Transposition of the great vessels: Difference between revisions
No edit summary |
No edit summary |
||
| Line 17: | Line 17: | ||
{{CMG}} | {{CMG}} | ||
'''Associate-Editors-In-Chief''': {{CZ}}; Keri Shafer, M.D. [mailto:kshafer@bidmc.harvard.edu] | '''Associate-Editors-In-Chief''': {{CZ}}; [[User:KeriShafer|Keri Shafer, M.D.]] [mailto:kshafer@bidmc.harvard.edu] | ||
{{Editor Join}} | {{Editor Join}} | ||
Revision as of 21:47, 7 January 2009
| Transposition of the great vessels | |
 | |
|---|---|
| Transposition of the Great Vessels; Aorta from Right Ventricle.; Pulmonary Artery from Left Ventricle; Patent Ductus Arteriosus. Image courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology | |
| ICD-10 | Q20.3 |
| ICD-9 | 745.1 |
| DiseasesDB | 13259 |
| eMedicine | ped/2548 |
| MeSH | D014188 |
| Cardiology Network |
 Discuss Transposition of the great vessels further in the WikiDoc Cardiology Network |
| Adult Congenital |
|---|
| Biomarkers |
| Cardiac Rehabilitation |
| Congestive Heart Failure |
| CT Angiography |
| Echocardiography |
| Electrophysiology |
| Cardiology General |
| Genetics |
| Health Economics |
| Hypertension |
| Interventional Cardiology |
| MRI |
| Nuclear Cardiology |
| Peripheral Arterial Disease |
| Prevention |
| Public Policy |
| Pulmonary Embolism |
| Stable Angina |
| Valvular Heart Disease |
| Vascular Medicine |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate-Editors-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]; Keri Shafer, M.D. [3]
Please Join in Editing This Page and Apply to be an Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [4] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Transposition of the great vessels (TGV) is a group of congenital heart defects (CHDs) involving an abnormal spatial arrangement of any of the primary blood vessels: superior and/or inferior vena cavae (SVC, IVC), pulmonary artery, pulmonary veins, and aorta. CHDs involving only the primary arteries (pulmonary artery and aorta) belong to a sub-group called transposition of the great arteries (TGA). The clinical signs and symptoms associated with TGV may range from a change in blood pressure to an interruption in circulation, depending on the nature and degree of the misplacement and which vessels are involved.
The term "TGV" is often used as a more specific reference to transposition of the great arteries TGA; however, TGA only relates to the aorta and the pulmonary artery, whereas TGV is a broader term which can relate to these vessels as well as the SVC, IVC, and pulmonary veins. In its strictest sense, transposition of vessels relates only to defects in which two or more vessels have "swapped" positions; in a broader sense, it may be taken to relate to any defect in which a vessel is in an abnormal position.
Although "transposed" literally means "swapped", many types of TGV involve vessels that are in abnormal positions, while not actually being swapped with each other. The terms TGV and TGA are most commonly used in reference to dextro-TGA (d-TGA) - in which the arteries are in swapped positions; however, both terms are also commonly used, though to a slightly lesser extent, in reference to levo-TGA (l-TGA) - in which both the arteries and the ventricles are swapped; while other defects in this category are almost never referred to by either of these terms. When no other heart defects are present it is called Simple TGV; when other defects are present it is called Complex TGV.
In TGA, the hallmark is ventriculoarterial discordance, in which the aorta (the main artery that carries blood to the body) arises from the morphologic right ventricle and the pulmonary artery (the artery that carries low oxygen blood to the lungs) arises from the morphologic left ventricle. This results in two separate and parallel circulations, which must communicate between them in order to survive after birth.
Most patients have an interatrial communication. Two-thirds have a patent ductus arteriosus, and about one-third have a ventricular septal defect VSD. Stenosis, or other defects, of valves and/or vessels may also be present.
