Gingivitis
| Gingivitis | |
 | |
|---|---|
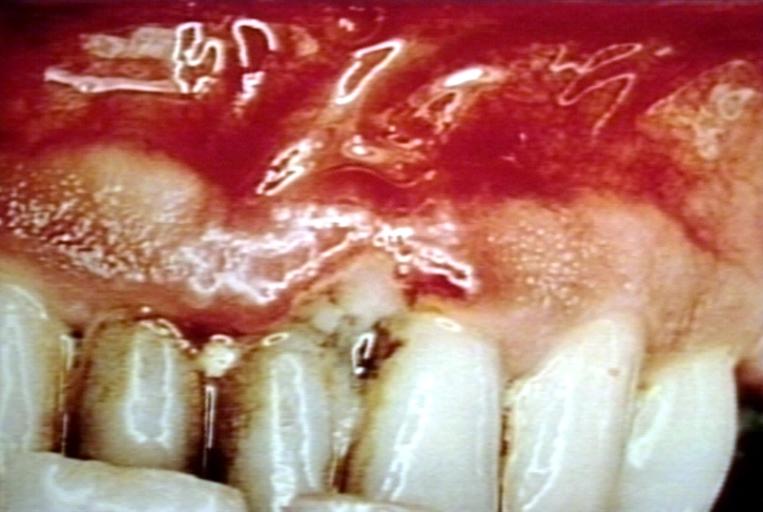
| Trench mouth. Necrotizing gingivitis Image courtesy of Professor Peter Anderson DVM PhD and published with permission. © PEIR, University of Alabama at Birmingham, Department of Pathology | |
| ICD-10 | K05.0-K05.1 |
|
WikiDoc Resources for Gingivitis |
|
Articles |
|---|
|
Most recent articles on Gingivitis |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Gingivitis at Clinical Trials.gov Clinical Trials on Gingivitis at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Gingivitis
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Gingivitis Discussion groups on Gingivitis Patient Handouts on Gingivitis Directions to Hospitals Treating Gingivitis Risk calculators and risk factors for Gingivitis
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Gingivitis |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ogheneochuko Ajari, MB.BS, MS [2] Jaspinder Kaur, MBBS[3]
Synonyms and keywords:
Overview
Gingivitis ("inflammation of the gums") is a terminology referring to the gingival inflammation induced by bacterial biofilms (also called plaque) adherent to tooth surfaces. It is characterized by a site-specific and reversible dental plaque‑induced inflammation of the gingiva without detectable bone loss or clinical attachment loss. It is frequently encountered in dental practice affecting people of all ages from children, adolescents to adults. The etiology of gingivitis is multi‑factorial and usually synergistic effect of more than one factor acting together which includes presence of bacteria biofilm, genetic, socioeconomic, demographic, iatrogenic, and behavioral factors. These plethora of factors seem to influence the process; thus, making it difficult to identify the risk factors. The most important factor that has been associated with gingivitis is plaque accumulation on the dental surface, resulting in an inflammatory reaction that were initially edematous and become more fibrotic as the condition persists. The earliest clinical sign of gingival inflammation is the transudation of gingival fluid. This thin and almost a cellular transudate is gradually supersceded by a fluid consisting of serum plus leucocytes. The redness of the gingival margin arises partly from the aggregation and enlargement of blood vessels in the immediate subepithelial connective tissue and the loss of keratinization of the facial aspects of gingiva. However, gingivitis is commonly painless, rarely leads to spontaneous bleeding, and is often characterized by subtle clinical changes, resulting in most patients being unaware of the disease or unable to recognize it. It holds a particular clinical significance because it is considered the precursor of periodontitis, a disease characterized by gingival inflammation combined with connective tissue attachment and bone loss. However, it is a reversible disease. Therapy is aimed primarily at reduction of etiologic factors to reduce or eliminate inflammation, thereby allowing gingival tissues to heal. Appropriate supportive periodontal maintenance that includes personal and professional care is important in preventing re-initiation of inflammation. Simple gingivitis is controlled by proper oral hygiene with or without an antibacterial mouth rinse and thorough scaling via professional cleaning with hand or ultrasonic instruments.
Classification
The 2017 World Workshop has classified the gingival diseases into two broad categories.
