Procarbazine: Difference between revisions
No edit summary |
No edit summary |
||
| (4 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag={{AV}} | |authorTag={{AV}} | ||
|genericName= | |genericName=Procarbazine | ||
|aOrAn=an | |aOrAn=an | ||
|drugClass=[[alkylating agent]] | |drugClass=[[alkylating agent]] | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=Stage III and IV | |indication=[[Hodgkin's disease|Stage III and IV Hodgkin's disease]] used as part of the [[MOPP regimen|MOPP]] ([[nitrogen mustard]], [[vincristine]], procarbazine, [[prednisone]]) regimen | ||
|hasBlackBoxWarning=Yes | |hasBlackBoxWarning=Yes | ||
|adverseReactions=[[nausea]], [[vomiting]], [[hemolysis]], [[myelosuppression]], [[neurotoxicity]], [[peripheral neuropathy]] | |adverseReactions=[[nausea]], [[vomiting]], [[hemolysis]], [[myelosuppression]], [[neurotoxicity]], [[peripheral neuropathy]] | ||
| Line 12: | Line 12: | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;"></span></i> | |blackBoxWarningBody=<i><span style="color:#FF0000;"></span></i> | ||
* It is recommended that | * It is recommended that procarbazine be given only by or under the supervision of a physician experienced in the use of potent antineoplastic drugs. Adequate clinical and laboratory facilities should be available to patients for proper monitoring of treatment. | ||
<!--Adult Indications and Dosage--> | <!--Adult Indications and Dosage--> | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult | |fdaLIADAdult= | ||
=====[[Hodgkin's disease]]===== | |||
*procarbazine is indicated for use in combination with other anticancer drugs for the treatment of [[Hodgkin's disease|Stage III and IV Hodgkin's disease]]. procarbazine is used as part of the [[MOPP]] ([[nitrogen mustard]], [[vincristine]], procarbazine, [[prednisone]]) regimen. | |||
=====Dosing Information===== | =====Dosing Information===== | ||
*The following doses are for administration of the drug as a single agent. When used in combination with other anticancer drugs, the | *The following doses are for administration of the drug as a single agent. When used in combination with other [[chemotherapy|anticancer drugs]], the procarbazine dose should be appropriately reduced, eg, in the [[MOPP regimen]], the procarbazine dose is 100 mg/m2 daily for 14 days. All dosages are based on the patient's actual weight. However, the estimated lean body mass (dry weight) is used if the patient is obese or if there has been a spurious weight gain due to [[edema]], [[ascites]] or other forms of abnormal [[fluid retention]]. | ||
*Adults: To minimize the [[nausea]] and [[vomiting]] experienced by a high percentage of patients beginning | *Adults: To minimize the [[nausea]] and [[vomiting]] experienced by a high percentage of patients beginning procarbazine therapy, single or divided doses of 2 to 4 mg/kg/day for the first week are recommended. Daily dosage should then be maintained at 4 to 6 mg/kg/day until maximum response is obtained or until the white blood count falls below 4000/cmm or the [[platelets]] fall below 100,000/cmm. When maximum response is obtained, the dose may be maintained at 1 to 2 mg/kg/day. Upon evidence of hematologic or other toxicity , the drug should be discontinued until there has been satisfactory recovery. After toxic side effects have subsided, therapy may then be resumed at the discretion of the physician, based on clinical evaluation and appropriate laboratory studies, at a dosage of 1 to 2 mg/kg/day. | ||
*Pediatric Patients: Very close clinical monitoring is mandatory. Undue toxicity, evidenced by [[tremors]], [[coma]] and [[convulsions]], has occurred in a few cases. Dosage, therefore, should be individualized. The following dosage schedule is provided as a guideline only. | *Pediatric Patients: Very close clinical monitoring is mandatory. Undue toxicity, evidenced by [[tremors]], [[coma]] and [[convulsions]], has occurred in a few cases. Dosage, therefore, should be individualized. The following dosage schedule is provided as a guideline only. | ||
| Line 31: | Line 33: | ||
*Fifty (50) mg per square meter of body surface per day is recommended for the first week. Dosage should then be maintained at 100 mg per square meter of body surface per day until maximum response is obtained or until [[leukopenia]] or [[thrombocytopenia]]occurs. When maximum response is attained, the dose may be maintained at 50 mg per square meter of body surface per day. Upon evidence of hematologic or other toxicity , the drug should be discontinued until there has been satisfactory recovery, based on clinical evaluation and appropriate laboratory tests. After toxic side effects have subsided, therapy may then be resumed. | *Fifty (50) mg per square meter of body surface per day is recommended for the first week. Dosage should then be maintained at 100 mg per square meter of body surface per day until maximum response is obtained or until [[leukopenia]] or [[thrombocytopenia]]occurs. When maximum response is attained, the dose may be maintained at 50 mg per square meter of body surface per day. Upon evidence of hematologic or other toxicity , the drug should be discontinued until there has been satisfactory recovery, based on clinical evaluation and appropriate laboratory tests. After toxic side effects have subsided, therapy may then be resumed. | ||