History
The TGA was first described in 1797 by Matthew Baillie as a "singular malformation" and later in 1814 by Farre as "transposition". However, no treatment was available until the middle of the 20th century. In the 1950s, surgical atrial septectomy was developed followed by balloon atrial septostomy in the 1960s. These surgical therapies were not curative, but were palliative. They were later followed by physiological procedures. Today, the survival rate for infants with TGA is greater than 90%.
Anatomy
Normally, the morphologic right atrium (RA) is connected to a morphologic right ventricle (RV). The morphologic left atrium (LA) is connected to the morphologic left ventricle (LV). This is called atrio-ventricular concordance.
In a normal heart, the great arteries (aorta and pulmonary arteries) are concordant with the morphologic LV and RV. This is termed ventriculo-arterial concordance. In addition, the aorta and pulmonary trunk ascend in a spiral relationship.
In the TGA the aorta arises from the morphologic right ventricle via a subaortic infundibulum and the pulmonary artery arises from the morphologic left ventricle, without a subpulmonary infundibulum. These ventriculoarterial connection is known as ventriculoarterial discordance. As a consequence, there is a a fibrous continuity between the mitral and pulmonary valve, but no continuity between the tricuspid and aortic valve.
The abnormal origin of the great arteries results in an altered spiral relationship. Therefore, the aorta and pulmonary artery run parallel to each other, producing circulation functioning in parallel, instead of normal circulation functioning in series.
d-TGA is also referred to as complete or uncorrected transposition of the great arteries identifying the single discordance between ventricles and great arteries, whereas l-TGA is referred to as congenitally corrected transposition, identifying a double discordance (atrioventricular and ventriculoarterial).
Great vessel defects
The following defects also involve abnormal spatial and/or structural arrangement of the great vessels:
- Total anomalous pulmonary venous connection (TAPVC)
- Partial anomalous pulmonary Venous Connection (PAPVC)
- Coarctation of the aorta
- Cor triatriatum
- dextro-Transposition of the great arteries (d-TGA)
- Double outlet right ventricle (DORV)
- Hypoplastic left heart syndrome (HLHS)
- levo-Transposition of the great arteries (l-TGA)
- Overriding aorta
- Patent ductus arteriosus (PDA)
- Pulmonary atresia (PA)
- Unilateral or bilateral Pulmonary arteriovenous malformation (PAVM)
- Pulmonary sequestration
- Scimitar syndrome
- Sinus venosus atrial septal defect (SVASD)
- Situs inversus
- Taussig-Bing syndrome
- Tetralogy of Fallot (TOF)
- Truncus arteriosus (TA)
- Vascular rings
Epidemiology and Demographics
Transposition of the great arteries TGA is one of the most common cyanotic congenital heart defects present in the first 24 hours of life. TGA represents 5-7% of all CHD with an incidence at birth of 20-30 in 100000 live births. TGA is more common in infants of diabetic mothers. Boys outnumber girls with an approximate ratio of 2:1. Without treatment, 30% of infants die within the first week of life, 50% will die in the first month, 70% will die in the first 6 months and 90% of infants will die before the end of the first year. 4.9% of patients have associated extracardiac abnormalities.
Genetics
Generally, TGA is not known to be associated with any specific single gene defect, but some studies have shown possible genetic association in some cases of TGA, involving deletions of chromosome 22q11.
The Two Major Forms of Transposition of the Great Vessels
In the sections that follow, two forms of the disorder are reviewed in detail:
D-Transposition of the Great Arteries (D-TGA)
Template:DiseaseDisorder infobox
Overview
This anomaly is also referred to as Complete Transposition of the Great Arteries. This is a cyanotic congenital heart defect (CHD) in which the primary arteries (the aorta and the pulmonary artery) are transposed, with the aorta anterior and to the right of the pulmonary artery. dextro-Transposition of the great arteries (d-Transposition of the great arteries, dextro-TGA, or d-TGA), sometimes also referred to as complete transposition of the great arteries, is a cyanotic congenital heart defect (CHD) in which the primary arteries (the aorta and the pulmonary artery) are transposed.
