Ticagrelor
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]; Ammu Susheela, M.D. [3]
Synonyms / Brand Names: Brilinta
Disclaimer
WikiDoc Drug Project is a constellation of drug information for healthcare providers and patients vigorously vetted on the basis of FDA package insert, MedlinePlus, Practice Guidelines, Scientific Statements, and scholarly medical literature. The information provided is not a medical advice or treatment. WikiDoc does not promote any medication or off-label use of drugs. Please read our full disclaimer here.
Black Box Warning
FDA Package Insert for Ticagrelor contains no information regarding Black Box Warning.
|
WARNING: (A) BLEEDING RISK, and (B) ASPIRIN DOSE AND Brilinta EFFECTIVENESS See full prescribing information for complete boxed warning. Condition Name:
|
Overview
Ticagrelor is a ADP-induced aggregation inhibitor, platelet aggregation inhibitor that is FDA approved for the treatment of acute coronary syndromes, There is a Black Box Warning for this drug as shown here. Common adverse reactions include bleeding, major and minor, headache, serum creatinine raised, cough, dyspnea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Acute coronary syndromes
- Dosing Information
- Initial dose: “Brilinta 180 mg PO once” with aspirin (325 mg) once.
- Maintenance dose: “Brilinta 90 mg PO bid” with aspirin 75-100 mg PO qd.
- Not recommended when aspirin maintenance dose is above 100 mg.
Percutaneous coronary intervention
- Dosing Information
- Loading dose: “Brilinta 180 mg PO” with aspirin (325 mg), once.
- Maintenance: “ticagrelor 90 mg PO bid” with aspirin 75-100 mg qd.
- Use of aspirin maintenance dose above 100 mg is not recommended.
- Consider carefully the continuation of therapy beyond 12 months (for drug-eluting stents).
Off-Label Use and Dosage (Adult)
There is limited information about Off-Label Use and Dosage of Ticagrelor tablet in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information about FDA-Labeled Indications and Dosageof Ticagrelor tablet in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information about Off-Label Use and Dosage of Ticagrelor tablet in pediatric patients.
Contraindications
- History of intracranial hemorrhage
- Active pathological bleeding
- Severe hepatic impairment
- Hypersensitivity to ticagrelor or any component of the product
Warnings
|
WARNING: (A) BLEEDING RISK, and (B) ASPIRIN DOSE AND ticagrelor EFFECTIVENESS See full prescribing information for complete boxed warning. Condition Name:
|
- Like other antiplatelet agents, ticagrelor increases the risk of bleeding.
- In PLATO, use of ticagrelor with maintenance doses of aspirin above 100 mg decreased the effectiveness of ticagrelor.
- Moderate Hepatic Impairment: Consider the risks and benefits of treatment, noting the probable increase in exposure to ticagrelor.
- Dyspnea: Dyspnea was reported more frequently with ticagrelor than with clopidogrel. Dyspnea resulting from ticagrelor is self-limiting. Rule out other causes.
- Discontinuation of ticagrelor: Premature discontinuation increases the risk of myocardial infarction, stent thrombosis, and death.
Adverse Reactions
Clinical Trials Experience
The following adverse reactions are also discussed elsewhere in the labeling: Warnings
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
ticagrelor has been evaluated for safety in more than 10000 patients, including more than 3000 patients treated for more than 1 year.
PLATO used the following bleeding severity categorization:
- Major bleed – fatal/life-threatening. Any one of the following: fatal; intracranial; intrapericardial bleed with cardiac tamponade; hypovolemic shock or severe hypotension due to bleeding and requiring pressors or surgery; clinically overt or apparent bleeding associated with a decrease in hemoglobin (Hb) of more than 5 g/dL; transfusion of 4 or more units (whole blood or packed red blood cells (PRBCs)) for bleeding.
- Major bleed – other. Any one of the following: significantly disabling (e.g., intraocular with permanent vision loss); clinically overt or apparent bleeding associated with a decrease in Hb of 3 g/dL; transfusion of 2-3 units (whole blood or PRBCs) for bleeding.
- Minor bleed. Requires medical intervention to stop or treat bleeding (e.g., epistaxis requiring visit to medical facility for packing).
- Minimal bleed. All others (e.g., bruising, bleeding gums, oozing from injection sites, etc.) not requiring intervention or treatment.
Figure 1 shows major bleeding events over time. Many events are early, at a time of coronary angiography, PCI, CABG, and other procedures, but the risk persists during later use of antiplatelet therapy.
Figure 1- Kaplan-Meier estimate of time to first PLATO-defined ‘Total Major’ bleeding event

Annualized rates of bleeding are summarized in Table 1 below. About half of the bleeding events were in the first 30 days.