*Procedures for proper handling and disposal of [[anticancer drugs]] should be considered. Several guidelines on this subject have been published.1-6 There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate. | *Procedures for proper handling and disposal of [[chemotherapy|anticancer drugs]] should be considered. Several guidelines on this subject have been published.1-6 There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate. | ||
<!--Off-Label Use and Dosage (Adult)--> | <!--Off-Label Use and Dosage (Adult)--> | ||
<!--Guideline-Supported Use (Adult)--> | <!--Guideline-Supported Use (Adult)--> | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of procarbazine in adult patients. | ||
<!--Non–Guideline-Supported Use (Adult)--> | <!--Non–Guideline-Supported Use (Adult)--> | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of procarbazine in adult patients. | ||
<!--Pediatric Indications and Dosage--> | <!--Pediatric Indications and Dosage--> | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed=There is limited information regarding <i>FDA-Labeled Use</i> of | |fdaLIADPed=There is limited information regarding <i>FDA-Labeled Use</i> of procarbazine in pediatric patients. | ||
<!--Off-Label Use and Dosage (Pediatric)--> | <!--Off-Label Use and Dosage (Pediatric)--> | ||
<!--Guideline-Supported Use (Pediatric)--> | <!--Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of procarbazine in pediatric patients. | ||
<!--Non–Guideline-Supported Use (Pediatric)--> | <!--Non–Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of procarbazine in pediatric patients. | ||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications=* | |contraindications=*procarbazine is contraindicated in patients with known [[Hypersensitivity]] to the drug or inadequate marrow reserve as demonstrated by [[bone marrow aspiration]]. Due consideration of this possible state should be given to each patient who has [[leukopenia]], [[thrombocytopenia]]or [[anemia]]. | ||
<!--Warnings--> | <!--Warnings--> | ||
|warnings=*To minimize [[CNS depression]] and possible potentiation, [[barbiturates]], [[antihistamines]], [[narcotics]], hypotensive agents or [[phenothiazines]] should be used with caution. [[Ethyl alcohol]] should not be used since there may be an Antabuse | |warnings=*To minimize [[CNS depression]] and possible potentiation, [[barbiturates]], [[antihistamines]], [[narcotics]], hypotensive agents or [[phenothiazines]] should be used with caution. [[Ethyl alcohol]] should not be used since there may be an Antabuse [[disulfiram|(disulfiram)-like reaction]]. Because procarbazine exhibits some [[monoamine oxidase inhibitory activity]], [[sympathomimetic drug]]s, [[tricyclic antidepressant drugs]] (eg, [[amitriptyline]] HCI, [[imipramine]] HCI) and other drugs and foods with known high [[tyramine]] content, such as wine, yogurt, ripe cheese and bananas, should be avoided. A further phenomenon of toxicity common to many [[hydrazine]] derivatives is [[hemolysis]] and the appearance of [[inclusion bodies|Heinz-Ehrlich inclusion bodies]] in [[erythrocytes]]. | ||
====Precautions==== | ====Precautions==== | ||
| Line 64: | Line 66: | ||
=====General===== | =====General===== | ||
*Undue toxicity may occur if | *Undue toxicity may occur if procarbazine is used in patients with impairment of renal and/or hepatic function. When appropriate, hospitalization for the initial course of treatment should be considered. | ||
*If radiation or a chemotherapeutic agent known to have marrow-depressant activity has been used, an interval of one month or longer without such therapy is recommended before starting treatment with | *If radiation or a [[chemotherapeutic agent]] known to have marrow-depressant activity has been used, an interval of one month or longer without such therapy is recommended before starting treatment with procarbazine . The length of this interval may also be determined by evidence of bone marrow recovery based on successive bone marrow studies. | ||
*Prompt cessation of therapy is recommended if any one of the following occurs: | *Prompt cessation of therapy is recommended if any one of the following occurs: | ||
| Line 89: | Line 91: | ||
=====Hematologic===== | =====Hematologic===== | ||
*[[Pancytopenia]]; [[eosinophilia]]; | *[[Pancytopenia]]; [[eosinophilia]]; [[anemia|hemolytic anemia]]; bleeding tendencies such as [[petechiae]], [[purpura]], [[epistaxis]] and [[hemoptysis]]. | ||
=====Gastrointestinal===== | =====Gastrointestinal===== | ||
| Line 139: | Line 141: | ||
*Intercurrent infections, [[hearing loss]], [[pyrexia]], [[diaphoresis]], [[lethargy]], [[weakness]], [[fatigue]], [[edema]], [[chills]], [[insomnia]], [[slurred speech]], [[hoarseness]], [[drowsiness]]. | *Intercurrent infections, [[hearing loss]], [[pyrexia]], [[diaphoresis]], [[lethargy]], [[weakness]], [[fatigue]], [[edema]], [[chills]], [[insomnia]], [[slurred speech]], [[hoarseness]], [[drowsiness]]. | ||