In segmental analysis, this condition is described as ventriculoarterial discordance with atrioventricular concordance, or just ventriculoarterial discordance.
d-TGA is often referred to simply as transposition of the great arteries (TGA); however, TGA is a more general term which may also refer to levo-transposition of the great arteries (l-TGA).
Another term commonly used to refer to both d-TGA and l-TGA is transposition of the great vessels (TGV), although this term might have an even broader meaning than TGA.
Pathophysiology

In a normal heart, oxygen-depleted ("blue") blood is pumped from the right side of the heart, through the pulmonary artery, to the lungs where it is oxygenated. The oxygen-rich ("red") blood then returns to the left heart, via the pulmonary veins, and is pumped through the aorta to the rest of the body, including the heart muscle itself.
With d-TGA, blue blood from the right heart is pumped immediately through the aorta and circulated to the body and the heart itself, bypassing the lungs altogether, while the left heart pumps red blood continuously back into the lungs through the pulmonary artery. In effect, two separate "circular" (parallel) circulatory systems are created, rather than the "figure 8" (in series) circulation of a normal cardio-pulmonary system.
Subclassification
Simple d-TGA: No other associated cardiac defects are present.
Complex d-TGA: d-TGA is often accompanied by other heart defects, the most common type being intracardiac shunts such as atrial septal defect (ASD) including patent foramen ovale (PFO), ventricular septal defect (VSD), and patent ductus arteriosus (PDA). Stenosis of valves or vessels may also be present. An accompanying VSD is present in 40% of these patients. Pulmonary stenosis and a VSD are present in 31% of patients.
Although it may seem counterintuitive, complex d-TGA presents better chance of survival and less developmental risks than simple d-TGA, as well as usually requiring fewer invasive palliative procedures. This is because the left-to-right and bidirectional shunting caused by the defects common to complex d-TGA allow a higher amount of oxygen-rich blood to enter the systemic circulation. However, complex d-TGA may be associated with a slight increase in the length and risk of the corrective surgery, as most or all other heart defects will normally be repaired at the same time.
Anatomy and Anatomic Variations
D-transposition of the great arteries d-TGA implies that the position of the aorta and the pulmonary artery are switched relative to the ventricular septum. The Atrio-Ventricular connections are normal. Differences in the shape of the atrial septum and/or ventricular outflow tracts affect the relative positions of the aorta and pulmonary artery. In the majority of d-TGA cases, the aorta is anterior and to the right of the pulmonary artery, but it can also be directly anterior or anterior and to the left. The aorta and pulmonary artery can also be side by side, with aorta on either side. This is a less common variant, and with this arrangement, an unusual coronary artery pattern is common. There are also some cases with aorta to the right and posterior to the pulmonary artery.[1]
The left coronary artery arises from the left aortic sinus and the right coronary artery from the posterior aortic sinus. In 31 of 149 cases, the circumflex originates from the posterior aortic sinus. [2]
Pathophysiology
In d-TGA, the pulmonary and the systemic circuits are in parallel circulation, rather than in series, which is incompatible with life if there is no mixing of the two systems. Therefore, in most cases, a complex d-TGA is the one that allows survival due to the presence of other heart defects like patent foramen ovale (PFO) for mixing blood between the two systems. Other possible mixing sites include a PDA or a VSD.
The course of TGA is determined by the degree of hypoxia, and the ability of each ventricle to sustain an increased work load in the presence of reduced coronary arterial oxygenation. It is also important the nature of associate heart defects, and the status of the pulmonary vascular circulation.
The pulmonic flow is increased in those cases with transposition and large VSD or large PDA without obstruction to left ventricular outflow. In these cases, pulmonary vascular obstruction develps by 1 to 2 years of age.
