Sandbox Lopressor: Difference between revisions
Gerald Chi (talk | contribs) mNo edit summary |
Gerald Chi (talk | contribs) mNo edit summary |
||
| Line 4: | Line 4: | ||
{{CMG}} | {{CMG}} | ||
==[[Drug project disclaimer|{{fontcolor|#000000|Disclaimer}}]]== | |||
'''''WikiDoc Drug Project is a constellation of drug information for healthcare providers and patients vigorously vetted on the basis of FDA package insert, MedlinePlus, Practice Guidelines, Scientific Statements, and scholarly medical literature. The information provided is not a medical advice or treatment. WikiDoc does not promote any medication or off-label use of drugs.''''' | '''''WikiDoc Drug Project is a constellation of drug information for healthcare providers and patients vigorously vetted on the basis of FDA package insert, MedlinePlus, Practice Guidelines, Scientific Statements, and scholarly medical literature. The information provided is not a medical advice or treatment. WikiDoc does not promote any medication or off-label use of drugs.''''' | ||
=={{fontcolor|#FF0000|Black Box Warning}}== | |||
{| style="border: 3px solid #696969;" | {| style="border: 3px solid #696969;" | ||
| Line 30: | Line 26: | ||
|} | |} | ||
==Dosing Information== | |||
====Dosage and Administration==== | ====Dosage and Administration==== | ||
| Line 108: | Line 102: | ||
To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088.<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = LOPRESSOR (METOPROLOL TARTRATE) TABLET [NOVARTIS PHARMACEUTICALS CORPORATION] | url =http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=bb05420c-fd24-4672-9f62-fdd313819287 | publisher = | date = | accessdate = }}</ref> | To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088.<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = LOPRESSOR (METOPROLOL TARTRATE) TABLET [NOVARTIS PHARMACEUTICALS CORPORATION] | url =http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=bb05420c-fd24-4672-9f62-fdd313819287 | publisher = | date = | accessdate = }}</ref> | ||
==Mechanism of Action== | |||
Lopressor is a [[beta1]]-selective (cardioselective) adrenergic receptor blocker. This preferential effect is not absolute, however, and at higher plasma concentrations, Lopressor also inhibits [[beta2]]-adrenoreceptors, chiefly located in the bronchial and vascular musculature. | Lopressor is a [[beta1]]-selective (cardioselective) adrenergic receptor blocker. This preferential effect is not absolute, however, and at higher plasma concentrations, Lopressor also inhibits [[beta2]]-adrenoreceptors, chiefly located in the bronchial and vascular musculature. | ||
| Line 142: | Line 134: | ||
The precise mechanism of action of Lopressor in patients with suspected or definite [[myocardial infarction]] is not known. | The precise mechanism of action of Lopressor in patients with suspected or definite [[myocardial infarction]] is not known. | ||
==Indications== | |||
====Hypertension==== | ====Hypertension==== | ||
| Line 185: | Line 175: | ||
* [[Migraine]] (class IIa, category B) | * [[Migraine]] (class IIa, category B) | ||
==Contraindications== | |||
====Hypertension and Angina==== | ====Hypertension and Angina==== | ||
| Line 203: | Line 191: | ||
Lopressor is contraindicated in patients with a heart rate <45 beats/min; [[second degree AV block|second]]- and [[third degree AV block|third-degree heart block]]; significant [[first degree AV block|first-degree heart block]] ([[P-R interval]] ≥0.24 sec); [[systolic blood pressure]] <100 mmHg; or moderate-to-severe [[cardiac failure]] ''(see [[Lopressor warnings and precautions|Warnings]])''.<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = LOPRESSOR (METOPROLOL TARTRATE) TABLET [NOVARTIS PHARMACEUTICALS CORPORATION] | url =http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=bb05420c-fd24-4672-9f62-fdd313819287 | publisher = | date = | accessdate = }}</ref> | Lopressor is contraindicated in patients with a heart rate <45 beats/min; [[second degree AV block|second]]- and [[third degree AV block|third-degree heart block]]; significant [[first degree AV block|first-degree heart block]] ([[P-R interval]] ≥0.24 sec); [[systolic blood pressure]] <100 mmHg; or moderate-to-severe [[cardiac failure]] ''(see [[Lopressor warnings and precautions|Warnings]])''.<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = LOPRESSOR (METOPROLOL TARTRATE) TABLET [NOVARTIS PHARMACEUTICALS CORPORATION] | url =http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=bb05420c-fd24-4672-9f62-fdd313819287 | publisher = | date = | accessdate = }}</ref> | ||