*Second nonlymphoid malignancies (including [[lung cancer]], [[acute myelocytic leukemia]] and | *Second nonlymphoid malignancies (including [[lung cancer]], [[acute myelocytic leukemia]] and [[myelosclerosis|malignant myelosclerosis]]) and [[azoospermia]] have been reported in patients with [[Hodgkin's disease]] treated with procarbazine in combination with other [[chemotherapy]] and/or [[radiation]]. The risks of secondary lung cancer from treatment appear to be multiplied by tobacco use. | ||
<!--Postmarketing Experience--> | <!--Postmarketing Experience--> | ||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of | |postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of procarbazine in the drug label. | ||
<!--Drug Interactions--> | <!--Drug Interactions--> | ||
| Line 148: | Line 150: | ||
<!--Use in Specific Populations--> | <!--Use in Specific Populations--> | ||
|FDAPregCat=D | |FDAPregCat=D | ||
|useInPregnancyFDA=* | |useInPregnancyFDA=*procarbazine hydrochloride can cause fetal harm when administered to a pregnant woman. While there are no adequate and well-controlled studies with procarbazine hydrochloride in pregnant women, there are case reports of malformations in the offspring of women who were exposed to procarbazine hydrochloride in combination with other [[chemotherapeutic agents|antineoplastic agents]] during [[pregnancy]]. procarbazine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant. procarbazine hydrochloride is [[teratogenic]] in the rat when given at doses approximately 4 to 13 times the maximum recommended human therapeutic dose of 6 mg/kg/day. | ||
=====Nonteratogenic Effects===== | =====Nonteratogenic Effects===== | ||
* | *procarbazine hydrochloride has not been adequately studied in animals for its effects on peri- and postnatal development. However, neurogenic tumors were noted in the offspring of rats given intravenous injections of 125 mg/kg of procarbazine hydrochloride on day 22 of gestation. Compounds which inhibit [[DNA]], [[RNA]] and [[protein synthesis]] might be expected to have adverse effects on peri- and postnatal development. | ||
|useInPregnancyAUS=* There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of | |useInPregnancyAUS=* There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of procarbazine in women who are pregnant. | ||
|useInLaborDelivery=There is no FDA guidance on use of | |useInLaborDelivery=There is no FDA guidance on use of procarbazine during labor and delivery. | ||
|useInNursing=*It is not known whether | |useInNursing=*It is not known whether procarbazine is excreted in human milk. Because of the potential for [[tumorigenicity]] shown for procarbazine hydrochloride in animal studies, mothers should not nurse while receiving this drug. | ||
|useInPed=*Undue toxicity, evidenced by tremors, coma and [[convulsions]], has occurred in a few cases. Dosage, therefore, should be individualized . Very close clinical monitoring is mandatory. | |useInPed=*Undue toxicity, evidenced by tremors, coma and [[convulsions]], has occurred in a few cases. Dosage, therefore, should be individualized . Very close clinical monitoring is mandatory. | ||
|useInGeri=There is no FDA guidance on the use of | |useInGeri=There is no FDA guidance on the use of procarbazine with respect to geriatric patients. | ||
|useInGender=There is no FDA guidance on the use of | |useInGender=There is no FDA guidance on the use of procarbazine with respect to specific gender populations. | ||
|useInRace=There is no FDA guidance on the use of | |useInRace=There is no FDA guidance on the use of procarbazine with respect to specific racial populations. | ||
|useInRenalImpair=There is no FDA guidance on the use of | |useInRenalImpair=There is no FDA guidance on the use of procarbazine in patients with [[renal impairment]]. | ||
|useInHepaticImpair=There is no FDA guidance on the use of | |useInHepaticImpair=There is no FDA guidance on the use of procarbazine in patients with [[hepatic impairment]]. | ||
|useInReproPotential=There is no FDA guidance on the use of | |useInReproPotential=There is no FDA guidance on the use of procarbazine in women of reproductive potentials and males. | ||
|useInImmunocomp=There is no FDA guidance one the use of | |useInImmunocomp=There is no FDA guidance one the use of procarbazine in patients who are [[immunocompromised]]. | ||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* Oral | |administration=* Oral | ||
|monitoring=*Baseline laboratory data should be obtained prior to initiation of therapy. The hematologic status as indicated by [[hemoglobin]], [[hematocrit]], white blood count (WBC), differential, [[reticulocytes]] and [[platelets]] should be monitored closely - at least every 3 or 4 days. | |monitoring=*Baseline laboratory data should be obtained prior to initiation of therapy. The hematologic status as indicated by [[hemoglobin]], [[hematocrit]], [[white blood count]] (WBC), differential, [[reticulocytes]] and [[platelets]] should be monitored closely - at least every 3 or 4 days. | ||