Diagnosis of d-TGA
Prenatal d-TGA
Prenatally, a baby with d-TGA experiences no symptoms as the lungs will not be used until after birth, and oxygen is provided by the mother via the placenta and umbilical cord; in order for the red blood to bypass the lungs in utero, the fetal heart has two shunts that begin to close when the newborn starts breathing; these are the foramen ovale and the ductus arteriosus. The foramen ovale is a hole in the atrial septum which allows blood from the right atrium to flow into the left atrium; after birth, the left atrium will be filled with blood returning from the lungs and the foramen ovale will close. The ductus arteriosus is a small, artery-like structure which allows blood to flow from the trunk of the pulmonary artery into the aorta; after birth, the blood in the pulmonary artery will flow into the lungs and the ductus arteriosus will close. Sometimes these shunts will fail to close after birth; these defects are called patent foramen ovale and patent ductus arteriosus, and either may occur independently, or in combination with one another, or with d-TGA or other heart and/or general defects.
In the presence of a d-TGA, the fetus will be asymptomatic due to the presence of shunts during the intrauterine period until after birth when several changes are produced in the circulation:
The following Fetal structures become the following Infant and adult structures :
- The foramen ovale becomes the fossa ovalis
- The ductus arteriosus becomes the ligamentum arteriousus
- The ductus venosus becomes the ligamentum venosus
- The umbilical vein becomes the ligamentum teres
- The umbilical arteries becomes the medial umbilical ligaments
Post natal d-TGA
After birth, the blood in the pulmonary artery will flow into the lungs, which have been expanded with the first breathing presenting less resistance, and the ductus arteriosus will close. Sometimes shunts will fail to close after birth and will result in a patent foramen ovale PFO and patent ductus arteriosus PDA. They may occur independently, or in combination with one another, or with d-TGA or other heart and/or general defects.
Diagnosis
Most of the time, diagnosis can be doned after 18 weeks gestation using an ultrasound. However, if it is not diagnosed in utero, cyanosis of the newborn (blue baby) should immediately indicate that there is a problem with the cardiovascular system.
Infants with d-TGA
Essentially, all these patients attract attention in infancy because of cyanosis or CHF or both.
Normally, the lungs are examined first, then the heart is examined if there are no apparent problems with the lungs. These examinations are typically performed using ultrasound, known as an echocardiogram when performed on the heart.
On the rare occasion, initial symptoms may go unnoticed, resulting in the infant being discharged without treatment in the event of a hospital or birthing center birth, or a delay in bringing the infant for diagnosis in the event of a home birth. On these occasions, a layperson is likely not to recognize symptoms until the infant is experiencing moderate to serious congestive heart failure (CHF) as a result of the heart working harder in a futile attempt to increase oxygen flow to the body; this overworking of the heart muscle eventually leads to hypertrophy and may result in cardiac arrest if left untreated.
Physical Examination in Infants with d-TGA
Symmetric cyanosis is the main characteristic in physical appearence of patients with d-TGA, inadequate mixing, and low pulmonary arterial blood flow. Delayed mild cyanosis, and the apearence of congestive heart failure accompanies non-restrictive VSD.
Reversed differential cyanosis (feet less cyanotic than hands) can be a manifestation in patients with d-TGA and large patent ductus arteriosus that has develop early pulmonary vascular disease, reversing the ductal flow if the patient has survived. At this poin in time, the pulmonary arterial blood of high oxygen content enters the aorta and is selectively distribuited to the lower extremities.
The murmur of a large PDA in d-TGA, is usually systolic, seldom continuos, due to the almost exclusive flow during systole from the aorta to the pulmonary artery.
There is a prominent impulse at the LLSB (the RV which is actually the morphologic LV).
The first heart sound (S1) is normal in intensity and splitting because the PR interval and ventricular activation is normal. Due to the anterior location of the aorta, the second heart sound (S2) is accentuated and is usually single.