As shown in Table 1, Ticagrelor was associated with a somewhat greater risk of non- CABG bleeding than was Clopidogrel. No baseline demographic factor altered the relative risk of bleeding with Ticagrelor compared to Clopidogrel.
In PLATO, 1584 patients underwent CABG surgery. The percentages of those patients who bled are shown in Table 2. Rates were very high but similar for Ticagrelor and Clopidogrel.

Although the platelet inhibition effect of Ticagrelor has a faster offset than Clopidogrel in in vitro tests and Ticagrelor is a reversibly binding P2Y12 inhibitor, PLATO did not show an advantage of Ticagrelor compared to Clopidogrel for CABG-related bleeding. When antiplatelet therapy was stopped 5 days before CABG, major bleeding occurred in 75% of Ticagrelor treated patients and 79% on Clopidogrel.
No data exist with Ticagrelor regarding a hemostatic benefit of platelet transfusions.
Drug Discontinuation
In PLATO, the rate of study drug discontinuation attributed to adverse reactions was 7.4% for Ticagrelor and 5.4% for Clopidogrel. Bleeding caused permanent discontinuation of study drug in 2.3% of Ticagrelor patients and 1.0% of Clopidogrel patients. Dyspnea led to study drug discontinuation in 0.9% of Ticagrelor and 0.1% of Clopidogrel patients.
Common Adverse Events
A variety of non-hemorrhagic adverse events occurred in PLATO at rates of 3% or more. These are shown in Table 3. In the absence of a placebo control, whether these are drug related cannot be determined in most cases, except where they are more common on Ticagrelor or clearly related to the drug’s pharmacologic effect (dyspnea).

