Acute myeloid leukemia natural history
|
Acute myeloid leukemia Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Acute myeloid leukemia natural history On the Web |
|
American Roentgen Ray Society Images of Acute myeloid leukemia natural history |
|
Risk calculators and risk factors for Acute myeloid leukemia natural history |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Raviteja Guddeti, M.B.B.S. [2] Carlos A Lopez, M.D. [3] Shyam Patel [4]
Overview
Common complications of acute myeloid leukemia include infections, disseminated intravascular coagulation, pyoderma gangrenosum, hemorrhage and complications due to side effects of chemotherapy. Prognosis of acute myelogenous leukemia depends on cytogenetics. Cytogenetics that indicate a good prognosis include inversions in chromosome 16 inv(16), translocations between chromosome 8 and 21 t(8;21) and translocations between chromosome 15 and 17 t(15;17).
Natural History
Acute myeloid leukemia usually begins with a variety of symptoms including fatigue, bleeding, and infections (such as upper respiratory tract infection). Patients typically present to their primary care physician with such symptoms, and a complete blood count usually reveals a low white blood cell count, low hemoglobin, and/or low platelet count. A bone marrow biopsy is usually done to work up the abnormal laboratory values, and a diagnosis of acute myeloid leukemia is made. The median survival in the absence of treatment is typically 6-8 weeks. In patients with acute promyelocytic leukemia, in the first few days to weeks of the disease, there is a high risk of bleeding due to disseminated intravascular coagulation, a condition characterized by abnormal thrombus formation and breakdown.[1] The median survival in the absence of treatment of acute promyelocytic leukemia is typically one week, due to bleeding complications contributing to mortality.[2] The high early mortality rate was previously a major part of the natural history of the disease, prior to the advent of rapid diagnostic and therapeutic interventions for this disease.[3] In areas of the United States with limited healthcare or highly specialized academic centers, bleeding diathesis continues to remain a major part of the natural history of the disease. Such bleeding complications include gingival bleeding (very common), bruising (very common), epistaxis, menorrhagia (less common). In areas of the United States with readily available healthcare and specialized academic medical centers, the natural history of the disease takes a favorable trajectory, as the cure rate is quite high if appropriate induction therapy is initiated.[3]
Complications
- Hemorrhage: Acute promyelocytic leukemia is frequently associated with bleeding caused by disseminated intravascular coagulation (DIC). Hemorrhagic and bleeding diathesis is the major cause of early complications that can lead to immediate death in patients with acute promyelocytic leukemia. For this reason, prompt treatment of the disease is required.
- Venous thromboembolism: Thrombus formation is a major cause of morbidity in acute promyelocytic leukemia. Thrombosis in the setting of acute promyelocytic leukemia is associated with a worse outcome compared to non-cancer-related thrombosis.[4] Studies have shown that nearly 80% of patients with venous thromboembolism and cancer die within 6 months of the diagnosis of venous thromboembolism. The reason for this correlation between thrombosis and death in acute promyelocytic leukemia is that thrombosis is a surrogate marker for disease progression.
- Procoagulants: There is increased production of procoagulant molecules such as thrombin from cancer cells. Furthermore, mucins and cytokines produced by malignant promyelocytes can induce endothelial cells to increase tissue factor production, and tissue factor functions in the extrinsic pathway to promote coagulation.
- Platelets: There is a increased platelet activation in acute promyelocytic leukemia.
- Fibrin: There is decreased fibrinolytic activity in acute promyelocytic leukemia, and this results in presence of excess fibrin. Fibrin is also known as factor I of the coagulation cascade and functions to binds platelets together via their GpIIb/IIIa receptors. This is one of the final steps in coagulation.
- Natural anticoagulants: There is decreased production of natural anticoagulants, and this results in increased propensity for thrombosis.
- Catheters: Central venous catheters can serve as a nidus for thrombosis since there is localized tissue and endothelial damage at the site of catheter insertion. and along the catheter within the body.[4] Patients with acute promyelocytic leukemia are more likely to have central venous catheters, compared to patients with other conditions, since chemotherapy usually requires the presence of a central catheter to be placed.