==Warnings and Precautions== | |||
====Warnings==== | ====Warnings==== | ||
| Line 354: | Line 340: | ||
The following adverse reactions have been reported during postapproval use of Lopressor: [[confusion|confusional state]], an increase in blood [[triglycerides]] and a decrease in [[HDL|High Density Lipoprotein (HDL)]]. Because these reports are from a population of uncertain size and are subject to confounding factors, it is not possible to reliably estimate their frequency.<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = LOPRESSOR (METOPROLOL TARTRATE) TABLET [NOVARTIS PHARMACEUTICALS CORPORATION] | url =http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=bb05420c-fd24-4672-9f62-fdd313819287 | publisher = | date = | accessdate = }}</ref> | The following adverse reactions have been reported during postapproval use of Lopressor: [[confusion|confusional state]], an increase in blood [[triglycerides]] and a decrease in [[HDL|High Density Lipoprotein (HDL)]]. Because these reports are from a population of uncertain size and are subject to confounding factors, it is not possible to reliably estimate their frequency.<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = LOPRESSOR (METOPROLOL TARTRATE) TABLET [NOVARTIS PHARMACEUTICALS CORPORATION] | url =http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=bb05420c-fd24-4672-9f62-fdd313819287 | publisher = | date = | accessdate = }}</ref> | ||
==Drug Interactions== | |||
======Catecholamine-depleting drugs====== | ======Catecholamine-depleting drugs====== | ||
| Line 399: | Line 383: | ||
In general, administration of a [[beta-blocker]] should be withheld before [[dipyridamole]] testing, with careful monitoring of heart rate following the [[dipyridamole]] injection.<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = LOPRESSOR (METOPROLOL TARTRATE) TABLET [NOVARTIS PHARMACEUTICALS CORPORATION] | url =http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=bb05420c-fd24-4672-9f62-fdd313819287 | publisher = | date = | accessdate = }}</ref> | In general, administration of a [[beta-blocker]] should be withheld before [[dipyridamole]] testing, with careful monitoring of heart rate following the [[dipyridamole]] injection.<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = LOPRESSOR (METOPROLOL TARTRATE) TABLET [NOVARTIS PHARMACEUTICALS CORPORATION] | url =http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=bb05420c-fd24-4672-9f62-fdd313819287 | publisher = | date = | accessdate = }}</ref> | ||
===[[Lopressor use in specific populations|Use in Specific Populations]]=== | ===[[Lopressor use in specific populations|Use in Specific Populations]]=== | ||
Revision as of 18:51, 14 March 2014
| Sandbox Lopressor® |
|---|
| Black Box Warning |
Adult Indications and Dosage
|
Pediatric Indications and Dosage
|
| Contraindications |
| Warnings |
Adverse Reactions
|
| Drug Interactions |
Use in Specific Populations
|
Routes and Preparations
|
| IV Compatibility |
| Overdosage |
Pharmacology
|
| Clinical Studies |
| How Supplied |
Images
|
Patient Information
|
| Combined Alcohol Use |
| Look-Alike Drug Names |
| Drug Shortage Status |
| Price |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Disclaimer
WikiDoc Drug Project is a constellation of drug information for healthcare providers and patients vigorously vetted on the basis of FDA package insert, MedlinePlus, Practice Guidelines, Scientific Statements, and scholarly medical literature. The information provided is not a medical advice or treatment. WikiDoc does not promote any medication or off-label use of drugs.
Black Box Warning
|
WARNING See full prescribing information for complete boxed warning.
|
Dosing Information
Dosage and Administration
Hypertension
Individualize the dosage of Lopressor tablets. Lopressor tablets should be taken with or immediately following meals.