In clinical studies Ticagrelor has been shown to increase the occurrence of Holter-detected bradyarrhythmias (including ventricular pauses). PLATO excluded patients at increased risk of bradycardic events (e.g., patients who have sick sinus syndrome, 2nd or 3rd degree AV block, or bradycardic-related syncope and not protected with a pacemaker). In PLATO, syncope, pre-syncope and loss of consciousness were reported by 1.7% and 1.5% of ticagrelor and Clopidogrel patients, respectively.
In a Holter substudy of about 3000 patients in PLATO, more patients had ventricular pauses with Ticagrelor (6.0%) than with Clopidogrel (3.5%) in the acute phase; rates were 2.2% and 1.6% respectively after 1 month.
In PLATO, gynecomastia was reported by 0.23% of men on ticagrelor and 0.05% on Clopidogrel. Other sex-hormonal adverse reactions, including sex organ malignancies, did not differ between the two treatment groups in PLATO.
Lab abnormalities
Serum Uric Acid:
Serum uric acid levels increased approximately 0.6 mg/dL from baseline on Ticagrelor and approximately 0.2 mg/dL on Clopidogrel in PLATO. The difference disappeared within 30 days of discontinuing treatment. Reports of gout did not differ between treatment groups in PLATO (0.6% in each group).
Serum Creatinine:
In PLATO, a >50% increase in serum creatinine levels was observed in 7.4% of patients receiving Ticagrelor compared to 5.9% of patients receiving Clopidogrel. The increases typically did not progress with ongoing treatment and often decreased with continued therapy. Evidence of reversibility upon discontinuation was observed even in those with the greatest on treatment increases. Treatment groups in PLATO did not differ for renal-related serious adverse events such as acute renal failure, chronic renal failure, toxic nephropathy, or oliguria. |postmarketing=The following adverse reactions have been identified during post-approval use of ticagrelor. Because these reactions are reported voluntarily from a population of an unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune system disorders – Hypersensitivity reactions including angioedema. See Contraindications.
Postmarketing Experience
There is limited information about Postmarketing Experience of Ticagrelor tablet in pediatric patients.
Drug Interactions
Effects of other drugs
Ticagrelor is predominantly metabolized by CYP3A4 and to a lesser extent by CYP3A5. Ticagrelor is also a p-glycoprotein (P-gp) substrate.
- CYP3A inhibitors
- Avoid use of strong inhibitors of CYP3A (e.g., ketoconazole, itraconazole, clarithromycin, nefazodone, ritonavir, saquinavir, nelfinavir, indinavir, atazanavir and telithromycin). See Warnings and Pharmacology.
- CYP3A inducers
- Avoid use with potent inducers of CYP3A (e.g., rifampin, dexamethasone, phenytoin, carbamazepine and phenobarbital) See Contraindications, Pharmacology and Warnings.
- See Pharmacology and Warnings.
Effect of ticagrelor on other drugs.
Ticagrelor is an inhibitor of CYP3A4/5 and the P-glycoprotein transporter.
- Ticagrelor will result in higher serum concentrations of simvastatin and lovastatin because these drugs are metabolized by CYP3A4. Avoid simvastatin and lovastatin doses greater than 40 mg. See Pharmacology.
- Digoxin
- Digoxin: Because of inhibition of the P-glycoprotein transporter, monitor digoxin levels with initiation of or any change in ticagrelor therapy. See Pharmacology
- Other Concomitant Therapy
- Ticagrelor can be administered with unfractionated or low-molecular-weight heparin, GPIIb/IIIa inhibitors, proton pump inhibitors, beta-blockers, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers.
Use in Specific Populations
Pregnancy
- Pregnancy Category (AUS): Ticagrelor is not included in Australian Drug Evaluation Committee (ADEC) Pregnancy Categories.
- There are no adequate and well-controlled studies of ticagrelor use in pregnant women. In animal studies, ticagrelor caused structural abnormalities at maternal doses about 5 to 7 times the maximum recommended human dose (MRHD) based on body surface area. ticagrelor should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- In reproductive toxicology studies, pregnant rats received ticagrelor during organogenesis at doses from 20 to 300 mg/kg/day. The lowest dose was approximately the same as the MRHD of 90 mg twice daily for a 60 kg human on a mg/m2 basis. Adverse outcomes in offspring occurred at doses of 300 mg/kg/day (16.5 times the MRHD on a mg/m2 basis) and included supernumerary liver lobe and ribs, incomplete ossification of sternebrae, displaced articulation of pelvis, and misshapen/misaligned sternebrae. When pregnant rabbits received ticagrelor during organogenesis at doses from 21 to 63 mg/kg/day, fetuses exposed to the highest maternal dose of 63 mg/kg/day (6.8 times the MRHD on a mg/m2 basis) had delayed gall bladder development and incomplete ossification of the hyoid, pubis and sternebrae occurred.
- In a prenatal/postnatal study, pregnant rats received ticagrelor at doses of 10 to 180 mg/kg/day during late gestation and lactation. Pup death and effects on pup growth were observed at 180 mg/kg/day (approximately 10 times the MRHD on a mg/m2 basis). Relatively minor effects such as delays in pinna unfolding and eye opening occurred at doses of 10 and 60 mg/kg (approximately one-half and 3.2 times the MRHD on a mg/m2 basis).
|useInNursing=It is not known whether ticagrelor or its active metabolites are excreted in human milk. Ticagrelor is excreted in rat milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from ticagrelor, a decision should be made whether to discontinue nursing or to discontinue drug, taking into account the importance of the drug to the mother.
Labor and Delivery
There is limited information about Labor and Delivery of Ticagrelor tablet in patients.
Nursing Mothers
There is limited information about Nursing Mothers of Ticagrelor tablet in patients.
Pediatric Use
The safety and effectiveness of ticagrelor in pediatric patients have not been established.
Geriatric Use
- In PLATO, 43% of patients were ≥65 years of age and 15% were ≥75 years of age. The relative risk of bleeding was similar in both treatment and age groups.
- No overall differences in safety or effectiveness were observed between these patients and younger patients. While this clinical experience has not identified differences in responses between the elderly and younger patients, greater sensitivity of some older individuals cannot be ruled out.
|useInRenalImpair=No dosage adjustment is needed in patients with renal impairment. Patients receiving dialysis have not been studied [see Clinical Pharmacology.
Gender
There is no FDA guidance on the use of Ticagrelor with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ticagrelor with respect to specific racial populations.
Renal Impairment
No dosage adjustment is needed in patients with renal impairment. Patients receiving dialysis have not been studied [see Clinical Pharmacology.
Hepatic Impairment
- Ticagrelor has not been studied in the patients with moderate or severe hepatic impairment. Ticagrelor is metabolized by the liver and impaired hepatic function can increase risks for bleeding and other adverse events. Hence, ticagrelor is contraindicated for use in patients with severe hepatic impairment and its use should be considered carefully in patients with moderate hepatic impairment. No dosage adjustment is needed in patients with mild hepatic impairment [see Contraindications, Warnings and Precautions (5.3) and Clinical Pharmacology.
Immunocompromised Patients
There is no FDA guidance one the use of Ticagrelor in patients who are immunocompromised.
Administration and Monitoring
Administration
- [[Orall.
- Initiate ticagrelor treatment with a 180 mg (two 90 mg tablets) loading dose and continue treatment with 90 mg twice daily.
- After the initial loading dose of aspirin (usually 325 mg), use ticagrelor with a daily maintenance dose of aspirin of 75-100 mg.
- ACS patients who have received a loading dose of clopidogrel may be started on ticagrelor.
- ticagrelor can be administered with or without food.
- A patient who misses a dose of ticagrelor should take one 90 mg tablet (their next dose) at its scheduled time.
Monitoring
IV Compatibility
Overdosage
Pharmacology
Mechanism of Action
- Ticagrelor and its major metabolite reversibly interact with the platelet P2Y12 ADP-receptor to prevent signal transduction and platelet activation. Ticagrelor and its active metabolite are approximately equipotent.
- The inhibition of platelet aggregation (IPA) by ticagrelor and clopidogrel was compared in a 6 week study examining both acute and chronic platelet inhibition effects in response to 20 μM ADP as the platelet aggregation agonist.
- The onset of IPA was evaluated on Day 1 of the study following loading doses of 180 mg ticagrelor or 600 mg clopidogrel. As shown in Figure 2, IPA was higher in the ticagrelor group at all time points. The maximum IPA effect of ticagrelor was reached at around 2 hours, and was maintained for at least 8 hours.
- The offset of IPA was examined after 6 weeks on ticagrelor 90 mg twice daily or clopidogrel 75 mg daily, again in response to 20 μM ADP.
- As shown in Figure 3, mean maximum IPA following the last dose of ticagrelor was 88% and 62% for clopidogrel. The insert in Figure 3 shows that after 24 hours, IPA in the ticagrelor group (58%) was similar to IPA in clopidogrel group (52%), indicating that patients who miss a dose of ticagrelor would still maintain IPA similar to the trough IPA of patients treated with clopidogrel. After 5 days, IPA in the ticagrelor group was similar to IPA in the placebo group. It is not known how either bleeding risk or thrombotic risk track with IPA, for either ticagrelor or clopidogrel.
Figure 2 - Mean inhibition of platelet aggregation (±SE) following single oral doses of placebo, 180 mg ticagrelor or 600 mg clopidogrel