*Hepatic and renal evaluation are indicated prior to beginning therapy. Urinalysis, transaminase, alkaline phosphatase and blood urea | *Hepatic and renal evaluation are indicated prior to beginning therapy. [[Urinalysis]], [[transaminase]], [[alkaline phosphatase]] and [[blood urea nitroge]]n tests should be repeated at least weekly. | ||
<!--IV Compatibility--> | <!--IV Compatibility--> | ||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of | |IVCompat=There is limited information regarding <i>IV Compatibility</i> of procarbazine in the drug label. | ||
<!--Overdosage--> | <!--Overdosage--> | ||
|overdose=*The major manifestations of overdosage with | |overdose=*The major manifestations of overdosage with procarbazine would be anticipated to be [[nausea]], [[vomiting]], [[enteritis]], [[Diarrhea]], [[hypotension]], [[tremors]], [[convulsions]] and [[coma]]. Treatment should consist of either the administration of an [[emetic]] or [[gastric lavage]]. General supportive measures such as [[intravenous fluids]] are advised. Since the major toxicity of procarbazine hydrochloride is hematologic and hepatic, patients should have frequent complete blood counts and liver function tests throughout their period of recovery and for a minimum of two weeks thereafter. Should abnormalities appear in any of these determinations, appropriate measures for correction and stabilization should be immediately undertaken. | ||
*The estimated mean lethal dose of | *The estimated mean lethal dose of procarbazine hydrochloride in laboratory animals varied from approximately 150 mg/kg in rabbits to 1300 mg/kg in mice. | ||
<!--Pharmacology--> | <!--Pharmacology--> | ||
<!--Drug box 2--> | <!--Drug box 2--> | ||
|drugBox=[[File:procarbazine00.png|thumb|none| | |drugBox=[[File:procarbazine00.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
<!--Mechanism of Action--> | <!--Mechanism of Action--> | ||
|mechAction=*The precise mode of cytotoxic action of | |mechAction=*The precise mode of cytotoxic action of procarbazine has not been clearly defined. There is evidence that the drug may act by inhibition of protein, [[RNA]] and [[DNA synthesis]]. Studies have suggested that procarbazine may inhibit transmethylation of methyl groups of [[methionine]] into t-[[RNA]]. The absence of functional t-RNA could cause the cessation of protein synthesis and consequently [[DNA]] and [[RNA synthesis]]. In addition, procarbazine may directly damage [[DNA]]. [[Hydrogen peroxide]], formed during the [[auto-oxidation]] of the drug, may attack protein sulfhydryl groups contained in residual protein which is tightly bound to [[DNA]]. | ||
<!--Structure--> | <!--Structure--> | ||
|structure=* | |structure=*procarbazine (procarbazine hydrochloride), a hydrazine derivative [[chemotherapy|antineoplastic agent]], is available as capsules containing the equivalent of 50 mg procarbazine as the hydrochloride. Each capsule also contains cornstarch, [[mannitol]] and talc. Gelatin capsule shells contain parabens (methyl and propyl), potassium [[sorbate]], [[titanium dioxide]], FD&C Yellow No. 6 and D&C Yellow No. 10. | ||
*Chemically, | *Chemically, procarbazine hydrochloride is N-isopropyl-α-(2-methylhydrazino)-p-toluamide monohydrochloride. It is a white to pale yellow crystalline powder which is soluble but unstable in water or aqueous solutions. The molecular weight of procarbazine hydrochloride is 257.76 and the structural formula is: | ||
: [[File:procarbazine01.png|thumb|none| | : [[File:procarbazine01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of | |PD=There is limited information regarding <i>Pharmacodynamics</i> of procarbazine in the drug label. | ||
<!--Pharmacokinetics--> | <!--Pharmacokinetics--> | ||
|PK=* | |PK=*procarbazine is metabolized primarily in the liver and kidneys. The drug appears to be auto-oxidized to the azo derivative with the release of hydrogen peroxide. The azo derivative isomerizes to the hydrazone, and following [[hydrolysis]] splits into a benzylaldehyde derivative and methylhydrazine. The methylhydrazine is further degraded to [[CO2]] and CH4 and possibly [[hydrazine]], whereas the aldehyde is oxidized to N-isopropylterephthalamic acid, which is excreted in the urine. | ||
* | *procarbazine is rapidly and completely absorbed. Following oral administration of 30 mg of 14C-labeled procarbazine, maximum peak plasma radioactive concentrations were reached within 60 minutes. | ||
*After intravenous injection, the plasma half-life of | *After intravenous injection, the plasma half-life of procarbazine is approximately 10 minutes. Approximately 70% of the [[radioactivity]] is excreted in the urine as N-isopropylterephthalamic acid within 24 hours following both oral and intravenous administration of 14C-labeled procarbazine. | ||
* | *procarbazine crosses the [[blood-brain barrier]] and rapidly equilibrates between plasma and [[cerebrospinal fluid]] after oral administration. | ||
<!--Nonclinical Toxicology--> | <!--Nonclinical Toxicology--> | ||
|nonClinToxic=====Carcinogenesis, Mutagenesis, Impairment of Fertility==== | |nonClinToxic=====Carcinogenesis, Mutagenesis, Impairment of Fertility==== | ||