The usual initial dosage of Lopressor tablets is 100 mg daily in single or divided doses, whether used alone or added to a diuretic. Increase the dosage at weekly (or longer) intervals until optimum blood pressure reduction is achieved. In general, the maximum effect of any given dosage level will be apparent after 1 week of therapy. The effective dosage range of Lopressor tablets is 100-450 mg per day. Dosages above 450 mg per day have not been studied. While once-daily dosing is effective and can maintain a reduction in blood pressure throughout the day, lower doses (especially 100 mg) may not maintain a full effect at the end of the 24-hour period, and larger or more frequent daily doses may be required. This can be evaluated by measuring blood pressure near the end of the dosing interval to determine whether satisfactory control is being maintained throughout the day. Beta1 selectivity diminishes as the dose of Lopressor is increased.
Angina Pectoris
The dosage of Lopressor tablets should be individualized. Lopressor tablets should be taken with or immediately following meals.
The usual initial dosage of Lopressor tablets is 100 mg daily, given in two divided doses. gradually increase the dosage at weekly intervals until optimum clinical response has been obtained or there is pronounced slowing of the heart rate. The effective dosage range of Lopressor tablets is 100-400 mg per day. Dosages above 400 mg per day have not been studied. If treatment is to be discontinued, gradually decrease the dosage over a period of 1-2 weeks (see WARNINGS).
Myocardial Infarction
Early Treatment: During the early phase of definite or suspected acute myocardial infarction, initiate treatment with Lopressor as soon as possible after the patient’s arrival in the hospital. Such treatment should be initiated in a coronary care or similar unit immediately after the patient’s hemodynamic condition has stabilized.
Begin treatment in this early phase with the intravenous administration of three bolus injections of 5 mg of Lopressor each; give the injections at approximately 2-minute intervals. During the intravenous administration of Lopressor, monitor blood pressure, heart rate, and electrocardiogram.
In patients who tolerate the full intravenous dose (15 mg), initiate Lopressor tablets, 50 mg every 6 hours, 15 minutes after the last intravenous dose and continue for 48 hours. Thereafter, the maintenance dosage is 100 mg twice daily (see Late Treatment below).
Start patients who appear not to tolerate the full intravenous dose on Lopressor tablets either 25 mg or 50 mg every 6 hours (depending on the degree of intolerance) 15 minutes after the last intravenous dose or as soon as their clinical condition allows. In patients with severe intolerance, discontinue Lopressor (see Warnings).
Late Treatment: Start patients with contraindications to treatment during the early phase of suspected or definite myocardial infarction, patients who appear not to tolerate the full early treatment, and patients in whom the physician wishes to delay therapy for any other reason on Lopressor tablets, 100 mg twice daily, as soon as their clinical condition allows. Continue therapy for at least 3 months. Although the efficacy of Lopressor beyond 3 months has not been conclusively established, data from studies with other beta blockers suggest that treatment should be continued for 1-3 years.
Special populations
Pediatric patients: No pediatric studies have been performed. The safety and efficacy of Lopressor in pediatric patients have not been established.
Renal impairment: No dose adjustment of Lopressor is required in patients with renal impairment.
Hepatic impairment: Lopressor blood levels are likely to increase substantially in patients with hepatic impairment. Therefore, Lopressor should be initiated at low doses with cautious gradual dose titration according to clinical response.
Geriatric patients (>65 years): In general, use a low initial starting dose in elderly patients given their greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Method of administration
Parenteral administration of Lopressor (ampoule) should be done in a setting with intensive monitoring.
Note: Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
For oral treatment, the tablets should be swallowed un-chewed with a glass of water. Lopressor should always be taken in standardized relation with meals. If the physician asks the patient to take Lopressor either before breakfast or with breakfast, then the patient should continue taking Lopressor with the same schedule during the course of therapy.[1]
Dosage Forms and Strengths
Lopressor® Tablets
metoprolol tartrate tablets, USP
Tablets 50 mg – capsule-shaped, biconvex, pink, scored (imprinted GEIGY on one side and 51 twice on the scored side)
Bottles of 100………………………………………………………………NDC 0078-0458-05
Tablets 100 mg – capsule-shaped, biconvex, light blue, scored (imprinted GEIGY on one side and 71 twice on the scored side)
Bottles of 100………………………………………………………………NDC 0078-0459-05
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature]. Protect from moisture and heat.