- Transitioning from clopidogrel to ticagrelor resulted in an absolute IPA increase of 26.4% and from ticagrelor to clopidogrel resulted in an absolute IPA decrease of 24.5%. Patients can be transitioned from clopidogrel to ticagrelor without interruption of antiplatelet effect [see dosage and Administration (2) .
Structure
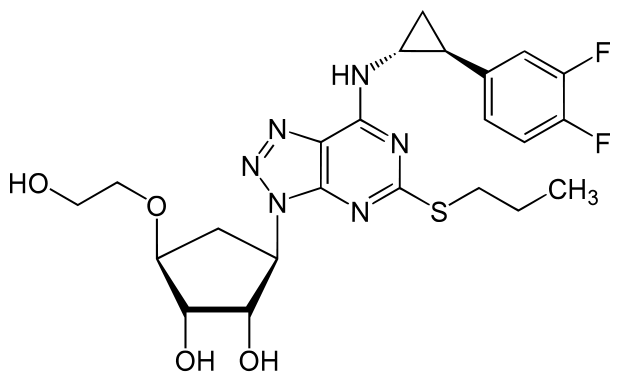
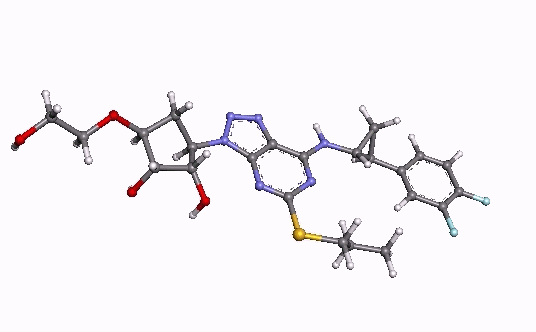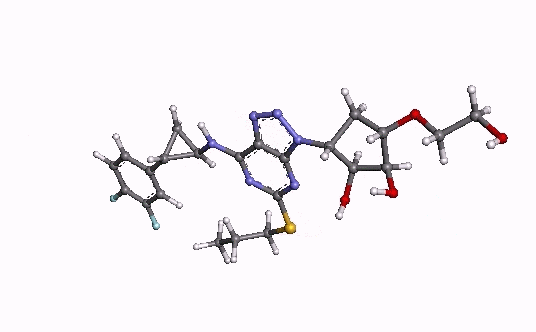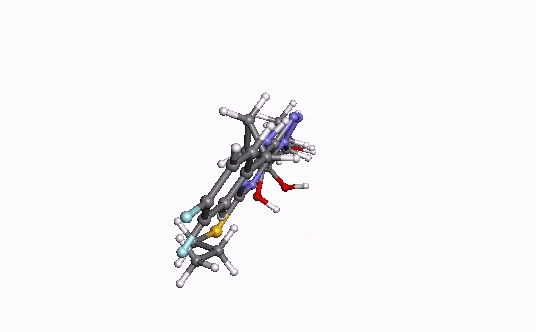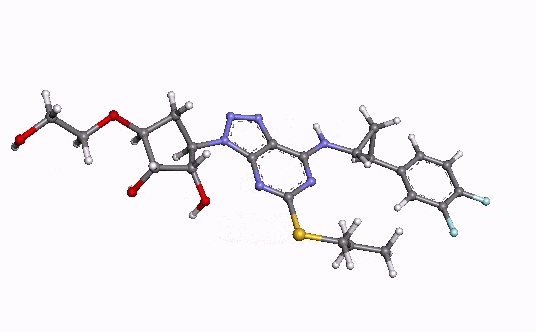
- Ticagrelor cyclopentyltriazolopyrimidine, inhibitor of platelet activation and aggregation mediated by the P2Y12 ADP-receptor. Chemically it is (1S,2S,3R,5S)-3-[7-{[(1R,2S)-2-(3,4-difluorophenyl)cyclopropyl]amino}-5-(propylthio)-3H-[1,2,3]-triazolo[4,5-d]pyrimidin-3-yl]-5-(2-hydroxyethoxy)cyclopentane-1,2-diol. The empirical formula of ticagrelor is C23H28F2N6O4S and its molecular weight is 522.57. The chemical structure of ticagrelor is:

- Ticagrelor is a crystalline powder with an aqueous solubility of approximately 10 μg/mL at room temperature.
- Ticagrelor tablets for oral administration contain 90 mg of ticagrelor and the following ingredients: mannitol, dibasic calcium phosphate, sodium starch glycolate, hydroxypropyl cellulose, magnesium stearate, hydroxypropyl methylcellulose, titanium dioxide, talc, polyethylene glycol 400, and ferric oxide yellow.
Pharmacodynamics
- The inhibition of platelet aggregation (IPA) by ticagrelor and clopidogrel was compared in a 6 week study examining both acute and chronic platelet inhibition effects in response to 20 μM ADP as the platelet aggregation agonist.
- The onset of IPA was evaluated on Day 1 of the study following loading doses of 180 mg ticagrelor or 600 mg clopidogrel. As shown in Figure 2, IPA was higher in the ticagrelor group at all time points. The maximum IPA effect of ticagrelor was reached at around 2 hours, and was maintained for at least 8 hours.
- The offset of IPA was examined after 6 weeks on ticagrelor 90 mg twice daily or clopidogrel 75 mg daily, again in response to 20 μM ADP.
- As shown in Figure 3, mean maximum IPA following the last dose of ticagrelor was 88% and 62% for clopidogrel. The insert in Figure 3 shows that after 24 hours, IPA in the ticagrelor group (58%) was similar to IPA in clopidogrel group (52%), indicating that patients who miss a dose of ticagrelor would still maintain IPA similar to the trough IPA of patients treated with clopidogrel. After 5 days, IPA in the ticagrelor group was similar to IPA in the placebo group. It is not known how either bleeding risk or thrombotic risk track with IPA, for either ticagrelor or clopidogrel.
Figure 2 - Mean inhibition of platelet aggregation (±SE) following single oral doses of placebo, 180 mg ticagrelor or 600 mg clopidogrel


- Transitioning from clopidogrel to ticagrelor resulted in an absolute IPA increase of 26.4% and from ticagrelor to clopidogrel resulted in an absolute IPA decrease of 24.5%. Patients can be transitioned from clopidogrel to ticagrelor without interruption of antiplatelet effect [see dosage and Administration (2) .
Pharmacokinetics
- Ticagrelor demonstrates dose proportional pharmacokinetics, which are similar in patients and healthy volunteers.
Absorption
- Absorption of ticagrelor occurs with a median tmax of 1.5 h (range 1.0–4.0). The formation of the major circulating metabolite AR-C124910XX (active) from ticagrelor occurs with a median tmax of 2.5 h (range 1.5-5.0).
- The mean absolute bioavailability of ticagrelor is about 36%, (range 30%-42%). Ingestion of a high-fat meal had no effect on ticagrelor Cmax, but resulted in a 21% increase in AUC. The Cmax of its major metabolite was decreased by 22% with no change in AUC. Ticagrelor can be taken with or without food.
Distribution
- The steady state volume of distribution of ticagrelor is 88 L. Ticagrelor and the active metabolite are extensively bound to human plasma proteins (>99%).
Metabolism
- CYP3A4 is the major enzyme responsible for ticagrelor metabolism and the formation of its major active metabolite. Ticagrelor and its major active metabolite are weak P-glycoprotein substrates and inhibitors. The systemic exposure to the active metabolite is approximately 30-40% of the exposure of ticagrelor.
Excretion
- The primary route of ticagrelor elimination is hepatic metabolism. When radiolabeled ticagrelor is administered, the mean recovery of radioactivity is approximately 84% (58% in feces, 26% in urine). Recoveries of ticagrelor and the active metabolite in urine were both less than 1% of the dose. The primary route of elimination for the major metabolite of ticagrelor is most likely to be biliary secretion. The mean t1/2 is approximately 7 hours for ticagrelor and 9 hours for the active metabolite.
Special Populations
- The effects of age, gender, ethnicity, renal impairment and mild hepatic impairment on the pharmacokinetics of ticagrelor are presented in Figure 4. Effects are modest and do not require dose adjustment.
- Figure 4 - Impact of intrinsic factors on the pharmacokinetics of ticagrelor