| Line 209: | Line 211: | ||
=====Carcinogenesis===== | =====Carcinogenesis===== | ||
*The carcinogenicity of | *The carcinogenicity of procarbazine hydrochloride in mice, rats and monkeys has been reported in a considerable number of studies. Instances of a second nonlymphoid malignancy, including [[lung cancer]] and [[acute myelocytic leukemia]], have been reported in patients with [[Hodgkin's disease]] treated with procarbazine in combination with other [[chemotherapy]] and/or radiation. The risks of secondary lung cancer from treatment appear to be multiplied by tobacco use. The International Agency for Research on Cancer (IARC) considers that there is “sufficient evidence” for the human [[carcinogenicity]] of procarbazine hydrochloride when it is given in intensive regimens which include other [[antineoplastic agents]] but that there is inadequate evidence of [[carcinogenicity]] in humans given procarbazine hydrochloride alone. | ||
=====Mutagenesis===== | =====Mutagenesis===== | ||
* | *procarbazine hydrochloride has been shown to be mutagenic in a variety of bacterial and mammalian test systems. | ||
=====Impairment of Fertility===== | =====Impairment of Fertility===== | ||
*[[Azoospermia]] and [[antifertility]] effects associated with | *[[Azoospermia]] and [[antifertility]] effects associated with procarbazine hydrochloride administration in combination with other [[chemotherapeutic agents]] for treating [[Hodgkin's disease]] have been reported in human clinical studies. Since these patients received multicombination therapy, it is difficult to determine to what extent procarbazine hydrochloride alone was involved in the male germ-cell damage. The usual Segment I [[fertility]]/reproduction studies in laboratory animals have not been carried out with procarbazine hydrochloride. However, compounds which inhibit [[DNA]], [[RNA]] and/or protein synthesis might be expected to have adverse effects on [[gametogenesis]]. Unscheduled [[DNA synthesis]] in the [[testis]] of rabbits and [[fertility|decreased fertility]] in male mice treated with procarbazine hydrochloride have been reported. | ||
<!--Clinical Studies--> | <!--Clinical Studies--> | ||
|clinicalStudies=There is limited information regarding <i>Clinical Studies</i> of | |clinicalStudies=There is limited information regarding <i>Clinical Studies</i> of procarbazine in the drug label. | ||
<!--How Supplied--> | <!--How Supplied--> | ||
|howSupplied=*Capsules, ivory, containing the equivalent of 50 mg | |howSupplied=*Capsules, ivory, containing the equivalent of 50 mg procarbazine as the hydrochloride; in bottles of 100 (NDC 54482-053-01). Imprint on capsules: procarbazine σ sigma-tau. | ||
<!--Patient Counseling Information--> | <!--Patient Counseling Information--> | ||
|fdaPatientInfo=*Patients should be warned not to drink alcoholic beverages while on | |fdaPatientInfo=*Patients should be warned not to drink alcoholic beverages while on procarbazine therapy since there may be an Antabuse ([[disulfiram]])-like reaction. They should also be cautioned to avoid foods with known high [[tyramine]] content such as wine, yogurt, ripe cheese and bananas. Over-the-counter drug preparations which contain [[antihistamines]] or [[sympathomimetic drugs]] should also be avoided. Patients taking procarbazine should also be warned against the use of prescription drugs without the knowledge and consent of their physician. Patients should be advised to discontinue tobacco use. | ||
<!--Precautions with Alcohol--> | <!--Precautions with Alcohol--> | ||
|alcohol=* Alcohol- | |alcohol=* Alcohol-procarbazine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames=* [[Matulane]] | |brandNames=* [[Matulane]] | ||
<!--Look-Alike Drug Names--> | <!--Look-Alike Drug Names--> | ||
|lookAlike= | |lookAlike= | ||
<!--Drug Shortage Status--> | <!--Drug Shortage Status--> | ||
| Line 247: | Line 248: | ||
}} | }} | ||
<!--Pill Image--> | <!--Pill Image--> | ||
<!--Label Display Image--> | <!--Label Display Image--> | ||
<!--Category--> | <!--Category--> | ||
[[Category: | [[Category:Chemotherapy]] | ||
[[Category:Chemotherapeutic agents]] | [[Category:Chemotherapeutic agents]] | ||
[[Category:Alkylating agents]] | |||
[[Category:Methylhydrazines]] | |||
Latest revision as of 21:29, 5 March 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
|
Overview
Procarbazine is an alkylating agent that is FDA approved for the treatment of Stage III and IV Hodgkin's disease used as part of the MOPP (nitrogen mustard, vincristine, procarbazine, prednisone) regimen. There is a Black Box Warning for this drug as shown here. Common adverse reactions include nausea, vomiting, hemolysis, myelosuppression, neurotoxicity, peripheral neuropathy.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Hodgkin's disease
- procarbazine is indicated for use in combination with other anticancer drugs for the treatment of Stage III and IV Hodgkin's disease. procarbazine is used as part of the MOPP (nitrogen mustard, vincristine, procarbazine, prednisone) regimen.