- Immobility: Patients with acute promyelocytic leukemia are frequently confined to a hospital bed during induction therapy, and venous stasis contributes to thrombosis. Obesity can also contribute to thrombosis.
- Erythropoiesis-stimulating agents: Patients with acute promyelocytic leukemia frequently have anemia. Some patients receive erythropoiesis-stimulating agents, such as erythropoietin, which can increase red blood cell production and exacerbate thrombotic complications.
In a 2015 study from MD Anderson Cancer Center, it was shown that the annual incidence of venous thromboembolism, which includes deep vein thrombosis and pulmonary embolism, was 6.1-42%, which is the highest amongst all leukemia subtypes.[4] In contrast, the incidence of venous thromboembolism in chronic myeloid leukemia was only 1.5%.
| Disease | Thrombotic Incidence |
|---|---|
|
Acute myeloid leukemia |
3.7% |
|
Acute promyelocytic leukemia |
6.1-48% |
|
Chronic lymphocytic leukemia |
2.7% |
|
Acute lymphoblastic leukemia |
2.1-13% |
|
Chronic myeloid leukemia |
1.5% |
- Therapy-related complications:: Treatment of acute myeloid leukemia can result in a variety of complications which are somewhat unique to the disease.
- Rash: Cytarabine-related rash is very common after induction chemotherapy. This can be treated with corticosteroids.
- Cerebellar toxicity: Patients treated with high-dose cytarabine (greater than 2-3g/m2) can develop cerebellar toxicity and thus require routine neurologic exams during consolidation chemotherapy.
- Conjunctival toxicity: Patients treated with high-dose cytarabine (greater than 2-3g/m2) can develop conjunctivitis and thus require routine ocular exams and prophylactic steroid eye drops during consolidation chemotherapy.
- Cardiomyopathy: Patients receiving chemotherapy with anthracyclines, such as idarubicin or daunorubicin, are at risk for short-term cardiac-related complications such as arrhythmias and long-term cardiac-related complications such as systolic dysfunction and heart failure. The highest risk of these complications occurs in patients with underlying cardiomyopathy such as congestive heart failure, atrial fibrillation, or other cardiac issues. The cardiotoxicity of anthracyclines is dose-dependent and generally irreversible.
- Differentiation syndrome: In patients with relapsed or refractory acute myeloid leukemia harboring the IDH2 mutation treated with enasidenib, differentiation syndrome can result. The incidence of differentiation syndrome overall after enasidenib treatment is about 10%, and the incidence of grade 3 or higher differentiation syndrome is 7%. It also occurs in patients with acute promyelocytic leukemia after treatment with all-trans retinoic acid.[5] This condition is characterized by weight gain, peripheral edema, hypoxia, dyspnea, renal failure, fever, and hypotension. The syndrome is thought to be due to systemic inflammation induced by the release of cytokines from malignant promyelocytes. This results in endothelial cell damage with resultant capillary leakage. Malignant promyelocytes are then able to adhere to tissue that is perfused by the microcirculation.[5] Patients with a high white blood cell count are at highest risk for differentiation syndrome, since all-trans retinoic acid will result in release of a large amount of cytokines if there is a high leukemia burden. Differentiation syndrome is a major complication that must be recognized early on, such that proper corrective measures can be taken. These include the use of dexamethasone 10mg PO twice daily, plus supportive treatment for any underlying respiratory distress. Diruesis may be needed to help eliminate excess fluid accumulation.
- QT interval prolongation: In patients with relapsed or refractory acute myeloid leukemia harboring the IDH1 mutation treated with ivosidenib, QT interval prolongation occurs in approximately 8% of patients. In patients with acute promyelocytic leukemia, arsenic trioxide can result in prolonged QT interval, which carries a risk for cardiac-related complications such as arrhythmias. Patients who are treated with arsenic trioxide must have routine electrocardiograms (EKGs) done to ensure that the corrected QT interval remains less than 500 milliseconds. In patients who are treated with concomitant chemotherapy and arsenic trioxide, such as patients with high-risk acute promyelocytic leukemia, there is a higher risk for cardiac-related complications. Chemotherapy and intravenous fluids can alter electrolyte such as potassium levels. Hypokalemia (low potassium) can exacerbate QT prolongation.
