Triglyceride
|
WikiDoc Resources for Triglyceride |
|
Articles |
|---|
|
Most recent articles on Triglyceride Most cited articles on Triglyceride |
|
Media |
|
Powerpoint slides on Triglyceride |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Triglyceride at Clinical Trials.gov Clinical Trials on Triglyceride at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Triglyceride
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Triglyceride Discussion groups on Triglyceride Patient Handouts on Triglyceride Directions to Hospitals Treating Triglyceride Risk calculators and risk factors for Triglyceride
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Triglyceride |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
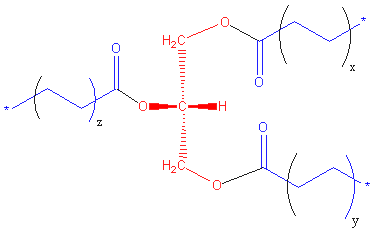
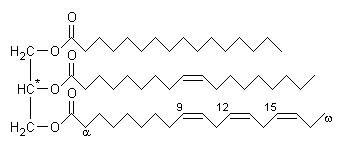
Triglyceride (more properly known as triacylglycerol, TAG or triacylglyceride) is glyceride in which the glycerol is esterified with three fatty acids.[1] It is the main constituent of vegetable oil and animal fats.
Chemical structure
The chemical formula is RCOO-CH2CH(-OOCR')CH2-OOCR", where R, R', and R" are longer alkyl chains. The three fatty acids RCOOH, R'COOH and R"COOH can be all different, all the same, or only two the same.
Chain lengths of the fatty acids in naturally occurring triglycerides can be of varying lengths but 16, 18 and 20 carbons are the most common. Natural fatty acids found in plants and animals are typically composed only of even numbers of carbon atoms due to the way they are bio-synthesised from acetyl CoA. Bacteria, however, possess the ability to synthesise odd- and branched-chain fatty acids. Consequently, ruminant animal fat contains odd numbered fatty acids, such as 15, due to the action of bacteria in the rumen.
Most natural fats contain a complex mixture of individual triglycerides; because of this, they melt over a broad range of temperatures. Cocoa butter is unusual in that it is composed of only a few triglycerides, one of which contains palmitic, oleic and stearic acids in that order. This gives rise to a fairly sharp melting point, causing chocolate to melt in the mouth without feeling greasy.
Metabolism
- See also fatty acid metabolism
Triglycerides, as major components of very low density lipoprotein (VLDL) and chylomicrons, play an important role in metabolism as energy sources and transporters of dietary fat. They contain more than twice as much energy (9 kcal/g) as carbohydrates and proteins. In the intestine, triglycerides are split into glycerol and fatty acids (this process is called lipolysis) (with the help of lipases and bile secretions), which are then moved into the cells lining the intestines (absorptive enterocytes). The triglycerides are rebuilt in the enterocytes from their fragments and packaged together with cholesterol and proteins to form chylomicrons. These are excreted from the cells and collected by the lymph system and transported to the large vessels near the heart before being mixed into the blood. Various tissues can capture the chylomicrons, releasing the triglycerides to be used as a source of energy. Fat and liver cells can synthesize and store triglycerides. When the body requires fatty acids as an energy source, the hormone glucagon signals the breakdown of the triglycerides by hormone-sensitive lipase to release free fatty acids. As the brain can not utilize fatty acids as an energy source, the glycerol component of triglycerides can be converted into glucose, via gluconeogenesis, for brain fuel when it is broken down. Fat cells may also be broken down for that reason, if the brain's needs ever outweigh the body's.
Triglycerides cannot pass through cell membranes freely. Special enzymes on the walls of blood vessels called lipoprotein lipases must breakdown triglycerides into fatty acids and glycerol. Fatty acids can then be taken up by cells via the fatty acid transporter (FAT).
Role in disease
- See also the main article hypertriglyceridemia
In the human body, high levels of triglycerides in the bloodstream have been linked to atherosclerosis, and, by extension, the risk of heart disease and stroke. The negative impact of raised levels of triglycerides is higher than that of LDL:HDL ratios. The risk can be partly accounted for by a strong inverse relationship between triglyceride level and HDL-cholesterol level.