Systolic murmus are absent in neonates unless a subpulmonic stenosis is present. Short midsystolic murmur originate in the anterior aorta when hypervolemia is present. When the pulmonary vascular resistence is low, a midsystolic murmur is originated in the posterior pulmonary artery, but the murmur is dump by the anterior aorta.
A VSD murmur (holosystolic)is absent at birth, until the pulmonary vascular resistence fall. A subsequent increase in pulmonary resistence shortens and later abolishes the murmur.
Symptoms
Cyanosis will appear soon, due to the low oxygen saturation of the blood. Peripheral areas such as around the mouth and lips, fingertips, and toes are affected first because they are furthest from the heart, and since the circulated blood is not fully oxygenated to begin with, very little oxygen reaches the peripheral arteries.
A d-TGA baby will exhibit indrawing beneath the ribcage and rapid breathing; this is likely a homeostatic reflex of the autonomic nervous system in response to hypoxic hypoxia. The infant will be easily fatigued and may experience weakness, particularly during feeding or playing; this interruption to feeding combined with hypoxia can cause failure to thrive. If d-TGA is not diagnosed and corrected early on, the infant may eventually experience syncopic episodes and develop clubbing of the fingers and toes.
Prognosis
The prognosis on simple d-TGA depends mainly on the presence of cardiac shunts such as FO, ASD, VSD, and DA. If one or more of these defects are present, blood will be mixed, allowing a small amount of oxygen to be delivered to the body, giving an opportunity to the newborn to survive long enough to receive corrective surgery.
With complex d-TGA, the infant will fail to thrive and is unlikely to survive longer than a year if corrective surgery is not performed. Generally, if the defect (d-TGA) is not corrected during the first year of life, the patient's condition will deteriorate to the point of inoperability.
Modern repair procedures within the ideal timeframe and without additional complications have a very high success rate.
Electrocardiogram
Ocasionally,the ECG is normal in the newborn , but in older patients there is RAD and RVH. After a Mustard operation there are bradyarrhythmias.
The right axis deviation is moderate or absent, in patients with a large VSD, low pulmonary vascular resistance, and LV volume overload. On the other hand, the right axis deviation is greater if there is a reduced pulmonary arterial blood flow and reduced LV volume as a result of pulmonary vascular disease or pulmonic stenosis.
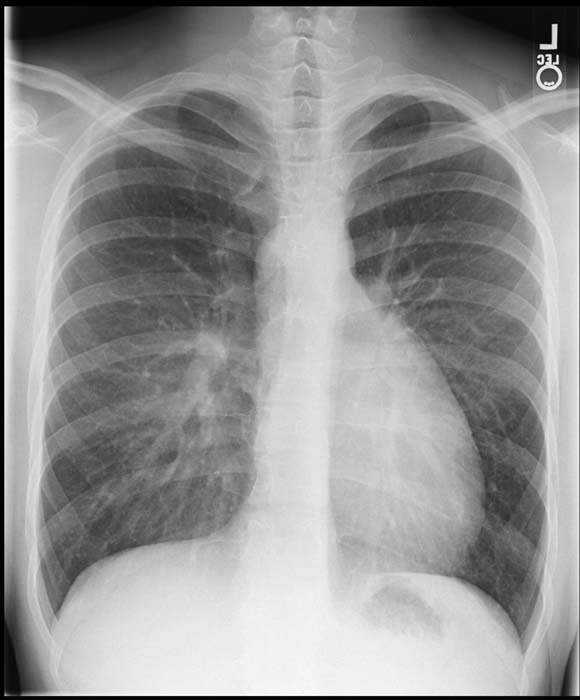
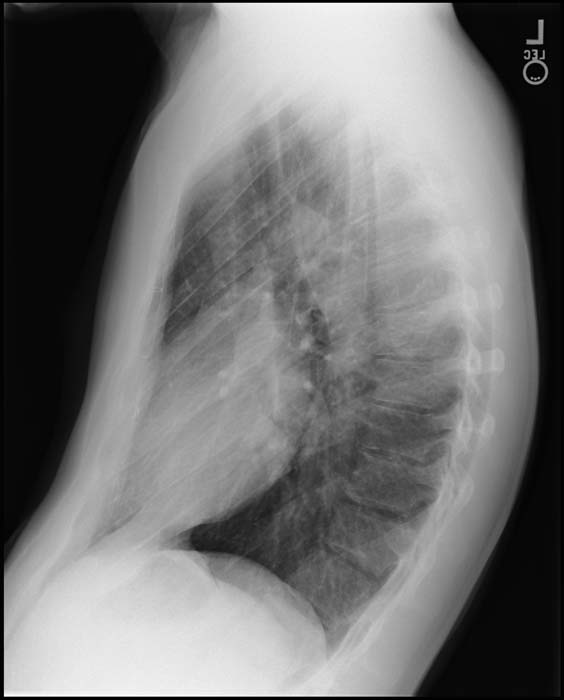
Chest X Ray
Generally, the superior mediastinum may be narrow due to the anterior-posterior relationship of the great vessels.