Dispense in tight, light-resistant container (USP).
Lopressor® Injection
metoprolol tartrate injection, USP
Ampuls 5 mL – each containing 5 mg of metoprolol tartrate
Carton of 10 ampuls……………………………………………………….NDC 0078-0400-01
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature]. Protect from light and heat.
To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088.[1]
Mechanism of Action
Lopressor is a beta1-selective (cardioselective) adrenergic receptor blocker. This preferential effect is not absolute, however, and at higher plasma concentrations, Lopressor also inhibits beta2-adrenoreceptors, chiefly located in the bronchial and vascular musculature.
Clinical pharmacology studies have demonstrated the beta-blocking activity of metoprolol, as shown by:
- Reduction in heart rate and cardiac output at rest and upon exercise
- Reduction of systolic blood pressure upon exercise
- Inhibition of isoproterenol-induced tachycardia
- Reduction of reflex orthostatic tachycardia
Hypertension
The mechanism of the antihypertensive effects of beta-blocking agents has not been fully elucidated. However, several possible mechanisms have been proposed:
- Competitive antagonism of catecholamines at peripheral (especially cardiac) adrenergic neuron sites, leading to decreased cardiac output.
- A central effect leading to reduced sympathetic outflow to the periphery.
- Suppression of renin activity
Angina Pectoris
By blocking catecholamine-induced increases in heart rate, in velocity and extent of myocardial contraction, and in blood pressure, Lopressor reduces the oxygen requirements of the heart at any given level of effort, thus making it useful in the long-term management of angina pectoris.
Myocardial Infarction
The precise mechanism of action of Lopressor in patients with suspected or definite myocardial infarction is not known.
Indications
Hypertension
Lopressor tablets are indicated for the treatment of hypertension. They may be used alone or in combination with other antihypertensive agents.
Angina Pectoris
Lopressor is indicated in the long-term treatment of angina pectoris.
Myocardial Infarction
Lopressor ampuls and tablets are indicated in the treatment of hemodynamically stable patients with definite or suspected acute myocardial infarction to reduce cardiovascular mortality. Treatment with intravenous Lopressor can be initiated as soon as the patient’s clinical condition allows (see Dosage and Administration, Contraindications, and Warnings).
Alternatively, treatment can begin within 3-10 days of the acute event (see Dosage and Administration).[1]
Off Label Indications
WikiDoc Drug Project is a constellation of drug information for healthcare providers and patients vigorously vetted on the basis of FDA package insert, MedlinePlus, Practice Guidelines, Scientific Statements, and scholarly medical literature. The information provided is not a medical advice or treatment. WikiDoc does not promote any medication or off-label use of drugs.
Adults
Acute
- Coronary artery disease (class IIb, category B)
- Pain at injection site (class IIb, category B)
Chronic
- Agressive behavior (class IIb, category B)
- Anxiety (class IIb, category B)
- Arrhythmia (class IIa, category B)
- Congestive heart failure (class I, category A)
- Glaucoma (class IIb, category B)
- Tremor (class IIb, category B)
Prophylaxis
- Coronary artery disease (class IIb, category B)
- Migraine (class IIa, category B)
Contraindications
Hypertension and Angina
Lopressor is contraindicated in sinus bradycardia, heart block greater than first degree, cardiogenic shock, and overt cardiac failure (see Warnings).
Hypersensitivity to Lopressor and related derivatives, or to any of the excipients; hypersensitivity to other beta blockers (cross sensitivity between beta blockers can occur).
Severe peripheral arterial circulatory disorders.
Myocardial Infarction
Lopressor is contraindicated in patients with a heart rate <45 beats/min; second- and third-degree heart block; significant first-degree heart block (P-R interval ≥0.24 sec); systolic blood pressure <100 mmHg; or moderate-to-severe cardiac failure (see Warnings).[1]
Warnings and Precautions
Warnings
Hypertension and Angina
Cardiac Failure: Sympathetic stimulation is a vital component supporting circulatory function in congestive heart failure, and beta blockade carries the potential hazard of further depressing myocardial contractility and precipitating more severe failure.