Another disease caused by high triglycerides is pancreatitis.
Guidelines
The American Heart Association has set guidelines for triglyceride levels:[2]
| Level mg/dL | Level mmol/L | Interpretation |
| <150 | <1.69 | Normal range, low risk |
| 150-199 | 1.70-2.25 | Borderline high |
| 200-499 | 2.26-5.65 | High |
| >500 | >5.65 | Very high: high risk |
Please note that this information is relevant to triglyceride levels as tested after fasting 8 to 12 hours. Triglyceride levels remain temporarily higher for a period of time after eating.
When some fatty acids are converted to ketone bodies, overproduction can result in ketoacidosis in diabetics.
Reducing triglyceride levels
Moderating the consumption of fats, alcohol and carbohydrates and partaking of aerobic exercise are considered essential to reducing triglyceride levels. Omega-3 fatty acids from fish, flax seed oil or other sources, (up to 3g per day in US, but up to 2g in Europe where it should be associated with Omega-6 with a ideal ω_6/ω_3 ratio near 5, unless under physician care)[3], Omega-6 fatty acids, one or more grams of niacin (mega-dose vitamin B-3) per day and some statins reduce triglyceride levels.
Unlike Japan, it is generally admitted that most populations in western countries are lacking omega-3 nutritional sources. As a result ingesting of excessively high levels of saturated or monounsaturated fatty acids in order to assimilate enough omega-6 fatty acids is common. The ideal ratio ω_6/ω_3 = 5 is almost never met and is most often too high (about 12 in France, up to 80 in the caucasian population of the US and Canada), and unused high levels of saturated or monounsaturated fatty acids accumulate in the body in the form of triglycerides that do not participate in the needed syntheses in the body.
In some cases Fibrates have been used as they can bring down TGs substantially. However they are not used as a first line measure as they can have unpleasant or dangerous side effects. In one case due to an increase in mortality, clofibrate was withdrawn from the North American market.
Alcohol abuse can cause elevated levels of triglycerides.
Triglycerides are formed from a single molecule of glycerol, combined with three fatty acids on each of the OH groups, and make up most of fats digested by humans. A triglyceride is shown in the diagram[2].
Ester bonds form between each fatty acid and the glycerol molecule.
This is where the enzyme pancreatic lipase acts, hydrolysing the bond and ‘releasing’ the fatty acid.
In triglyceride form, lipids cannot be absorbed by the duodenum. Fatty acids, monoglycerides (one glycerol, one fatty acid) and some diglycerides are absorbed by the duodenum, once the triglycerides have been broken down.
Industrial uses
Triglycerides are also split into their components via transesterification during the manufacture of biodiesel. The fatty acid monoalkyl ester can be used as fuel in diesel engines. The glycerin has many uses, such as in the manufacture of food and in the production of pharmaceuticals.
Other examples are the Triglyceride process in the decaffeination of coffee beans.
Staining
Staining for fatty acids, triglycerides, lipoproteins, and other lipids is done through the use of lysochromes (fat-soluble dyes). These dyes can allow the qualification of a certain fat of interest by staining the material a specific color. Some examples: Sudan IV, Oil Red O, and Sudan Black B.
References
- ↑ "Nomenclature of Lipids". IUPAC-IUB Commission on Biochemical Nomenclature (CBN). Retrieved 2007-03-08.
- ↑ What Your Cholesterol Levels Mean
- ↑ Fish and Omega-3 Fatty Acids. American Heart Association.
See also
- Diglyceride acyltransferase - enzyme responsible for triglyceride biosynthesis
- Medium chain triglycerides
bg:Мазнина ca:Triacilglicèrid da:Triglycerid de:Triglyceride eo:Triglicerido hr:Trigliceridi id:Trigliserida it:Trigliceride he:טריגליצריד lt:Trigliceridas mk:Триглицерид nl:Triglyceride fi:Triglyseridi sv:Triglycerid tl:Trayglisirayd