Dosing Information
- The following doses are for administration of the drug as a single agent. When used in combination with other anticancer drugs, the procarbazine dose should be appropriately reduced, eg, in the MOPP regimen, the procarbazine dose is 100 mg/m2 daily for 14 days. All dosages are based on the patient's actual weight. However, the estimated lean body mass (dry weight) is used if the patient is obese or if there has been a spurious weight gain due to edema, ascites or other forms of abnormal fluid retention.
- Adults: To minimize the nausea and vomiting experienced by a high percentage of patients beginning procarbazine therapy, single or divided doses of 2 to 4 mg/kg/day for the first week are recommended. Daily dosage should then be maintained at 4 to 6 mg/kg/day until maximum response is obtained or until the white blood count falls below 4000/cmm or the platelets fall below 100,000/cmm. When maximum response is obtained, the dose may be maintained at 1 to 2 mg/kg/day. Upon evidence of hematologic or other toxicity , the drug should be discontinued until there has been satisfactory recovery. After toxic side effects have subsided, therapy may then be resumed at the discretion of the physician, based on clinical evaluation and appropriate laboratory studies, at a dosage of 1 to 2 mg/kg/day.
- Pediatric Patients: Very close clinical monitoring is mandatory. Undue toxicity, evidenced by tremors, coma and convulsions, has occurred in a few cases. Dosage, therefore, should be individualized. The following dosage schedule is provided as a guideline only.
- Fifty (50) mg per square meter of body surface per day is recommended for the first week. Dosage should then be maintained at 100 mg per square meter of body surface per day until maximum response is obtained or until leukopenia or thrombocytopeniaoccurs. When maximum response is attained, the dose may be maintained at 50 mg per square meter of body surface per day. Upon evidence of hematologic or other toxicity , the drug should be discontinued until there has been satisfactory recovery, based on clinical evaluation and appropriate laboratory tests. After toxic side effects have subsided, therapy may then be resumed.
- Procedures for proper handling and disposal of anticancer drugs should be considered. Several guidelines on this subject have been published.1-6 There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of procarbazine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of procarbazine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of procarbazine in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of procarbazine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of procarbazine in pediatric patients.
Contraindications
- procarbazine is contraindicated in patients with known Hypersensitivity to the drug or inadequate marrow reserve as demonstrated by bone marrow aspiration. Due consideration of this possible state should be given to each patient who has leukopenia, thrombocytopeniaor anemia.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
|
- To minimize CNS depression and possible potentiation, barbiturates, antihistamines, narcotics, hypotensive agents or phenothiazines should be used with caution. Ethyl alcohol should not be used since there may be an Antabuse (disulfiram)-like reaction. Because procarbazine exhibits some monoamine oxidase inhibitory activity, sympathomimetic drugs, tricyclic antidepressant drugs (eg, amitriptyline HCI, imipramine HCI) and other drugs and foods with known high tyramine content, such as wine, yogurt, ripe cheese and bananas, should be avoided. A further phenomenon of toxicity common to many hydrazine derivatives is hemolysis and the appearance of Heinz-Ehrlich inclusion bodies in erythrocytes.
Precautions
General
- Undue toxicity may occur if procarbazine is used in patients with impairment of renal and/or hepatic function. When appropriate, hospitalization for the initial course of treatment should be considered.
- If radiation or a chemotherapeutic agent known to have marrow-depressant activity has been used, an interval of one month or longer without such therapy is recommended before starting treatment with procarbazine . The length of this interval may also be determined by evidence of bone marrow recovery based on successive bone marrow studies.
- Prompt cessation of therapy is recommended if any one of the following occurs:
- Central nervous system signs or symptoms such as paresthesias, neuropathies or confusion.
- leukopenia (white blood count under 4000).
- thrombocytopenia(platelets under 100,000).
- Hypersensitivity reaction.
- Stomatitis - The first small ulceration or persistent spot soreness around the oral cavity is a signal for cessation of therapy.
- Diarrhea - Frequent bowel movements or watery stools.
- Hemorrhage or bleeding tendencies.
- Bone marrow depression often occurs 2 to 8 weeks after the start of treatment. If leukopenia occurs, hospitalization of the patient may be needed for appropriate treatment to prevent systemic infection.
Adverse Reactions
Clinical Trials Experience
- leukopenia, anemia and thrombopenia occur frequently. nausea and vomiting are the most commonly reported side effects.