Initially, cardiac size is normal, but soon enlarges with the cardiac apex shifted to the left and inferiorly, producing the typically ovale-shaped or egg-on-side pattern.
If a VSD is present, there will be an increase of the pulmonar vascular margins.
Echocardiography
Two-dimensional echocardiography identify the spatial relationship between the great arteries and their ventricular origins. In complete TGA, the aortic and pulmonary valve appear as double circles, with the aorta anterior and to the right or side by side and to the right of the pulmonary artery. It is also important to look for right and left branches of the pulmonary artery, and the brachiocaphalic branches of the aortic arch.
MRI and CT
Other Diagnostic Studies
Treatment
Newborns with d-TGA need the special monitoring and care of an ICU.
Palliative treatment
Critically ill newborns benefit from the infusion of Prostaglanding E1, which is used to prevent closure of the PDA (additional shunt) through which the systemic circulation is provided with a higher level of oxygen.
Balloon atrial septostomy, enlargement of the interatrial communication using a balloon, allowing better atrial-level mixing between the two parallel circulations. The catheter is introduced via femoral venous or umbilical venous approach, and passed into the right atrium, across the oval foramen and into the left atrium (LA) under echocardiographic or angiographic guidance. Once the ballon is in the LA, is inflated and drawned back across the oval foramen, producing a tear of the interatrial septum.
Antibiotics are often used because the patient is invaded with arterial and vein lines, nasogastric tube, urethral catheter, etc, that makes the patient very susceptible to infections.
Diuretics aid in flushing excess fluid from the body, thereby easing strain on the heart.
Cardiac glycosides, used to maintain proper heart rhythm while increasing the strength of each contraction.
Sedatives may be used palliatively to prevent a young child from pulling out any of their lines.
Corrective Surgery
Arterial switch or Jatene Operation
The successful anatomical correction of TGA was first described in 1975 by Jatene et al(1). In the absence of left ventricular outflow tract obstruction, the arterial switch operation is the standard therapy for d-TGA. Most infants undergo definitive repair within the first 2 weeks of life.
During the procedure, the baby will be placed under general anesthesia and special monitoring IVs will be used. The heart and vessels are accessed via median sternotomy. The heart/lung machine (cardiopulmonary bypass machine) is connected. As this machine needs its "circulation" to be filled with blood, a child will require a blood transfusion for this surgery. The patient is cooled for 20 minutes to 20 Celsius degree rectal temperature.
Once the heart is stopped and emptied, the aorta and the pulmonary artery are divided. The site of the aortic transection is marked before the cross clamp is applied. The aorta and pulmonary artery are transected at a level above the valve sinuses. The ostium of the coronary arteries are excised along with a large segment of surrounding aortic wall and sutured into place in the neo-aorta (basal part of the pulmonary artery). The pulmonary trunk is moved forward into its new position anterior to the aorta. Finally, the switched great arteries are sutured into place.