Table 1: Classification of the gingivitis
| Periodontal Health |
|---|
|
| Gingivitis—Dental Plaque-induced |
|
| Gingival Disease—Non-dental Plaque-induced |
|
Stages
The condition gingivitis undergoes through four different stages before progressing to periodontitis if not treated. The different stages of gingivitis were first explained by Page and Schroeder in 1976.
Table 2: Progression of the gingivitis through different level of stages
| Stage | Differentiating features |
|---|---|
| Initial: 24-48 hours |
|
| Early: 4-7 days |
|
| Established: 2-3 weeks |
|
| Advanced |
|
Pathophysiology
- Gingivitis is usually caused by bacterial plaque that accumulates in the spaces between the gums and the teeth and in calculus (tartar) that forms on the teeth.
- When the teeth are not cleaned properly by regular brushing and flossing, bacterial plaque accumulates, and becomes mineralized by calcium and other minerals in the saliva transforming it into a hard material called calculus (tartar) which harbors bacteria and irritates the gingiva (gums).
- As the bacterial plaque biofilm becomes thicker this creates an anoxygenic environment which allows more pathogenic bacteria to flourish and release toxins and cause gingival inflammation.
- Alternatively, excessive injury to the gums caused by very vigorous brushing may lead to recession, inflammation and infection.
- This inflammation can cause deep pockets over the years between the teeth and gums and loss of bone around teeth otherwise known as periodontitis.
- The superseded infection usually begins when the immune system of the body gets reduced due to some local or systemic conditions.
Local factors
- Crowding of teeth makes the plaque removal difficult.
- Malaligned teeth often require orthodontic correction, which adds on to the difficulty in cleansing.
- A dental prosthesis that does not have an adequate fit or is not properly finished can act as a nidus for plaque accumulation.
- In children, tooth eruption is also frequently associated with gingivitis as plaque accumulation tends to increase in the area where primary teeth are exfoliating, and permanent teeth are erupting as oral hygiene may be difficult to be maintained in these areas which is referred as eruption gingivitis.
Infectious gingivitis
- It can occur as a reaction to some low-grade injury to the local tissues such as fractured teeth, overhanging restorations, overextended flanges of the denture, and faulty fixed dental prosthesis with poor pontic design (saddle pontic) or over contoured margins.
Hypersensitive reaction
- An allergen in the form of chewing gum, certain components of toothpaste, cinnamon, mint, red pepper, etc. can trigger the infiltration of plasma cells in the gingiva and causing plasma cell gingivitis.
Nutritional gingivitis
- Modern lifestyle with the intake of an increased amount of refined carbohydrates and an increased ratio of omega-6 to omega-3 fatty acids can initiate the inflammatory process. The mechanism by which carbohydrates with a high glycemic index promotes inflammatory process is through activation of NFkB and oxidative stress.
Hormonal gingivitis
- This form of gingivitis occurs during pregnancy, puberty, or steroid therapy.
- Pregnancy: An increase in the level of circulating female sex hormones are responsible for causing pregnancy gingivitis.
- Puberty: Gingival inflammation occurs even without the presence of plaque. This is referred to as puberty gingivitis. It has been observed that during adolescence, gingivitis appears earlier in girls (eleven to thirteen years) than in boys (thirteen to fourteen years).
It has been found that in the cytoplasm of the cells of the gingiva, receptors for both estrogens and testosterone that have a high affinity for these hormones are present. The receptors for estrogen are specifically present in the basal and spinous layers of the epithelium. In the connective tissue, such receptors are found in the fibroblasts and endothelial cells of small vessels. Therefore, the gingiva is an easy target organ for these steroid hormones resulting in gingivitis.
Drug induced gingivitis
- The mechanism behind this gingival inflammation is thought to be the ability of the metabolites of these drugs to induce the proliferation of fibroblasts.
- An imbalance between the synthesis and the degradation of the extracellular matrix leads to the accumulation of immature proteins in the extracellular matrix, particularly collagen which subsequently results in gingivitis.
Causes
Life Threatening Causes
Life-threatening causes include conditions which may result in death or permanent disability within 24 hours if left untreated.