Pediatric
- Ticagrelor has not been evaluated in a pediatric population [see Use in Specific Populations (8.4)].
Body Weight
- No dose adjustment is necessary for ticagrelor based on weight.
Smoking
- Habitual smoking increased population mean clearance of ticagrelor by approximately 22% when compared to non-smokers. No dose adjustment is necessary for ticagrelor based on smoking status.
Effects of Other Drugs on Ticagrelor
- CYP3A4 is the major enzyme responsible for ticagrelor metabolism and the formation of its major active metabolite. The effects of other drugs on the pharmacokinetics of ticagrelor are presented in Figure 5 as change relative to ticagrelor given alone (test/reference). Strong CYP3A inhibitors (e.g., ketoconazole, itraconazole, and clarithromycin) substantially increase ticagrelor exposure. Moderate CYP3A inhibitors have lesser effects (e.g., diltiazem). CYP3A inducers (e.g., rifampin) substantially reduce ticagrelor blood levels. P-gp inhibitors (e.g. cyclosporine) increase ticagrelor exposure.

Effects of Brilinta on Other Drugs
- In vitro metabolism studies demonstrate that ticagrelor and its major active metabolite are weak inhibitors of CYP3A4, potential activators of CYP3A5 and inhibitors of the P-gp transporter. Ticagrelor and AR-C124910XX were shown to have no inhibitory effect on human CYP1A2, CYP2C19, and CYP2E1 activity. For specific in vivo effects on the pharmacokinetics of simvastatin, atorvastatin, ethinyl estradiol, levonorgesterol, tolbutamide, digoxin and cyclosporine, see Figure 6.

Nonclinical Toxicology
Carcinogenesis
- Ticagrelor was not carcinogenic in the mouse at doses up to 250 mg/kg/day or in the male rat at doses up to 120 mg/kg/day (19 and 15 times the MRHD of 90 mg twice daily on the basis of AUC, respectively). Uterine carcinomas, uterine adenocarcinomas and hepatocellular adenomas were seen in female rats at doses of 180 mg/kg/day (29-fold the maximally recommended dose of 90 mg twice daily on the basis of AUC), whereas 60 mg/kg/day (8-fold the MRHD based on AUC) was not carcinogenic in female rats.
Mutagenesis
- Ticagrelor did not demonstrate genotoxicity when tested in the Ames bacterial mutagenicity test, mouse lymphoma assay and the rat micronucleus test. The active O-demethylated metabolite did not demonstrate genotoxicity in the Ames assay and mouse lymphoma assay.
Impairment of Fertility
- Ticagrelor had no effect on male fertility at doses up to 180 mg/kg/day or on female fertility at doses up to 200 mg/kg/day (>15-fold the MRHD on the basis of AUC). Doses of ≥10 mg/kg/day given to female rats caused an increased incidence of irregular duration estrus cycles (1.5-fold the MRHD based on AUC).
Clinical Studies
- The clinical evidence for the effectiveness of Brilinta is derived from PLATO, a randomized double-blind study comparing Brilinta (N=9333) to clopidogrel (N=9291), both given in combination with aspirin and other standard therapy, in patients with acute coronary syndromes (ACS). Patients were treated for at least 6 months and for up to 12 months. Study endpoints were obtained until the study was complete, even if drug was discontinued.
- Patients who presented within 24 hours of onset of the most recent episode of chest pain or symptoms were randomized to receive Brilinta or clopidogrel. Patients who had already been treated with clopidogrel could be enrolled and randomized to either study treatment. Patients could be included whether there was intent to manage the ACS medically or invasively, but patient randomization was not stratified by this intent. Subjects in the clopidogrel arm were treated with an initial loading dose of clopidogrel 300 mg, if previous clopidogrel therapy had not been given prior to randomization. Patients undergoing PCI could receive an additional 300 mg of clopidogrel at investigator discretion. All subjects randomized to Brilinta received a loading dose of 180 mg followed by a maintenance dose of 90 mg twice daily. Concomitant aspirin was recommended at a loading dose of 160-500 mg. A daily maintenance dose of aspirin 75-100 mg was recommended, but higher maintenance doses of aspirin were allowed according to local judgment.
- Because of ticagrelor’s metabolism by CYP3A enzymes, the protocol recommended limiting the maximum dosage of simvastatin and lovastatin to 40 mg in both study arms. * Because of an increased bleeding risk, the study excluded patients with previous intracranial hemorrhage, a gastrointestinal bleed within the past 6 months, or other factors that predispose to bleeding.
- PLATO patients were predominantly male (72%) and Caucasian (92%). About 43% of patients were >65 years and 15% were >75 years.
- The study’s primary endpoint was the composite of first occurrence of cardiovascular death, non-fatal MI (excluding silent MI), or non-fatal stroke. The components were assessed as secondary endpoints.
- Median exposure to study drug was 277 days. About half of the patients received pre-study clopidogrel and about 99% of the patients received aspirin at some time during PLATO. About 35% of patients were receiving a statin at baseline and 93% received a statin sometime during PLATO.
- Table 4 shows the study results for the primary composite endpoint and the contribution of each component to the primary endpoint. Separate secondary endpoint analyses are shown for the overall occurrence of CV death, MI, and stroke and overall mortality.