Complications
Complications may include:
- Infections
- Disseminated intravascular coagulation
- Pyoderma gangrenosum
- Hemorrhage
- Complications due to side effects of chemotherapy
Prognosis
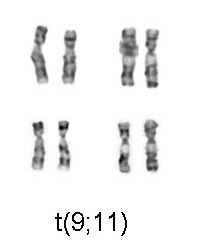
Acute myeloid leukemia is a curable disease; the chance of cure for a specific patient depends on a number of prognostic factors.[6]
Cytogenetics and prognosis in acute myeloid leukemia
The first publication to address cytogenetics and prognosis was the MRC trial of 1998:[7]
In this table we can see the staging concerning the risk category of acute myeloid leukemia cytogenetics.
| Risk Category | Abnormality | 5-year survival | Relapse rate |
|---|---|---|---|
| Favorable | t(8;21), t(15;17), inv(16) | 70% | 33% |
| Intermediate | Normal, +8, +21, +22, del(7q), del(9q), Abnormal 11q23, all other structural or numerical changes | 48% | 50% |
| Adverse | -5, -7, del(5q), Abnormal 3q, Complex cytogenetics | 15% | 78% |
Later, the Southwest Oncology Group and Eastern Cooperative Oncology Group,[11] and later still, Cancer and Leukemia Group B published other, mostly overlapping lists of cytogenetics prognostication in leukemia[12]
Cytogenetics that indicate a good prognosis
- t(8; 21)[13]
- inv(16) or t(16;16)
- t(15;17)
Cytogenetics That Indicate Poor Prognosis
- Factors that indicate poor prognosis in patients with acute myeloid leukemia, include:[13]
- Deletions of the long arms or monosomies of chromosomes 5 or 7
- Translocations or inversions of chromosome 3
- Translocations or inversions of t(6; 9), t(9; 22)
- Abnormalities of chromosome 11q23
- Translocations or inversions of (1;22)(p13;q13) (M7)
- Note: Normal cytogenetics portends average-risk of acute myeloid leukemia.
Antecedent MDS and prognosis
Acute myeloid leukemia which arises from a pre-existing myelodysplastic syndrome or myeloproliferative disease (so-called secondary AML) has a worse prognosis, as does treatment-related AML arising after chemotherapy for another previous malignancy. Both of these entities are associated with a high rate of unfavorable cytogenetic abnormalities.[14][15][16]
Other prognostic markers
In some studies, age > 60 years and elevated lactate dehydrogenase level were also associated with poorer outcomes.[17] As with most forms of cancer, performance status (i.e. the general physical condition and activity level of the patient) plays a major role in prognosis as well.
- FLT3 internal tandem duplications (ITDs) have been shown to confer a poorer prognosis in acute myeloid leukemia.[18]
- Treating these patients with more aggressive therapy, such as stem-cell transplantation in first remission, has not been shown to enhance long-term survival, so this prognostic feature is of uncertain clinical significance at this point.[19]
- Researchers are investigating the clinical significance of c-KIT mutations[20] in acute myeloid leukemia. These are prevalent, and clinically relevant because of the availability of tyrosine kinase inhibitors, such as sunitinib and imatinib that can block the activity of c-KIT pharmacologically.
- Other genes being investigated as prognostic factors or therapeutic targets include CEBPA, BAALC, ERG, and NPM1.
Overall expectation of cure
- Cure rates in clinical trials have ranged from 20–45%;[21][22] however, it should be noted that clinical trials often include only younger patients and those able to tolerate aggressive therapies. The overall cure rate for all patients with acute myeloid leukemia (including the elderly and those unable to tolerate aggressive therapy) is likely lower. Cure rates for promyelocytic leukemia can be as high as 98%.[23]
Survival
- More than 25% of adults with acute myeloid leukemia (about 45% of those who attain CR) can be expected to survive 3 or more years and may be cured.