In Patients Without a History of Cardiac Failure: Continued depression of the myocardium with beta-blocking agents over a period of time can, in some cases, lead to cardiac failure. At the first sign or symptom of impending cardiac failure, fully digitalize patients and/or give a diuretic. The response should be observed closely. If cardiac failure continues, despite adequate digitalization and diuretic therapy, withdraw Lopressor.
Bronchospastic Diseases: PATIENTS WITH BRONCHOSPASTIC DISEASES SHOULD, IN GENERAL, NOT RECEIVE BETA BLOCKERS, including Lopressor. Because of its relative beta-1 selectivity, however, Lopressor may be used with caution in patients with bronchospastic disease who do not respond to, or cannot tolerate, other antihypertensive treatment. Since beta-1 selectivity is not absolute, a beta2-stimulating agent should be administered concomitantly, and the lowest possible dose of Lopressor should be used. In these circumstances it would be prudent initially to administer Lopressor in smaller doses three times daily, instead of larger doses two times daily, to avoid the higher plasma levels associated with the longer dosing interval (see Dosage and Administration).
Major Surgery: Chronically administered beta-blocking therapy should not be routinely withdrawn prior to major surgery; however, the impaired ability of the heart to respond to reflex adrenergic stimuli may augment the risks of general anesthesia and surgical procedures.
Diabetes and Hypoglycemia: Beta blockers may mask tachycardia occurring with hypoglycemia, but other manifestations such as dizziness and sweating may not be significantly affected.
Pheochromocytoma: If Lopressor is used in the setting of pheochromocytoma, it should be given in combination with an alpha blocker, and only after the alpha blocker has been initiated. Administration of beta blockers alone in the setting of pheochromocytoma has been associated with a paradoxical increase in blood pressure due to the attenuation of beta-mediated vasodilatation in skeletal muscle.
Thyrotoxicosis: Beta-adrenergic blockade may mask certain clinical signs (e.g., tachycardia) of hyperthyroidism. Avoid abrupt withdrawal of beta blockade, which might precipitate a thyroid storm.
Myocardial Infarction
Cardiac Failure: Sympathetic stimulation is a vital component supporting circulatory function, and beta blockade carries the potential hazard of depressing myocardial contractility and precipitating or exacerbating minimal cardiac failure.
During treatment with Lopressor, monitor the hemodynamic status of the patient. If heart failure occurs or persists despite appropriate treatment, discontinue Lopressor.
Bradycardia: Lopressor produces a decrease in sinus heart rate in most patients; this decrease is greatest among patients with high initial heart rates and least among patients with low initial heart rates. Acute myocardial infarction (particularly inferior infarction) may in itself produce significant lowering of the sinus rate. If the sinus rate decreases to <40 beats/min, particularly if associated with evidence of lowered cardiac output, atropine (0.25-0.5 mg) should be administered intravenously. If treatment with atropine is not successful, discontinue Lopressor and consider cautious administration of isoproterenol or installation of a cardiac pacemaker.
AV Block: Lopressor slows AV conduction and may produce significant first- (PR interval ≥0.24 sec), second-, or third-degree heart block. Acute myocardial infarction also produces heart block.
If heart block occurs, discontinue Lopressor and administer atropine (0.25-0.5 mg) intravenously. If treatment with atropine is not successful, consider administration of isoproterenol or installation of a cardiac pacemaker.
Hypotension: If hypotension (systolic blood pressure ≤90 mmHg) occurs, discontinue Lopressor, and assess the hemodynamic status of the patient and the extent of myocardial damage. Invasive monitoring of central venous, pulmonary capillary wedge, and arterial pressures may be required. Institute appropriate therapy with fluids, positive inotropic agents, balloon counterpulsation, or other treatment modalities. If hypotension is associated with sinus bradycardia or AV block, direct treatment at reversing these (see above).
Precautions
General
Start at a low dose and uptitrate slowly in patients with impaired hepatic function.