- The difference between treatments on the composite resulted from effects on CV death and MI; each was statistically significant when considered as a secondary endpoint and there was no beneficial effect on strokes. For all-cause mortality the benefit was also statistically significant (p = 0.0003) with a hazard ratio of 0.78.
- Among 11289 patients with PCI receiving any stent during PLATO, there was a lower risk of stent thrombosis (1.3% for adjudicated “definite”) than with clopidogrel (1.9%) (HR 0.67, 95% CI 0.50-0.91; p=0.0091). The results were similar for drug-eluting and bare metal stents.
The Kaplan-Meier curve (Figure 7) shows time to first occurrence of the primary composite endpoint of CV death, non-fatal MI or non-fatal stroke in the overall study. Figure 7 - Time to First Occurrence of CV death, MI, or Stroke in PLATO

- The curves separate by 30 days (RRR 12%) and continue to diverge throughout the 12 month treatment period (RRR 16%).
- A wide range of demographic, concurrent baseline medications, and other treatment differences were examined for their influence on outcome. Many of these are shown in Figure 8. Such analyses must be interpreted cautiously, as differences can reflect the play of chance among a large number of analyses. Most of the analyses show effects consistent with the overall results, but there are two marked exceptions: a finding of heterogeneity by region and a strong influence of the maintenance dose of aspirin. * These are considered further below.
- Most of the characteristics shown are baseline characteristics, but some reflect post-randomization determinations (e.g., final diagnosis, aspirin maintenance dose, use of PCI). Patients were not stratified by initial diagnosis, but the effect in the unstable angina subset (determined after randomization) appeared smaller than the effect in the NSTEMI and STEMI subsets. The results in the subsets based on final diagnosis (STEMI, NSTEMI and unstable angina) are also presented in Figure 8.
Figure 8 - Subgroup analyses of PLATO

Regional Differences
- Results in the rest of the world compared to effects in North America (US and Canada) show a smaller effect in North America, numerically inferior to the control and driven by the US subset. The statistical test for the US/non-US comparison is statistically significant (p=0.009), and the same trend is present for both CV death and non-fatal MI. The individual results and nominal p-values, like all subset analyses, need cautious interpretation, and they could represent chance findings. The consistency of the differences in both the CV mortality and non-fatal MI components, however, supports the possibility that the finding is reliable.
- A wide variety of baseline and procedural differences between the US and non-US (including intended invasive vs. planned medical management, use of GPIIb/IIIa inhibitors, use of drug eluting vs. bare-metal stents) were examined to see if they could account for regional differences, but with one exception, aspirin maintenance dose, these differences did not appear to lead to differences in outcome.
aspirin Dose
- The PLATO protocol left the choice of aspirin maintenance dose up to the investigator and use patterns were very different in the US and elsewhere, with about 8% of non-US investigators using aspirin doses above 100 mg, and about 2% using doses above 300 mg, in contrast with US practice, where 57% of patients received doses above 100 mg and 54% received doses above 300 mg. Overall results favored Brilinta when used with low maintenance doses (≤ 100 mg) of aspirin, and results analyzed by aspirin dose were similar in the US and elsewhere. Figure 8 shows overall results by median aspirin dose. Table 5 shows results by region and dose.
Table 5 - PLATO: CV Death, MI, Stroke by maintenance aspirin dose in the US and outside the US