- Remission rates in adult acute myeloid leukemia are inversely related to age, with an expected remission rate of more than 65% for those younger than 60 years.
- Data suggest that once attained, duration of remission may be shorter in older patients.
5-Year Survival
- Between 2004 and 2010, the 5-year relative survival of patients with acute myeloid leukemia was 25.4%.[24]
- When stratified by age, the 5-year relative survival of patients with acute myeloid leukemia was 42.8% and 5.6% for patients <65 and ≥ 65 years of age respectively.[24]
References
- ↑ Franchini M, Lippi G, Manzato F (2006). "Recent acquisitions in the pathophysiology, diagnosis and treatment of disseminated intravascular coagulation". Thromb J. 4: 4. doi:10.1186/1477-9560-4-4. PMC 1402263. PMID 16504043.
- ↑ Chen C, Huang X, Wang K, Chen K, Gao D, Qian S (2018). "Early mortality in acute promyelocytic leukemia: Potential predictors". Oncol Lett. 15 (4): 4061–4069. doi:10.3892/ol.2018.7854. PMC 5835847. PMID 29541170.
- ↑ 3.0 3.1 Coombs CC, Tavakkoli M, Tallman MS (2015). "Acute promyelocytic leukemia: where did we start, where are we now, and the future". Blood Cancer J. 5: e304. doi:10.1038/bcj.2015.25. PMC 4450325. PMID 25885425.
- ↑ 4.0 4.1 4.2 Vu K, Luong NV, Hubbard J, Zalpour A, Faderl S, Thomas DA; et al. (2015). "A retrospective study of venous thromboembolism in acute leukemia patients treated at the University of Texas MD Anderson Cancer Center". Cancer Med. 4 (1): 27–35. doi:10.1002/cam4.332. PMC 4312115. PMID 25487644.
- ↑ 5.0 5.1 McCulloch D, Brown C, Iland H (2017). "Retinoic acid and arsenic trioxide in the treatment of acute promyelocytic leukemia: current perspectives". Onco Targets Ther. 10: 1585–1601. doi:10.2147/OTT.S100513. PMC 5359123. PMID 28352191.
- ↑ Estey E (2001). "Prognostic factors in acute myelogenous leukemia". Leukemia. 15 (4): 670–2. PMID 11368376.
- ↑ Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, Rees J, Hann I, Stevens R, Burnett A, Goldstone A. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998 Oct 1;92(7):2322–33.
- ↑ Wheatley K, Burnett A, Goldstone A, Gray R, Hann I, Harrison C, Rees J, Stevens R, Walker H (1999). "A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. United Kingdom Medical Research Council's Adult and Childhood Leukaemia Working Parties". Br J Haematol. 107 (1): 69–79. PMID 10520026.
- ↑ Slovak M, Kopecky K, Cassileth P, Harrington D, Theil K, Mohamed A, Paietta E, Willman C, Head D, Rowe J, Forman S, Appelbaum F (2000). "Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study". Blood. 96 (13): 4075–83. PMID 11110676.
- ↑ Byrd J, Mrózek K, Dodge R, Carroll A, Edwards C, Arthur D, Pettenati M, Patil S, Rao K, Watson M, Koduru P, Moore J, Stone R, Mayer R, Feldman E, Davey F, Schiffer C, Larson R, Bloomfield C (2002). "Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461)". Blood. 100 (13): 4325–36. PMID 12393746.
- ↑ Slovak ML; Kopecky KJ; Cassileth PA; Harrington DH; Theil KS; Mohamed A; Paietta E; Willman CL; Head DR; Rowe JM; Forman SJ; Appelbaum FR Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood 2000 Dec 15;96(13):4075–83.
- ↑ Byrd J, Mrózek K, Dodge R, Carroll A, Edwards C, Arthur D, Pettenati M, Patil S, Rao K, Watson M, Koduru P, Moore J, Stone R, Mayer R, Feldman E, Davey F, Schiffer C, Larson R, Bloomfield C (2002). "Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461)". Blood. 100 (13): 4325–36. PMID 12393746.