- Other adverse reactions are:
Hematologic
- Pancytopenia; eosinophilia; hemolytic anemia; bleeding tendencies such as petechiae, purpura, epistaxis and hemoptysis.
Gastrointestinal
- Hepatic dysfunction, jaundice, Stomatitis, hematemesis, melena, Diarrhea, dysphagia, anorexia, abdominal pain, constipation, dry mouth.
Neurologic
- Coma, convulsions, neuropathy, ataxia, paresthesia, nystagmus, diminished reflexes, falling, foot drop, headache, dizziness, unsteadiness.
Cardiovascular
Ophthalmic
- Retinal Hemorrhage, papilledema, photophobia, diplopia, inability to focus.
Respiratory
Dermatologic
Allergic
- Generalized allergic reactions.
Genitourinary
Musculoskeletal
- Pain, including myalgia and arthralgia; tremors.
Psychiatric
Endocrine
- Gynecomastia in prepubertal and early pubertal boys.
Miscellaneous
- Intercurrent infections, hearing loss, pyrexia, diaphoresis, lethargy, weakness, fatigue, edema, chills, insomnia, slurred speech, hoarseness, drowsiness.
- Second nonlymphoid malignancies (including lung cancer, acute myelocytic leukemia and malignant myelosclerosis) and azoospermia have been reported in patients with Hodgkin's disease treated with procarbazine in combination with other chemotherapy and/or radiation. The risks of secondary lung cancer from treatment appear to be multiplied by tobacco use.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of procarbazine in the drug label.
Drug Interactions
- No cross-resistance with other chemotherapeutic agents, radiotherapy or steroids has been demonstrated.
Use in Specific Populations
Pregnancy
- procarbazine hydrochloride can cause fetal harm when administered to a pregnant woman. While there are no adequate and well-controlled studies with procarbazine hydrochloride in pregnant women, there are case reports of malformations in the offspring of women who were exposed to procarbazine hydrochloride in combination with other antineoplastic agents during pregnancy. procarbazine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant. procarbazine hydrochloride is teratogenic in the rat when given at doses approximately 4 to 13 times the maximum recommended human therapeutic dose of 6 mg/kg/day.
Nonteratogenic Effects
- procarbazine hydrochloride has not been adequately studied in animals for its effects on peri- and postnatal development. However, neurogenic tumors were noted in the offspring of rats given intravenous injections of 125 mg/kg of procarbazine hydrochloride on day 22 of gestation. Compounds which inhibit DNA, RNA and protein synthesis might be expected to have adverse effects on peri- and postnatal development.
- There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of procarbazine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of procarbazine during labor and delivery.
Nursing Mothers
- It is not known whether procarbazine is excreted in human milk. Because of the potential for tumorigenicity shown for procarbazine hydrochloride in animal studies, mothers should not nurse while receiving this drug.
Pediatric Use
- Undue toxicity, evidenced by tremors, coma and convulsions, has occurred in a few cases. Dosage, therefore, should be individualized . Very close clinical monitoring is mandatory.
Geriatic Use
There is no FDA guidance on the use of procarbazine with respect to geriatric patients.
Gender
There is no FDA guidance on the use of procarbazine with respect to specific gender populations.
Race
There is no FDA guidance on the use of procarbazine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of procarbazine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of procarbazine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of procarbazine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of procarbazine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
- Baseline laboratory data should be obtained prior to initiation of therapy. The hematologic status as indicated by hemoglobin, hematocrit, white blood count (WBC), differential, reticulocytes and platelets should be monitored closely - at least every 3 or 4 days.
- Hepatic and renal evaluation are indicated prior to beginning therapy. Urinalysis, transaminase, alkaline phosphatase and blood urea nitrogen tests should be repeated at least weekly.
IV Compatibility
There is limited information regarding IV Compatibility of procarbazine in the drug label.
Overdosage
- The major manifestations of overdosage with procarbazine would be anticipated to be nausea, vomiting, enteritis, Diarrhea, hypotension, tremors, convulsions and coma. Treatment should consist of either the administration of an emetic or gastric lavage. General supportive measures such as intravenous fluids are advised. Since the major toxicity of procarbazine hydrochloride is hematologic and hepatic, patients should have frequent complete blood counts and liver function tests throughout their period of recovery and for a minimum of two weeks thereafter. Should abnormalities appear in any of these determinations, appropriate measures for correction and stabilization should be immediately undertaken.
- The estimated mean lethal dose of procarbazine hydrochloride in laboratory animals varied from approximately 150 mg/kg in rabbits to 1300 mg/kg in mice.
