations of artemether or the artemether/DHA ratio. In the same study, there was a 30% reduction in Cmax and 40% reduction in AUC of lumefantrine, possibly due to lower absorption secondary to a mefloquine-induced decrease in bile production.
- Intravenous administration of a single dose of quinine (10 mg/kg bodyweight) concurrent with the last dose of a 6-dose regimen of artemether and lumefantrine Tablets had no effect on systemic exposure of DHA, lumefantrine or quinine in 14 healthy volunteers. Mean AUC of artemether were 46% lower when administered with quinine compared to artemether and lumefantrine Tablets alone. This decrease in artemether exposure is not thought to be clinically significant. However, quinine should be used cautiously in patients following treatment with artemether and lumefantrine Tablets due to the long elimination half-life of lumefantrine and the potential for additive effects on the QT interval; ECG monitoring is advised if use of quinine is medically required .
Antiretroviral Drugs
- The oral administration of lopinavir/ritonavir (400 mg/100 mg twice daily for 26 days) in 10 healthy volunteers coadministered with artemether and lumefantrine Tablets (6-dose regimen over 3 days), resulted in a decrease in systemic exposures, in terms of AUC, to artemether and DHA by approximately 40%, but an increase in exposure to lumefantrine by approximately 2.3-fold. The oral administration of efavirenz (600 mg once daily for 26 days) in 12 healthy volunteers coadministered with artemether and lumefantrine Tablets (6-dose regimen over 3 days), resulted in a decrease in exposures to artemether, DHA, and lumefantrine by approximately 50%, 45%, and 20%, respectively. Exposures to lopinavir/ritonavir and efavirenz were not significantly affected by concomitant use of artemether and lumefantrine Tablets. artemether and lumefantrine Tablets should be used cautiously in patients on antiretroviral drugs such as HIV protease inhibitors and non-nucleoside reverse transcriptase inhibitors because decreased artemether, DHA, and/or lumefantrine concentrations may result in a decrease of antimalarial efficacy of artemether and lumefantrine Tablets, and increased lumefantrine concentrations may cause QT prolongation
Hormonal Contraceptives
- No clinical drug-drug interaction studies between artemether and lumefantrine Tablets and hormonal contraceptives have been performed. In vitro studies revealed that the metabolism of ethinyl estradiol and levonorgestrel was not induced by artemether, DHA or lumefantrine. However, artemether has been reported to weakly induce, in humans, the activity of CYP2C19, CYP2B6, and CYP3A. Therefore, coadministration of artemether and lumefantrine Tablets may potentially reduce the effectiveness of hormonal contraceptives
Effects on the Electrocardiogram
- In a healthy adult volunteer parallel-group study including a placebo and moxifloxacin control-group (n=42 per group), the administration of the 6-dose regimen of artemether and lumefantrine Tablets was associated with prolongation of QTcF (Fridericia). Following administration of a 6-dose regimen of artemether and lumefantrine Tablets consisting of 4 tablets per dose (total of 4 tablets of 80 mg artemether/480 mg lumefantrine) taken with food, the maximum mean change from baseline and placebo adjusted QTcF was 7.5 msec (1-sided 95% Upper CI: 11 msec). There was a concentration-dependent increase in QTcF for lumefantrine.
- In clinical trials conducted in children, no patient had QTcF >500 msec. Over 5% of patients had an increase in QTcF of over 60 msec.
- In clinical trials conducted in adults, QTcF prolongation of >500 msec was reported in 3 (0.3%) patients. Over 6% of adults had a QTcF increase of over 60 msec from baseline.
|nonClinToxic=====Carcinogenesis, Mutagenesis, Impairment of Fertility====
Carcinogenesis
- Carcinogenicity studies were not conducted.
Mutagenesis
- No evidence of mutagenicity was detected. The artemether: lumefantrine combination was evaluated using the Salmonella and Escherichia/mammalian-microsome mutagenicity test, the gene mutation test with Chinese hamster cells V79, the cytogenetic test on Chinese hamster cells in vitro, and the rat micronucleus test, in vivo.
of Fertility
- Pregnancy rates were reduced by about one-half in female rats dosed for 2 to 4 weeks with the artemether-lumefantrine combination at 1000 mg/kg (about 9 times the clinical dose based on body surface area comparisons). Male rats dosed for 70 days showed increases in abnormal sperm (87% abnormal) and increased testes weights at 30 mg/kg doses (about one-third the clinical dose). Higher doses (about 9 times the clinical dose) resulted in decreased sperm motility and 100% abnormal sperm cells.