Common Causes
Causes by Organ System
Table 3: System wise causative factors of the gingivitis
Causes in Alphabetical Order
Table 4: Alphabetical presentation of the causative factors of the gingivitis
| A | B | C | D | E |
|---|---|---|---|---|
| F | G | H | I | K |
| L | M | N | O | P |
| R | S | T | V | Z |
Differentiating Gingivitis from other Diseases
Table 5: Enumerate the conditions mimicking the gingivitis
| Differentiating condition | Differentiating sign and symptoms | Differentiating features |
|---|---|---|
| Oral lichen planus |
|
|
| Pemphigoid |
|
|
| Pemphigus |
|
|
| Lupus erythematosus |
|
|
| Desquamative gingivitis |
|
|
| Drug-influenced gingival enlargement |
|
|
| Primary herpetic gingivostomatitis |
|
|
| Allergic reactions |
|
|
| Leukemia |
|
|
| Gingival candidosis |
|
|
| Primary and metastatic carcinoma |
|
|
| Foreign body gingivitis |
|
|
| Orofacial granulomatosis |
|
|
| Pyostomatitis vegetans |
|
|
| Linear IgA disease |
|
|
| Wegener granulomatosis |
|
|
| Erythema multiforme |
|
|
| Agranulocytosis |
|
|
| Histoplasmosis |
|
|
| Cyclic neutropenia |
|
|
Epidemiology and Demographics
- Gingivitis is the commonest periodontal disease that is found to be more prevalent in males as compared to females as it has been found that females tend to follow better oral care regimes and thus in maintaining oral hygiene.
- Women: There have been studies that found gingivitis to be more prevalent in pregnant women as compared to non-pregnant women. Also, the severe form of gingivitis has been found to be predominant in pregnant women.
- It is commonly seen in children and adults and prevalent worldwide. .
- Young population: Gingivitis occurs in half the population by the age of 4 or 5 years, and the incidence continues to increase with age. The prevalence of gingivitis peaks at close to 100% at puberty, but after puberty it declines slightly and stays constant into adulthood. Some children exhibit severe gingivitis at puberty. Puberty-associated gingivitis is related to increases in steroid hormones.
- Socioeconomic status: Studies have found gingivitis to be more prevalent in people with low socioeconomic status as people with high socioeconomic status tend to show a more positive attitude towards the maintenance of oral hygiene. Also, they have better access to health care options.
Risk Factors
- Risk factor is defined as an environmental, behavioral, or biologic factor which directly increases the probability of a disease occurring and if absent or removed, reduces the disease probability.
| Modifiable risk factors | Non-modifiable risk factors |
|---|---|
|
|
Complications
- Recurrence of gingivitis
- Periodontitis
- Infection or abscess of the gingiva or the jaw bones
- Trench mouth (bacterial infection and ulceration of the gums)
Acute Necrotizing Ulcerative Gingitivitis (ANUG or Trench mouth)
- Chronic gingivitis can progress to ANUG if not treated and when the patient neglects oral hygiene completely or when the immune system of the patient is compromised.
- The condition is commonly seen in developing countries where the living conditions are poor.
- IT occurs most frequently in smokers and debilitated patients who are under stress. Other risk factors are poor oral hygiene, nutritional deficiencies, immunodeficiency (eg, HIV/AIDS, use of immunosuppressive drugs), and sleep deprivation. Some patients also have oral candidiasis.
- The aetiology of ANUG is the overgrowth of a particular type of pathogenic bacteria (fusiform-spirochete variety) but risk factors such as stress, poor nutrition and a compromised immune system can exacerbate the infection
- It is categorized under a severe form of gingivitis associated with pain, gingival bleeding, and ulceration. It is characterized by marked gingival edema, spontaneous bleeding, or bleeding in response to minimal local trauma. It may be associated with localized pain, altered taste (metallic taste mostly), and halitosis.
- Ulcerations, which are pathognomonic, are present on the dental papillae and marginal gingiva. These ulcerations have a characteristically punched-out appearance and are covered by a gray pseudomembrane. Similar lesions on the buccal mucosa and tonsils are rare. Swallowing and talking may be painful. Regional lymphadenopathy often is present.
- Treatment: It includes debridement, rinses (eg, hydrogen peroxide, chlorhexidine) and improved oral hygiene. If debridement is delayed (eg, if a dentist or the instruments necessary for debridement are unavailable), oral antibiotics (eg, amoxicillin 500 mg every 8 hours, erythromycin 250 mg every 6 hours, or tetracycline 250 mg every 6 hours) may help to provide relief and can be continued until 72 hours after symptoms resolve.
Prognosis
- Gingivitis, if identified and treated, can easily be resolved as the condition is reversible in its early stages.
- However, chronic gingivitis, if left untreated, can progress to periodontitis and can ultimately result in bone destruction, causing tooth loss.