Information for Patients
Advise patients to take Lopressor regularly and continuously, as directed, with or immediately following meals. If a dose should be missed, the patient should take only the next scheduled dose (without doubling it). Patients should not discontinue Lopressor without consulting the physician.
Advise patients to:
- Avoid operating automobiles and machinery or engaging in other tasks requiring alertness until the patient’s response to therapy with Lopressor has been determined.
- Contact the physician if any difficulty in breathing occurs.
- Inform the physician or dentist before any type of surgery that he or she is taking Lopressor.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have been conducted to evaluate carcinogenic potential. In a 2-year study in rats at three oral dosage levels of up to 800 mg/kg per day, there was no increase in the development of spontaneously occurring benign or malignant neoplasms of any type. The only histologic changes that appeared to be drug related were an increased incidence of generally mild focal accumulation of foamy [[macrophages in pulmonary alveoli and a slight increase in biliary hyperplasia. In a 21-month study in Swiss albino mice at three oral dosage levels of up to 750 mg/kg per day, benign lung tumors (small adenomas) occurred more frequently in female mice receiving the highest dose than in untreated control animals. There was no increase in malignant or total (benign plus malignant) lung tumors, or in the overall incidence of tumors or malignant tumors. This 21-month study was repeated in CD-1 mice, and no statistically or biologically significant differences were observed between treated and control mice of either sex for any type of tumor.
All mutagenicity tests performed (a dominant lethal study in mice, chromosome studies in somatic cells, a Salmonella/mammalian-microsome mutagenicity test, and a nucleus anomaly test in somatic interphase nuclei) were negative.
Reproduction toxicity studies in mice, rats and rabbits did not indicate teratogenic potential for metoprolol tartrate. Embryotoxicity and/or fetotoxicity in rats and rabbits were noted starting at doses of 50 mg/kg in rats and 25 mg/kg in rabbits, as demonstrated by increases in preimplantation loss, decreases in the number of viable fetuses per dose, and/or decreases in neonatal survival. High doses were associated with some maternal toxicity, and growth delay of the offspring in utero, which was reflected in minimally lower weights at birth. The oral NOAELs for embryo-fetal development in mice, rats, and rabbits were considered to be 25, 200, and 12.5 mg/kg. This corresponds to dose levels that are approximately 0.3, 4, and 0.5 times, respectively, when based on surface area, the maximum human oral dose (8 mg/kg/day) of metoprolol tartrate. Metoprolol tartrate has been associated with reversible adverse effects on spermatogenesis starting at oral dose levels of 3.5 mg/kg in rats (a dose that is only 0.1-times the human dose, when based on surface area), although other studies have shown no effect of metoprolol tartrate on reproductive performance in male rats.
Pregnancy Category: C
Upon confirming the diagnosis of pregnancy, women should immediately inform the doctor.
Lopressor has been shown to increase postimplantation loss and decrease neonatal survival in rats at doses up to 11 times the maximum daily human dose of 450 mg, when based on surface area. Distribution studies in mice confirm exposure of the fetus when Lopressor is administered to the pregnant animal. These limited animal studies do not indicate direct or indirect harmful effects with respect to teratogenicity (see Carcinogenesis, Mutagenesis, Impairment of Fertility).
There are no adequate and well-controlled studies in pregnant women. The amount of data on the use of metoprolol in pregnant women is limited. The risk to the fetus/mother is unknown. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Lopressor is excreted in breast milk in a very small quantity. An infant consuming 1 liter of breast milk daily would receive a dose of less than 1 mg of the drug.
Fertility
The effects of Lopressor on the fertility of humans have not been studied.
Lopressor showed effects on spermatogenesis in male rats at a therapeutic dose level, but had no effect on rates of conception at higher doses in animal fertility studies (see Carcinogenesis, Mutagenesis, Impairment of Fertility).
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
Clinical trials of Lopressor in hypertension did not include sufficient numbers of elderly patients to determine whether patients over 65 years of age differ from younger subjects in their response to Lopressor. Other reported clinical experience in elderly hypertensive patients has not identified any difference in response from younger patients.