Animal Toxicology and/or Pharmacology
- Neonatal rats (7 to 21 days old) were more sensitive to the toxic effects of artemether (a component of artemether and lumefantrine Tablets) than older juvenile rats or adults. Mortality and severe clinical signs were observed in neonatal rats at doses which were well tolerated in pups above 22 days old.
|clinicalStudies=
Treatment of Acute, Uncomplicated P. falciparum Malaria
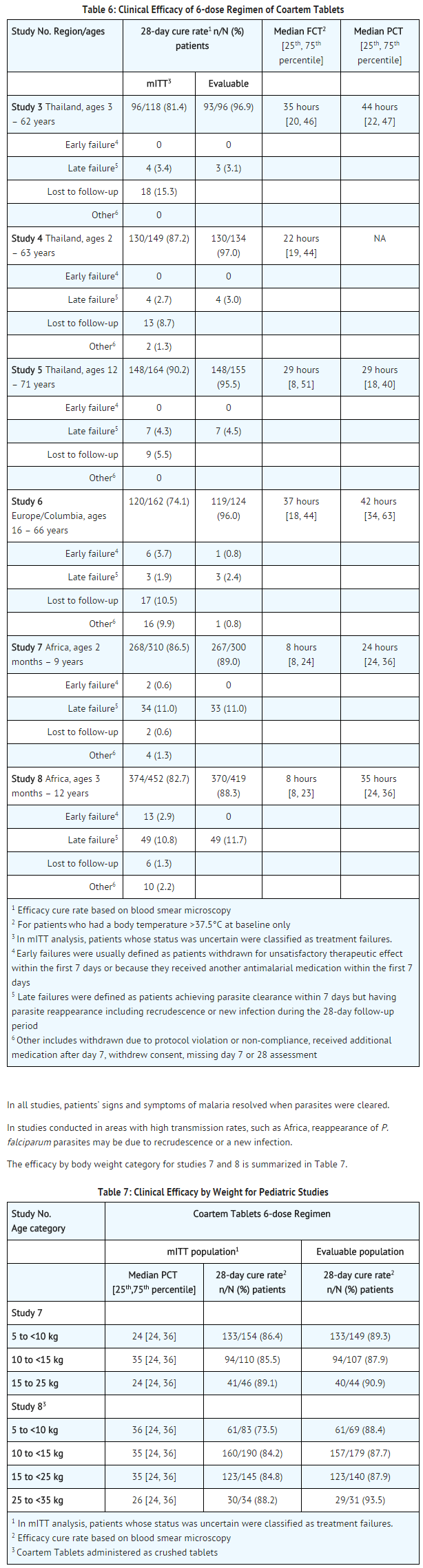
- The efficacy of artemether and lumefantrine Tablets was evaluated for the treatment of acute, uncomplicated malaria caused by P. falciparum in HIV negative patients in 8 clinical studies. Uncomplicated malaria was defined as symptomatic P. falciparum malaria without signs and symptoms of severe malaria or evidence of vital organ dysfunction. Baseline parasite density ranged from 500/mcL to 200,000/mcL (0.01% to 4% parasitemia) in the majority of patients. Studies were conducted in partially immune and non-immune adults and children (≥5kg body weight) with uncomplicated malaria in China, Thailand, sub-Saharan Africa, Europe, and South America. Patients who had clinical features of severe malaria, severe cardiac, renal, or hepatic impairment were excluded.
- The studies include two 4-dose studies assessing the efficacy of the components of the regimen, a study comparing a 4-dose versus a 6-dose regimen, and 5 additional 6-dose regimen studies.