The heart is then allowed to fill and take over its normal function. Temporary pacemaker wires and drainage tubes are then placed and the chest is closed.
Some arterial switch recipients may present with post-operative pulmonary stenosis, which would then be repaired with angioplasty, pulmonary stenting via heart cath or median sternotomy, and/or xenograft.
References
- Michael Gatzolius. Diagnosis and management of Adult with Congenital Heart Disease. 2004
Atrial Switch Repair
Mustard and Senning Operations
In 1959, Senning described the first definitive operation (physiological repair) for patients with TGA. In 1964, Mustard published his experience with the atrial switch. This operation became very popular due to an increase in survival of over 90%. Both of these procedures "correct" the physiologic abnormality of the TGA by forming a baffle within the atria in order to switch the flow of blood at inflow level. As a consequence the heart and lungs will be in series.
The Mustard Operation consist of an atrial septectomy and placement of a baffle that directs caval blood to the mitral valve, allowing the pulmonary veins to drain into the tricuspid valve. The baffle is created from pericardium or synthetic material.
The Senning operation, utilized right atrial wall and atrial septal tissue (without the use of extrinsic materials), to create the baffle or wall of the caval tunnel in order to achieve the same goal as in Mustard.
Although the early mortality rate for both procedures is low, between 1 and 10% in experienced hands, the long-term outcome is affected by late complications such as atrial dysrhythmia (with the highest incidence of more than 50% within 10 years), and a late right ventricular (systemic ventricular) dysfunction (approximately 10%).
The Seening repair is becoming more promising than Mustard due to the better long term outcomes in terms of venous obstruction and atrial haemodynamics. However, the procedure of choice for treatment of patients with d-TGA is the Arterial Switch or Jatene Operation.
Rastelli Operation
Is the most frequently used surgical option for patients with TGA, VSD, and pulmonary outflow tract obstruction. It depend on appropriate VSD anatomy (large and subaortic) because the it will be used as part of the left ventricular outflow tract (LVOT), involving placement of a baffle within the RV to direct blood flow from the VSD to the aorta. A conduit is inserted between the RV and the pulmonary artery, which has been oversewn.
The main advantage of this procedure is that the LV becomes the systemic ventricle, but the conduit will likely need to be replaced several times during the patient's life. The appropriate age for this operation is still debated, due to the higher risk with the early repair. The younger the patient the smaller the conduit, needing earlier reoperation.
L-TGA or Congenitally Corrected Transposition of the Great Arteries (CCTGA)
| Levo-Transposition of the great arteries | |
| ICD-10 | Q20.5 |
|---|---|
| ICD-9 | 745.12 |
| DiseasesDB | 13259 |
Overview
levo-Transposition of the great arteries (l-Transposition of the great arteries, levo-TGA, or l-TGA), also commonly referred to as congenitally corrected transposition of the great arteries (CC-TGA), is an acyanotic congenital heart defect (CHD) in which the primary arteries (the aorta and the pulmonary artery) are transposed, with the aorta anterior and to the left of the pulmonary artery; and the morphological left and right ventricles are also transposed.
Use of the term "corrected" has been disputed by many due to the frequent occurrence of other abnormalities and or acquired disorders in l-TGA patients.
In segmental analysis, this condition is described as atrioventricular discordance (ventricular inversion) with ventriculoarterial discordance.
l-TGA is often referred to simply as transposition of the great arteries (TGA); however, TGA is a more general term which may also refer to dextro-transposition of the great arteries (d-TGA).
Another term commonly used to refer to both l-TGA and d-TGA is transposition of the great vessels (TGV), although this term can have an even broader meaning than TGA.
The letter L in the terms l-TGA or L-TGA refers to the a leftward or Levo aorta, versus the normal dextro or rightward aorta.