Pharmacology

Mechanism of Action
- The precise mode of cytotoxic action of procarbazine has not been clearly defined. There is evidence that the drug may act by inhibition of protein, RNA and DNA synthesis. Studies have suggested that procarbazine may inhibit transmethylation of methyl groups of methionine into t-RNA. The absence of functional t-RNA could cause the cessation of protein synthesis and consequently DNA and RNA synthesis. In addition, procarbazine may directly damage DNA. Hydrogen peroxide, formed during the auto-oxidation of the drug, may attack protein sulfhydryl groups contained in residual protein which is tightly bound to DNA.
Structure
- procarbazine (procarbazine hydrochloride), a hydrazine derivative antineoplastic agent, is available as capsules containing the equivalent of 50 mg procarbazine as the hydrochloride. Each capsule also contains cornstarch, mannitol and talc. Gelatin capsule shells contain parabens (methyl and propyl), potassium sorbate, titanium dioxide, FD&C Yellow No. 6 and D&C Yellow No. 10.
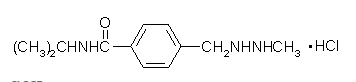
- Chemically, procarbazine hydrochloride is N-isopropyl-α-(2-methylhydrazino)-p-toluamide monohydrochloride. It is a white to pale yellow crystalline powder which is soluble but unstable in water or aqueous solutions. The molecular weight of procarbazine hydrochloride is 257.76 and the structural formula is:
Pharmacodynamics
There is limited information regarding Pharmacodynamics of procarbazine in the drug label.
Pharmacokinetics
- procarbazine is metabolized primarily in the liver and kidneys. The drug appears to be auto-oxidized to the azo derivative with the release of hydrogen peroxide. The azo derivative isomerizes to the hydrazone, and following hydrolysis splits into a benzylaldehyde derivative and methylhydrazine. The methylhydrazine is further degraded to CO2 and CH4 and possibly hydrazine, whereas the aldehyde is oxidized to N-isopropylterephthalamic acid, which is excreted in the urine.
- procarbazine is rapidly and completely absorbed. Following oral administration of 30 mg of 14C-labeled procarbazine, maximum peak plasma radioactive concentrations were reached within 60 minutes.
- After intravenous injection, the plasma half-life of procarbazine is approximately 10 minutes. Approximately 70% of the radioactivity is excreted in the urine as N-isopropylterephthalamic acid within 24 hours following both oral and intravenous administration of 14C-labeled procarbazine.
- procarbazine crosses the blood-brain barrier and rapidly equilibrates between plasma and cerebrospinal fluid after oral administration.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
- The carcinogenicity of procarbazine hydrochloride in mice, rats and monkeys has been reported in a considerable number of studies. Instances of a second nonlymphoid malignancy, including lung cancer and acute myelocytic leukemia, have been reported in patients with Hodgkin's disease treated with procarbazine in combination with other chemotherapy and/or radiation. The risks of secondary lung cancer from treatment appear to be multiplied by tobacco use. The International Agency for Research on Cancer (IARC) considers that there is “sufficient evidence” for the human carcinogenicity of procarbazine hydrochloride when it is given in intensive regimens which include other antineoplastic agents but that there is inadequate evidence of carcinogenicity in humans given procarbazine hydrochloride alone.
Mutagenesis
- procarbazine hydrochloride has been shown to be mutagenic in a variety of bacterial and mammalian test systems.
Impairment of Fertility
- Azoospermia and antifertility effects associated with procarbazine hydrochloride administration in combination with other chemotherapeutic agents for treating Hodgkin's disease have been reported in human clinical studies. Since these patients received multicombination therapy, it is difficult to determine to what extent procarbazine hydrochloride alone was involved in the male germ-cell damage. The usual Segment I fertility/reproduction studies in laboratory animals have not been carried out with procarbazine hydrochloride. However, compounds which inhibit DNA, RNA and/or protein synthesis might be expected to have adverse effects on gametogenesis. Unscheduled DNA synthesis in the testis of rabbits and decreased fertility in male mice treated with procarbazine hydrochloride have been reported.
Clinical Studies
There is limited information regarding Clinical Studies of procarbazine in the drug label.
How Supplied
- Capsules, ivory, containing the equivalent of 50 mg procarbazine as the hydrochloride; in bottles of 100 (NDC 54482-053-01). Imprint on capsules: procarbazine σ sigma-tau.
Storage
There is limited information regarding Procarbazine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Procarbazine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Procarbazine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients should be warned not to drink alcoholic beverages while on procarbazine therapy since there may be an Antabuse (disulfiram)-like reaction. They should also be cautioned to avoid foods with known high tyramine content such as wine, yogurt, ripe cheese and bananas. Over-the-counter drug preparations which contain antihistamines or sympathomimetic drugs should also be avoided. Patients taking procarbazine should also be warned against the use of prescription drugs without the knowledge and consent of their physician. Patients should be advised to discontinue tobacco use.
Precautions with Alcohol
- Alcohol-procarbazine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Procarbazine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Procarbazine
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Procarbazine |Label Name=procarbazine02.png
}}
{{#subobject:
|Label Page=Procarbazine |Label Name=procarbazine03.png
}}