- artemether and lumefantrine Tablets were administered at 0, 8, 24, and 48 hours in the 4-dose regimen, and at 0, 8, 24, 36, 48, and 60 hours in the 6-dose regimen. Efficacy endpoints consisted of:
- 28-day cure rate, defined as clearance of asexual parasites (the erythrocytic stage) within 7 days without recrudescence by day 28
parasite clearance time (PCT), defined as time from first dose until first total and continued disappearance of asexual parasite which continues for a further 48 hours fever clearance time (FCT), defined as time from first dose until the first time body temperature fell below 37.5°C and remained below 37.5°C for at least a further 48 hours (only for patients with temperature >37.5°C at baseline)
- The modified intent to treat (mITT) population includes all patients with malaria diagnosis confirmation who received at least 1 dose of study drug. Evaluable patients generally are all patients who had a day 7 and a day 28 parasitological assessment or experienced treatment failure by day 28.
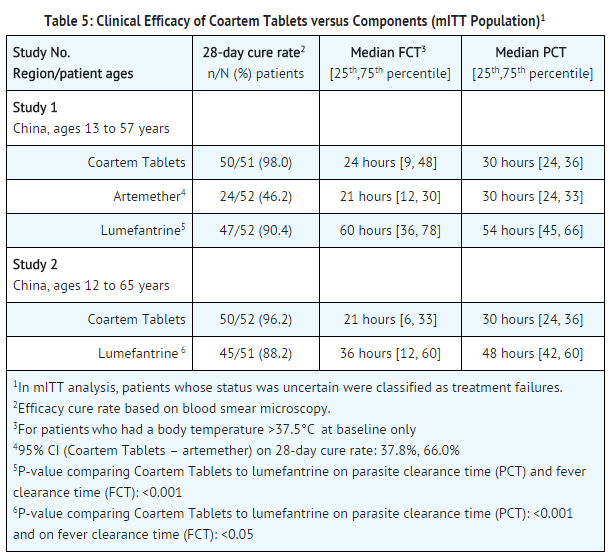
- Studies 1 and 2: The 2 studies which assessed the efficacy of artemether and lumefantrine Tablets (4 doses of 4 tablets of 20 mg artemether/120 mg lumefantrine) compared to each component alone were randomized, double-blind, comparative, single center, conducted in China. The efficacy results (Table 5) support that the combination of artemether and lumefantrine in artemether and lumefantrine Tablets had a significantly higher 28-day cure rate compared to artemether and had a significantly faster parasite clearance time (PCT) and fever clearance time (FCT) compared to lumefantrine.
This image is provided by the National Library of Medicine.
- Results of 4-dose studies conducted in areas with high resistance such as Thailand during 1995-96 showed lower efficacy results than the above studies. Therefore, Study 3 was conducted.
- Study 3: Study 3 was a randomized, double-blind, 2-center study conducted in Thailand in adults and children (aged ≥2 years), which compared the 4-dose regimen (administered over 48 hours) of artemether and lumefantrine Tablets to a 6-dose regimen (administered over 60 hours). Twenty-eight day cure rate in mITT subjects was 81% (96/118) for the artemether and lumefantrine Tablets 6-dose arm as compared to 71% (85/120) in the 4-dose arm.
- Studies 4, 5, 6, 7, and 8: In these studies, artemether and lumefantrine Tablets were administered as the 6-dose regimen.
- In study 4, a total of 150 adults and children aged ≥2 years received artemether and lumefantrine Tablets. In study 5, a total 164 adults and children ≥12 years received artemether and lumefantrine Tablets. Both studies were conducted in Thailand.
- Study 6 was a study of 165 non-immune adults residing in regions non-endemic for malaria (Europe and Colombia) who contracted acute uncomplicated falciparum malaria when traveling in endemic regions.
- Study 7 was conducted in Africa in 310 infants and children aged 2 months to 9 years, weighing 5 kg to 25 kg, with an axillary temperature ≥37.5ºC.
- Study 8 was conducted in Africa in 452 infants and children, aged 3 months to 12 years, weighing 5 kg to <35 kg, with fever (≥37.5°C axillary or ≥38°C rectally) or history of fever in the preceding 24 hours.
- Results of 28-day cure rate, median parasite clearance time (PCT), and fever clearance time (FCT) for Studies 3 to 8 are reported in Table 6.