In worldwide clinical trials of Lopressor in myocardial infarction, where approximately 478 patients were over 65 years of age (0 over 75 years of age), no age-related differences in safety and effectiveness were found. Other reported clinical experience in myocardial infarction has not identified differences in response between the elderly and younger patients. However, greater sensitivity of some elderly individuals taking Lopressor cannot be categorically ruled out. Therefore, in general, it is recommended that dosing proceed with caution in this population.[1]
Adverse Reactions
Hypertension and Angina
Most adverse effects have been mild and transient.
Central Nervous System: Tiredness and dizziness have occurred in about 10 of 100 patients. Depression has been reported in about 5 of 100 patients. Mental confusion and short-term memory loss have been reported. Headache, nightmares, and insomnia have also been reported.
Cardiovascular: Shortness of breath and bradycardia have occurred in approximately 3 of 100 patients. Cold extremities; arterial insufficiency, usually of the Raynaud type; palpitations; congestive heart failure; peripheral edema; and hypotension have been reported in about 1 of 100 patients. Gangrenein patients with pre-existing severe peripheral circulatory disorders has also been reported very rarely. (See Contraindications, Warnings, and Precautions)
Respiratory: Wheezing (bronchospasm) and dyspnea have been reported in about 1 of 100 patients (see Warnings). Rhinitis has also been reported.
Gastrointestinal: Diarrhea has occurred in about 5 of 100 patients. Nausea, dry mouth, gastric pain, constipation, flatulence, and heartburn have been reported in about 1 of 100 patients. Vomiting was a common occurrence. Postmarketing experience reveals very rare reports of hepatitis, jaundice and non-specific hepatic dysfunction. Isolated cases of transaminase, alkaline phosphatase, and lactic dehydrogenase elevations have also been reported.
Hypersensitive Reactions: Pruritus or rash have occurred in about 5 of 100 patients. Very rarely, photosensitivity and worsening of psoriasis has been reported.
Miscellaneous: Peyronie’s disease has been reported in fewer than 1 of 100,000 patients. Musculoskeletal pain, blurred vision, and tinnitus have also been reported.
There have been rare reports of reversible alopecia, agranulocytosis, and dry eyes. Discontinuation of the drug should be considered if any such reaction is not otherwise explicable.
There have been very rare reports of weight gain, arthritis, and retroperitoneal fibrosis (relationship to Lopressor has not been definitely established).
The oculomucocutaneous syndrome associated with the beta blocker practolol has not been reported with Lopressor.
Myocardial Infarction
Central Nervous System: Tiredness has been reported in about 1 of 100 patients. Vertigo, sleep disturbances, hallucinations, headache, dizziness, visual disturbances, confusion, and reduced libido have also been reported, but a drug relationship is not clear.
Cardiovascular: In the randomized comparison of Lopressor and placebo described in the Clinical Pharmacology section, the following adverse reactions were reported:
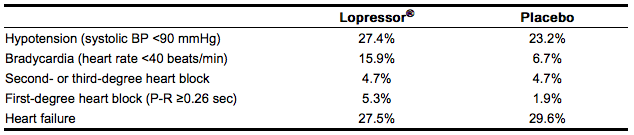 |
Respiratory: Dyspnea of pulmonary origin has been reported in fewer than 1 of 100 patients.
Gastrointestinal: Nausea and abdominal pain have been reported in fewer than 1 of 100 patients.
Dermatologic: Rash and worsened psoriasis have been reported, but a drug relationship is not clear.
Miscellaneous: Unstable diabetes and claudication have been reported, but a drug relationship is not clear.
Potential Adverse Reactions
A variety of adverse reactions not listed above have been reported with other beta-adrenergic blocking agents and should be considered potential adverse reactions to Lopressor.
Central Nervous System: Reversible mental depression progressing to catatonia; an acute reversible syndrome characterized by disorientation for time and place, short-term memory loss, emotional lability, slightly clouded sensorium, and decreased performance on neuropsychometrics.
Cardiovascular: Intensification of AV block (see Contraindications).
Hematologic: Agranulocytosis, nonthrombocytopenic purpura, and thrombocytopenic purpura.