Clinical presentation
- Onset: It can be acute or chronic, and can be either localized or generalized which is categorized as follows:
- Marginal gingivitis: An inflammation confined to the gingival margin.
- Papillary gingivitis: It involves interdental papillae.
- Diffuse gingivitis: It has diffuse involvement of the gingival margin, attached gingiva, and interdental papillae.
Clinical symptoms
The symptoms of gingivitis are as follows:
- Swollen gums
- Mouth sores
- Bright-red, or purple gums
- Shiny gums
- Gums that are painless, except when pressure is applied
- Gums that bleed easily, even with gentle brushing,and especially when you floss
- Gums that itch with varying degrees of severity
- Receding gumline
Clinical signs
| Medical condition | Clinical signs on examination |
|---|---|
| Bacterial dental biofilm only |
|
| Plaque-induced gingivitis |
|
| Puberty |
|
| Menstrual cycle |
|
| Pregnancy |
|
| Oral contraceptives |
|
| Hyperglycemia |
|
| Leukemia |
|
| Smoking |
|
| Malnutrition |
|
| Prominent subgingival restoration margins |
|
| Hyposalivation |
|
| Drug-influenced gingival enlargements |
|
Diagnosis
- A detailed history taking and physical examination (Table) should be performed.
- Clinical evaluation: Finding erythematous and friable tissue at the gum lines confirms the diagnosis of gingivitis. To detect early gingival disease, some dentists frequently measure the depth of the pocket around each tooth. Depths < 3 mm are normal; deeper pockets are at high risk of gingivitis and periodontitis.
- Laboratory test: Not routinely required.
- Radiographs: As gingivitis is a soft tissue disease, radiographic evaluation is not helpful. However, it should be done to rule out periodontitis or other differential disorder.
Treatment
- Treatment approach: An interprofessional approach is required to identify the causes of gingivitis and to intervene at an early stage before its progression to periodontitis.
- Aim: To restore the inflamed tissues to clinical health and then to maintain clinically healthy gingivae and subsequently preventing periodontitis. Periodontitis has also been linked to diabetes, arteriosclerosis, osteoporosis, pancreatic cancer and pre-term low birth weight babies.
- Stepwise approach:
- A dentist or dental hygienist will perform a thorough cleaning of the teeth and gums and remove localized factors initiating the inflammatory response. This includes scaling to thoroughly remove biofilm and deposits on the tooth structure and laser decontamination of the sulcus if possible. The removal of plaque is usually not painful, and the inflammation of the gums should be gone between one and two weeks.
- Ensure oral hygiene reinforcement by twice daily tooth brushing and once daily interdental cleaning (with an interdental brush or dental floss) and adjunctive chemical plaque control agents (such as chlorhexidine or essential oil-containing mouthwash).
- Address the modifiable systemic or local factors by changing the medication if drug induced; prescribing supplements in case of nutritional deficiency; and an indentification of faulty prosthesis should be done and replaced.
- In severe cases, patients can also be prescribed antibiotics.
Prevention
- Oral hygiene: Maintenance of a good oral hygiene can prevent the formation of plaque and gingivitis. Patients should be taught the correct brushing technique, frequency of brushing (twice daily) along with the use of floss.
- Brushing: Brushing after meals helps remove food debris and plaque trapped between your teeth and gums. Don’t forget to include your tongue, bacteria loves to hide there.
- Floss: Flossing at least once a day helps remove food particles and plaque between teeth and along the gum line that your toothbrush can’t quite reach.
- Swish with mouthwash: Mouthwash and gel containing antiseptic and anti-inflammatory properties can also be advised to the patient.
- Balanced diet: An importance of a balanced diet should be emphasized.
- Dentist visit: A routine cleaning by a dentist or hygienist at 6-month to 1-year intervals can help minimize gingivitis. Patients with systemic disorders predisposing to gingivitis require more frequent professional cleanings (from every 2 weeks to every 3 months).
- Know your risk: Age, smoking, diet and genetics can all increase your risk for periodontal disease. If you are at increased risk, be sure to talk with your dental professional.
References
External links
- Gingivitis - Medline plus
Template:Periodontology
Template:Oral pathology
zh-min-nan:Khí-hoāⁿ-iām
de:Gingivitis
el:Ουλίτιδα
it:Gengivite
nl:Tandvleesontsteking
simple:Gingivitis
fi:Ientulehdus
sv:Gingivit