- ↑ 13.0 13.1 "National Cancer Institute".
- ↑ Thirman M, Larson R (1996). "Therapy-related myeloid leukemia". Hematol Oncol Clin North Am. 10 (2): 293–320. PMID 8707757.
- ↑ Rowley J, Golomb H, Vardiman J (1981). "Nonrandom chromosome abnormalities in acute leukemia and dysmyelopoietic syndromes in patients with previously treated malignant disease". Blood. 58 (4): 759–67. PMID 7272506.
- ↑ Pedersen-Bjergaard J, Andersen M, Christiansen D, Nerlov C (2002). "Genetic pathways in therapy-related myelodysplasia and acute myeloid leukemia". Blood. 99 (6): 1909–12. PMID 11877259.
- ↑ Haferlach T, Schoch C, Löffler H, Gassmann W, Kern W, Schnittger S, Fonatsch C, Ludwig W, Wuchter C, Schlegelberger B, Staib P, Reichle A, Kubica U, Eimermacher H, Balleisen L, Grüneisen A, Haase D, Aul C, Karow J, Lengfelder E, Wörmann B, Heinecke A, Sauerland M, Büchner T, Hiddemann W (2003). "Morphologic dysplasia in de novo acute myeloid leukemia (AML) is related to unfavorable cytogenetics but has no independent prognostic relevance under the conditions of intensive induction therapy: results of a multiparameter analysis from the German AML Cooperative Group studies". J Clin Oncol. 21 (2): 256–65. PMID 12525517.
- ↑ Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C, Löffler H, Sauerland C, Serve H, Büchner T, Haferlach T, Hiddemann W (2002). "Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease". Blood. 100 (1): 59–66. PMID 12070009.
- ↑ Gale R, Hills R, Kottaridis P, Srirangan S, Wheatley K, Burnett A, Linch D (2005). "No evidence that FLT3 status should be considered as an indicator for transplantation in acute myeloid leukemia (AML): an analysis of 1135 patients, excluding acute promyelocytic leukemia, from the UK MRC AML10 and 12 trials". Blood. 106 (10): 3658–65. PMID 16076872.
- ↑ Paschka P, Marcucci G, Ruppert A, Mrózek K, Chen H, Kittles R, Vukosavljevic T, Perrotti D, Vardiman J, Carroll A, Kolitz J, Larson R, Bloomfield C (2006). "Adverse prognostic significance of KIT mutations in adult acute myeloid leukemia with inv(16) and t(8;21): a Cancer and Leukemia Group B Study". J Clin Oncol. 24 (24): 3904–11. PMID 16921041.
- ↑ Cassileth P, Harrington D, Appelbaum F, Lazarus H, Rowe J, Paietta E, Willman C, Hurd D, Bennett J, Blume K, Head D, Wiernik P (1998). "Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukemia in first remission". N Engl J Med. 339 (23): 1649–56. PMID 9834301.
- ↑ Matthews J, Bishop J, Young G, Juneja S, Lowenthal R, Garson O, Cobcroft R, Dodds A, Enno A, Gillett E, Hermann R, Joshua D, Ma D, Szer J, Taylor K, Wolf M, Bradstock K (2001). "Patterns of failure with increasing intensification of induction chemotherapy for acute myeloid leukaemia". Br J Haematol. 113 (3): 727–36. PMID 11380464.
- ↑ Sanz M, Lo Coco F, Martín G, Avvisati G, Rayón C, Barbui T, Díaz-Mediavilla J, Fioritoni G, González J, Liso V, Esteve J, Ferrara F, Bolufer P, Bernasconi C, Gonzalez M, Rodeghiero F, Colomer D, Petti M, Ribera J, Mandelli F (2000). "Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups". Blood. 96 (4): 1247–53. PMID 10942364.
- ↑ 24.0 24.1 Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2011, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission, posted to the SEER web site, April 2014.