- Like any unplanned subset analysis, especially one where the characteristic is not a true baseline characteristic (but may be determined by usual investigator practice), the above analyses must be treated with caution. It is notable, however, that aspirin dose predicts outcome in both regions with a similar pattern, and that the pattern is similar for the two major components of the primary endpoint, CV death and non-fatal MI.
Despite the need to treat such results cautiously, there appears to be good reason to restrict aspirin maintenance dosage accompanying ticagrelor to 100 mg. Higher doses do not have an established benefit in the ACS setting, and there is a strong suggestion that use of such doses reduces the effectiveness of Brilinta. Pharmacogenetics
- In a genetic substudy of PLATO (n=10,285), the effects of Brilinta compared to clopidogrel on thrombotic events and bleeding were not significantly affected by CYP2C19 genotype.
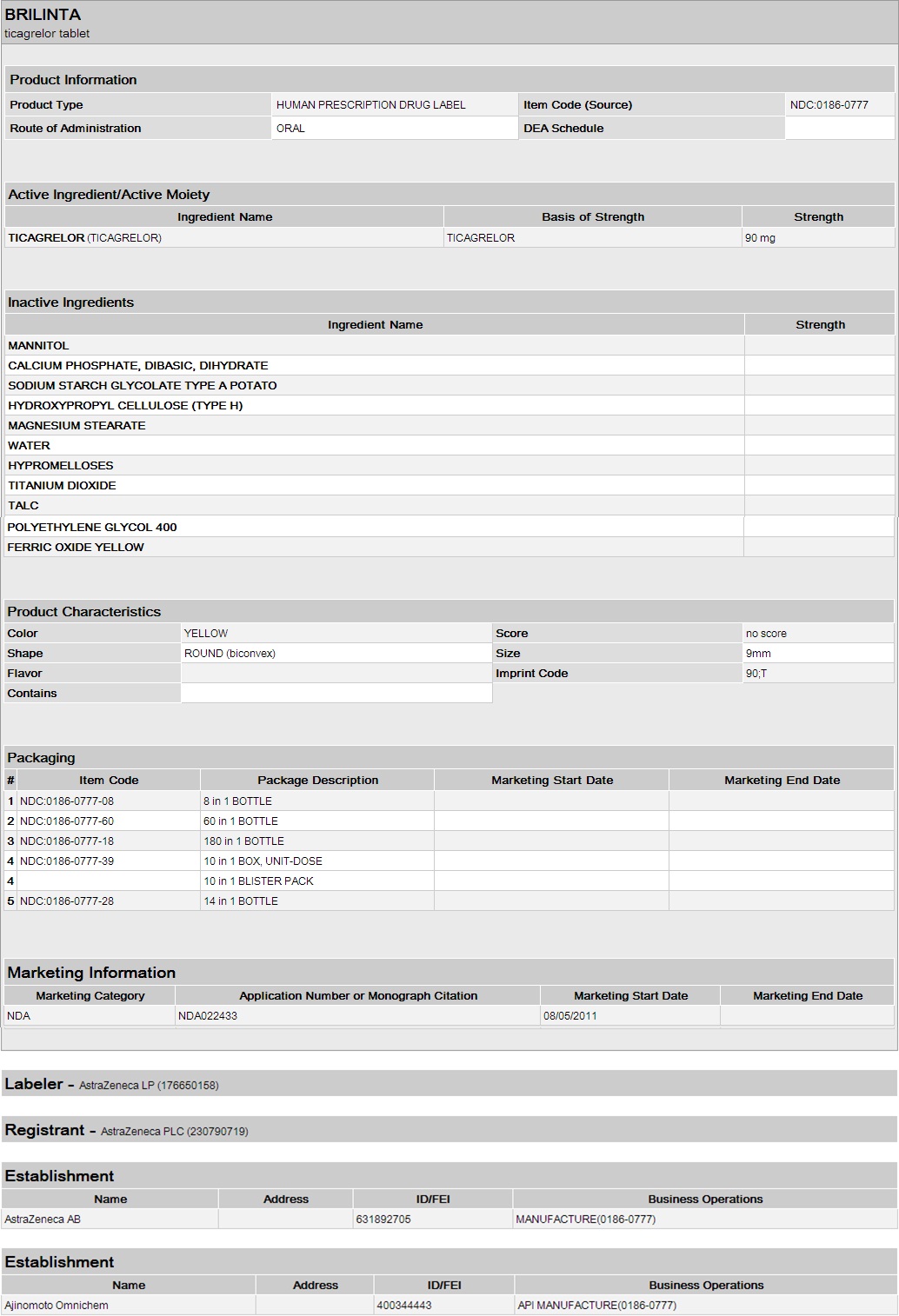
How Supplied
- Brilinta (ticagrelor) 90 mg is supplied as a round, biconvex, yellow, film-coated tablet marked with a “90” above “T” on one side.
- Bottles of 14 – NDC 0186-0777-28.
- Bottles of 60 – NDC 0186-0777-60.
- Bottles of 180 – NDC 0186-0777-18.
- 100 count Hospital Unit Dose – NDC 0186-0777-39.
- Store at 25°C (77°F); excursions permitted to 15°-30°C (59°- 86°F).
Images
Drug Images
 Drug Name: Brilinta 90 MG Oral Tablet |
Package and Label Display Panel

Patient Information
For patient information about ticagrelor, click here.
Precautions with Alcohol
Alcohol-Ticagrelor interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Brilinta®