- The efficacy of artemether and lumefantrine Tablets for the treatment P. falciparum infections mixed with P. vivax was assessed in a small number of patients. artemether and lumefantrine Tablets are only active against the erythrocytic phase of P. vivax malaria. Of the 43 patients with mixed infections at baseline, all cleared their parasitemia within 48 hours. However, parasite relapse occurred commonly (14/43; 33%). Relapsing malaria caused by P. vivax requires additional treatment with other antimalarial agents to achieve radical cure i.e., eradicate any hypnozoite forms that may remain dormant in the liver.
|howSupplied=*artemether and lumefantrine (artemether/lumefantrine) Tablets 20 mg/120 mg Tablets - yellow, round flat tablets with beveled edges and scored on one side. Tablets are imprinted with N/C on one side and CG on the other.
- Bottle of 24 NDC 0078-0568-45
- Store at 25ºC (77ºF); excursions permitted to 15ºC to 30ºC (59ºF to 86ºF) [see USP Controlled Room Temperature].
- Dispense in tight container (USP).
|fdaPatientInfo=*Advise patients to read the FDA-Approved Patient Labeling.
- Information for Safe Use
- Instruct patients to take artemether and lumefantrine Tablets with food. Patients who do not have an adequate intake of food are at risk for recrudescence of malaria.
- Patients with known hypersensitivity to artemether, lumefantrine, or to any of the excipients should not receive artemether and lumefantrine Tablets.
- Instruct patients to inform their physician of any personal or family history of QT prolongation or proarrhythmic conditions such as hypokalemia, bradycardia, or recent myocardial ischemia.
- Instruct patients to inform their physician if they are taking any other medications that prolong the QT interval, such as class IA (quinidine, procainamide, disopyramide), or class III (amiodarone, sotalol) antiarrhythmic agents; antipsychotics (pimozide, ziprasidone); antidepressants; certain antibiotics (macrolide antibiotics, fluoroquinolone antibiotics, imidazole, and triazole antifungal agents).
- Instruct patients to notify their physicians if they have any symptoms of prolongation of the QT interval, including prolonged heart palpitations or a loss of consciousness.
- Instruct patients to avoid medications that are metabolized by the cytochrome enzyme CYP2D6 while receiving artemether and lumefantrine Tablets since these drugs also have cardiac effects (e.g., flecainide, imipramine, amitriptyline, clomipramine).
- Inform patients that based on animal data, artemether and lumefantrine Tablets administered during pregnancy may result in fetal loss. Fetal defects have been reported when artemisinins are administered to animals.
- Halofantrine and artemether and lumefantrine Tablets should not be administered within 1 month of each other due to potential additive effects on the QT interval.
- Antimalarials should not be given concomitantly with artemether and lumefantrine Tablets, unless there is no other treatment option, due to limited safety data.
- QT prolonging drugs, including quinine and quinidine, should be used cautiously following artemether and lumefantrine Tablets due to the long elimination half-life of lumefantrine and the potential for additive effects on the QT interval. ECG monitoring is advised if use of drugs that prolong the QT interval is medically required.
- Closely monitor food intake in patients who received mefloquine immediately prior to treatment with artemether and lumefantrine Tablets.
- Use artemether and lumefantrine Tablets cautiously in patients receiving other drugs that are substrates, inhibitors or inducers of CYP3A4, including grapefruit juice, especially those that prolong the QT interval or are antiretroviral drugs.
- Coadministration of strong inducers of CYP3A4 such as rifampin, carbamazepine, phenytoin, and St. John’s wort is contraindicated with artemether and lumefantrine Tablets.
- Artemether and lumefantrine Tablets may reduce the effectiveness of hormonal contraceptives. Therefore, patients using oral, transdermal patch, or other systemic hormonal contraceptives should be advised to use an additional non-hormonal method of birth control.
- Inform patients that artemether and lumefantrine Tablets can cause hypersensitivity reactions. Instruct patients to discontinue the drug at the first sign of a skin rash, hives or other skin reactions, a rapid heartbeat, difficulty in swallowing or breathing, any swelling suggesting angioedema (e.g., swelling of the lips, tongue, face, tightness of the throat, hoarseness), or other symptoms of an allergic reaction.
|alcohol=* Alcohol-Artemether lumefantrine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
|brandNames=*Coartem
|lookAlike= |drugShortage= }}
{{#subobject:
|Label Page=Artemether lumefantrine |Label Name=Artemether lumefantrine08.png
}}
{{#subobject:
|Label Page=Artemether lumefantrine |Label Name=Artemether lumefantrine09.png
}}