Hypersensitive Reactions: Fever combined with aching and sore throat, laryngospasm and respiratory distress.
Postmarketing Experience
The following adverse reactions have been reported during postapproval use of Lopressor: confusional state, an increase in blood triglycerides and a decrease in High Density Lipoprotein (HDL). Because these reports are from a population of uncertain size and are subject to confounding factors, it is not possible to reliably estimate their frequency.[1]
Drug Interactions
Catecholamine-depleting drugs
Catecholamine-depleting drugs (e.g., reserpine) may have an additive effect when given with beta-blocking agents or monoamine oxidase (MAO) inhibitors.
Observe patients treated with Lopressor plus a catecholamine depletor for evidence of hypotension or marked bradycardia, which may produce vertigo, syncope, or postural hypotension. In addition, possibly significant hypertension may theoretically occur up to 14 days following discontinuation of the concomitant administration with an irreversible MAO inhibitor.
Digitalis glycosides and beta blockers
Both digitalis glycosides and beta blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia. Monitor heart rate and PR interval.
Calcium channel blockers
Concomitant administration of a beta-adrenergic antagonist with a calcium channel blocker may produce an additive reduction in myocardial contractility because of negative chronotropic and inotropic effects.
Risk of Anaphylactic Reaction
While taking beta blockers, patients with a history of severe anaphylactic reaction to a variety of allergens may be more reactive to repeated challenge, either accidental, diagnostic, or therapeutic. Such patients may be unresponsive to the usual doses of epinephrine used to treat allergic reaction.
General Anesthetics
Some inhalation anesthetics may enhance the cardiodepressant effect of beta blockers (see WARNINGS, Major Surgery).
CYP2D6 Inhibitors
Potent inhibitors of the CYP2D6 enzyme may increase the plasma concentration of Lopressor which would mimic the pharmacokinetics of CYP2D6 poor metabolizer (see pharmacokinetics section). Increase in plasma concentrations of metoprolol would decrease the cardioselectivity of metoprolol. Known clinically significant potent inhibitors of CYP2D6 are antidepressants such as fluvoxamine, fluoxetine, paroxetine, sertraline, bupropion, clomipramine, and desipramine; antipsychotics such as chlorpromazine, fluphenazine, haloperidol, and thioridazine; antiarrhythmics such as quinidine or propafenone; antiretrovirals such as ritonavir; antihistamines such as diphenhydramine; antimalarials such as hydroxychloroquine or quinidine; antifungals such as terbinafine.
Hydralazine
Concomitant administration of hydralazine may inhibit presystemic metabolism of metoprolol leading to increased concentrations of metoprolol.
Alpha-adrenergic agents
Antihypertensive effect of alpha-adrenergic blockers such as guanethidine, betanidine, reserpine, alpha-methyldopa or clonidine may be potentiated by beta-blockers including Lopressor. Beta-adrenergic blockers may also potentiate the postural hypotensive effect of the first dose of prazosin, probably by preventing reflex tachycardia. On the contrary, beta adrenergic blockers may also potentiate the hypertensive response to withdrawal of clonidine in patients receiving concomitant clonidine and beta-adrenergic blocker. If a patient is treated with clonidine and Lopressor concurrently, and clonidine treatment is to be discontinued, stop Lopressor several days before clonidine is withdrawn. Rebound hypertension that can follow withdrawal of clonidine may be increased in patients receiving concurrent beta-blocker treatment.
Ergot alkaloid
Concomitant administration with beta-blockers may enhance the vasoconstrictive action of ergot alkaloids.
Dipyridamole
In general, administration of a beta-blocker should be withheld before dipyridamole testing, with careful monitoring of heart rate following the dipyridamole injection.[1]
Use in Specific Populations
Overdosage
Description
Clinical Pharmacology
Nonclinical Toxicology
Clinical Studies
How Supplied/Storage and Handling
Patient Counseling Information
Labels and Packages
Pill Images and Characteristics
Pricing
Black box warning Dosing information Mechanism of action Indications Contraindications Warnings and Precautions Adverse reactions Drug Interactions Overdosage Pharmacology Clinical studies Patient counseling information Pill images and characteristics