Ramatroban
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
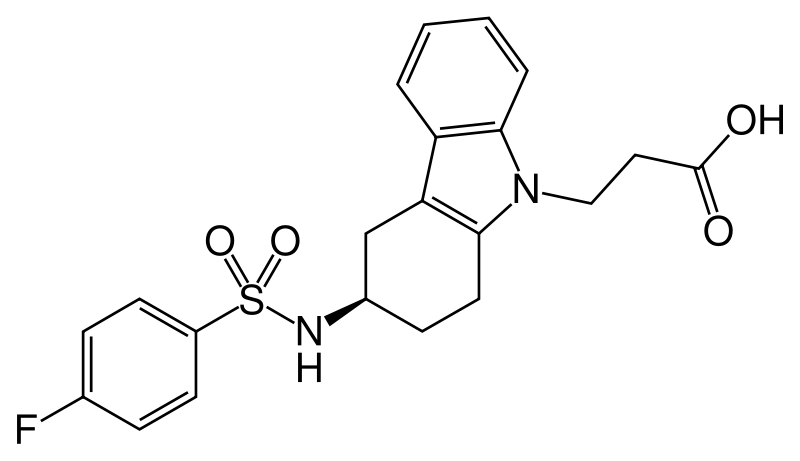
| Formula | C21H21FN2O4S |
| Molar mass | 416.46 g/mol |
| 3D model (JSmol) | |
| |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [2] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Ramatroban (INN) is a thromboxane receptor antagonist indicated for the treatment of coronary artery disease.[1] It has also been used for the treatment of asthma.[2]
It was developed by the German pharmaceutical company Bayer AG and is co-marketed in Japan by Bayer and Nippon Shinyaku Co. Ltd. under the tradename Baynas.
References
- ↑ Fiedler VB, Seuter F, Perzborn E (1990). "Effects of the novel thromboxane antagonist Bay U 3405 on experimental coronary artery disease". Stroke. 21 (12 Suppl): IV149–51. PMID 2260140. Unknown parameter
|month=ignored (help) - ↑ Endo S, Akiyama K (1996). "[Thromboxane A2 receptor antagonist in asthma therapy]". Nippon Rinsho (in Japanese). 54 (11): 3045–8. PMID 8950952. Unknown parameter
|month=ignored (help)
- Pages with script errors
- Pages with citations using unsupported parameters
- CS1 maint: Multiple names: authors list
- CS1 maint: Unrecognized language
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Articles containing unverified chemical infoboxes
- Antiplatelet drugs