Subclassification of TGA
Simple and complex l-TGA
l-TGA is often accompanied by other heart defects, the most common type being intracardiac shunts such as atrial septal defect (ASD) including patent foramen ovale (PFO), ventricular septal defect (VSD), and patent ductus arteriosus (PDA). Stenosis of valves such as pulmonary stenosis or atresia may also be present. Tricuspid regurgitation may be present as well.
When no other heart defects are present it is called 'simple' l-TGA; when other defects are present it is called 'complex' l-TGA.
Pathophysiology
Levo-transposition of the great arteries is a defect in which atrial and ventricular morphologies are discordant, and also the morphology of each ventricle is discordant with the great artery that comes from it. In other words this anomaly is a "double discordance" with both atrioventricular and ventriculoarterial discordance, which essentially "corrects" the physiologic abnormality. The atria are in normal position and received appropriate venous return, but the atria are connected to the opposite ventricle (RA to the LV and LA to the RV). In addition the ventricles are inversely connected to the wrong great artery.
In a normal heart, oxygen-depleted ("blue") blood is pumped from the right atrium into the right ventricle, then through the pulmonary artery to the lungs where it is oxygenated. The oxygen-rich ("red") blood then returns, via the pulmonary veins, to the left atrium from which it is pumped into the left ventricle, then through the aorta to the rest of the body, including the heart muscle itself.
With l-TGA, blue blood is pumped from the right atrium into the morphological left ventricle (which lies on the right side of the heart), then through the pulmonary artery to the lungs. The red blood then returns, via the pulmonary veins, to the left atrium from which it is pumped into the morphological right ventricle, then ejected into the aorta.
History
Congenitally corrected transposition of the great arteries (CCTGA), was first described by Von Rokitansky in 1875.
Epidemiology
Among patients with congenital heart disease, CCTGA has an incidence of 0.5%, with a slight male predominance. 95% of CCTGA occurs in "situs solitus".
Genetics
An increased prevalence in families has been reported.
Diagnosis
Simple l-TGA may be "accidentally" diagnosed in adulthood, as an incidental finding as part of the evaluation or treatment of other conditions.
Symptoms
Simple l-TGA may not yield symptoms in infancy. However, since the morphologic right ventricle normally functions in a low pressure system, the right ventricle may eventually hypertrophy due to increased pressure of ejecting into the systemic circulation of the aorta, and produce symptoms such as dyspnea or fatigue may develop.
Complex l-TGA in contrast, may be associated with symptoms earlier in the natural history of the disase depending on the nature, degree and number of accompanying defect(s). If a right-to-left or bidirectional shunt is present, the list of signs and symptoms may include mild cyanosis. Infants and children can present with congestive heart failure CHF, due to a large VSD or severe tricuspid regurgitation.
Physical Examination
Heart
If there is an associated large VSD, or severe tricuspid regurgitation, these may be detected on physical examination.
Laboratory Findings
Chest X ray
Depending upon underlying associated defects such as a VSD, or severe tricuspid regurgitation, the chest x ray may be abnormal and consistent with these associated defects.
Electrocardiogram
Premature development of heart block may be present.
Echocardiography
l-TGA can sometimes be diagnosed in utero with an ultrasound after 18 weeks gestation.
The following links demonstrate the findings on echocardiography in ccTGA:
Prognosis
Simple l-TGA has a very good prognosis. Most patients are asymptomatic and not require surgical correction.
Treatment
In some cases, the technically challenging "double switch operation" has been successfully performed to restore the normal blood flow through the appropriate morphologic ventricles.
References
Acknowledgements and Initial Contributors to Page
Leida Perez, M.D.
External links
- Diagram at kumc.edu
- Diagram and description at umich.edu
- Overview at pediheart.org
- Royal Children's Hospital, Melbourne
- Mayo Clinic, Arizona - Florida - Minnesota, USA
de:Transposition der großen Arterien nl:Transpositie van de grote vaten nn:Transposisjon av dei store arteriane