Pioglitazone/Glimepiride: Difference between revisions
No edit summary |
Rabin Bista (talk | contribs) No edit summary |
||
| (10 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag={{SS}} | |authorTag={{SS}}, {{RB}} | ||
|genericName=Pioglitazone/Glimepiride | |genericName=Pioglitazone/Glimepiride | ||
|aOrAn=a | |aOrAn=a | ||
|drugClass=Thiazolidinedione | |drugClass=[[Thiazolidinedione]] | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=glycemic control in adults with [[type 2 diabetes mellitus]] who are already treated with a [[thiazolidinedione]] and sulfonylurea or who have inadequate glycemic control on a [[thiazolidinedione]] alone or a sulfonylurea alone | |indication=glycemic control in adults with [[type 2 diabetes mellitus]] who are already treated with a [[thiazolidinedione]] and [[sulfonylurea]] or who have inadequate glycemic control on a [[thiazolidinedione]] alone or a [[sulfonylurea]] alone | ||
|hasBlackBoxWarning=Yes | |hasBlackBoxWarning=Yes | ||
|adverseReactions=[[ | |adverseReactions=[[edema]], [[edema]] of lower extremity, [[hypoglycemia]], [[Weight gain|weight increased]], [[diarrhea]], [[nausea]], [[backache]], [[myalgia]], pain in limb, [[headache]], [[Urinary tract infection|urinary tract infectious disease]], [[pharyngitis]], [[sinusitis]], [[upper respiratory infection]], [[Motor vehicle accident|accidental injury]], [[influenza]] | ||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">WARNING: CONGESTIVE HEART FAILURE</span></b> | |blackBoxWarningTitle=<b><span style="color:#FF0000;">WARNING: CONGESTIVE HEART FAILURE</span></b> | ||
|blackBoxWarningBody=<span style="color:#FF0000;"> | |blackBoxWarningBody=<span style="color:#FF0000;"> | ||
Thiazolidinediones, including pioglitazone, which is a component of DUETACT, cause or exacerbate | Thiazolidinediones, including pioglitazone, which is a component of DUETACT, cause or exacerbate Congestive heart failure in some patients. | ||
* After initiation of DUETACT, and after dose increases, monitor patients carefully for signs and symptoms of heart failure (e.g., excessive, rapid weight gain, dyspnea, and/or edema). If heart failure develops, it should be managed according to current standards of care and discontinuation or dose reduction of DUETACT must be considered. | * After initiation of DUETACT, and after dose increases, monitor patients carefully for signs and symptoms of heart failure (e.g., excessive, rapid weight gain, dyspnea, and/or edema). If heart failure develops, it should be managed according to current standards of care and discontinuation or dose reduction of DUETACT must be considered. | ||
* DUETACT is not recommended in patients with symptomatic heart failure. | * DUETACT is not recommended in patients with symptomatic heart failure. | ||
* Initiation of DUETACT in patients with established New York Heart Association (NYHA) Class III or IV heart failure is contraindicated. | * Initiation of DUETACT in patients with established New York Heart Association (NYHA) Class III or IV heart failure is contraindicated.</span> | ||
|fdaLIADAdult=<h4> | |fdaLIADAdult=<h4>Type 2 Diabetes Mellitus</h4> | ||
* Recommendations for All Patients | * Recommendations for All Patients | ||
:* DUETACT should be taken once daily with the first main meal. | :* DUETACT should be taken once daily with the first main meal. | ||
:* DUETACT tablets are available as a 30 mg pioglitazone plus 2 mg glimepiride or a 30 mg pioglitazone plus 4 mg glimepiride tablet. If therapy with a combination tablet containing pioglitazone and glimepiride is considered appropriate the recommended starting dose is: | :* DUETACT tablets are available as a 30 mg pioglitazone plus 2 mg glimepiride or a 30 mg pioglitazone plus 4 mg glimepiride tablet. If therapy with a combination tablet containing [[pioglitazone]] and [[glimepiride]] is considered appropriate the recommended starting dose is: | ||
::* '''30 mg/2 mg''' or '''30 mg/4 mg once daily''' and gradually titrated, as needed, after assessing adequacy of therapeutic response and tolerability, | ::* '''30 mg/2 mg''' or '''30 mg/4 mg once daily''' and gradually titrated, as needed, after assessing adequacy of therapeutic response and tolerability, | ||
| Line 29: | Line 29: | ||
::* for patients inadequately controlled on pioglitazone monotherapy: '''30 mg/2 mg once daily''' and gradually titrated, as needed, after assessing adequacy of therapeutic response and tolerability, | ::* for patients inadequately controlled on pioglitazone monotherapy: '''30 mg/2 mg once daily''' and gradually titrated, as needed, after assessing adequacy of therapeutic response and tolerability, | ||
::* for patients who are changing from combination therapy of pioglitazone plus glimepiride as separate tablets: DUETACT should be taken at doses that are as close as possible to the dose of pioglitazone and glimepiride already being taken, | ::* for patients who are changing from combination therapy of pioglitazone plus glimepiride as separate tablets: DUETACT should be taken at doses that are as close as possible to the dose of [[pioglitazone]] and [[glimepiride]] already being taken, | ||
::* for patients currently on a different sulfonylurea monotherapy or switching from combination therapy of pioglitazone plus a different sulfonylurea (e.g., glyburide, glipizide, chlorpropamide, tolbutamide, acetohexamide): 30 mg/2 mg once daily and adjusted after assessing adequacy of therapeutic response. Observe for [[hypoglycemia]] for one to two weeks due to the potential overlapping drug effect. | ::* for patients currently on a different sulfonylurea monotherapy or switching from combination therapy of pioglitazone plus a different sulfonylurea (e.g., [[glyburide]], [[glipizide]], [[chlorpropamide]], [[tolbutamide]], [[acetohexamide]]): 30 mg/2 mg once daily and adjusted after assessing adequacy of therapeutic response. Observe for [[hypoglycemia]] for one to two weeks due to the potential overlapping drug effect. | ||
::* for patients with systolic dysfunction, the lowest approved dose of DUETACT should be prescribed only after titration from 15 mg to 30 mg of pioglitazone has been safely tolerated. | ::* for patients with systolic dysfunction, the lowest approved dose of DUETACT should be prescribed only after titration from 15 mg to 30 mg of pioglitazone has been safely tolerated. | ||
* After initiation of DUETACT or with dose increase, monitor patients carefully for [[hypoglycemia]] and adverse reactions related to fluid retention such as weight gain, edema, and signs and symptoms of [[congestive heart failure]] . | * After initiation of DUETACT or with dose increase, monitor patients carefully for [[hypoglycemia]] and adverse reactions related to fluid retention such as weight gain, [[edema]], and signs and symptoms of [[congestive heart failure]] . | ||
* Liver tests (serum alanine and aspartate aminotransferases, alkaline phosphatase, and total bilirubin) should be obtained prior to initiating DUETACT. Routine periodic monitoring of liver tests during treatment with DUETACT is not recommended in patients without liver disease. Patients who have liver test abnormalities prior to initiation of DUETACT or who are found to have abnormal liver tests while taking DUETACT should be managed as described under Warnings and Precautions . | * Liver tests (serum alanine and [[aspartate aminotransferases]], [[alkaline phosphatase]], and total [[bilirubin]]) should be obtained prior to initiating DUETACT. Routine periodic monitoring of liver tests during treatment with DUETACT is not recommended in patients without liver disease. Patients who have liver test abnormalities prior to initiation of DUETACT or who are found to have abnormal liver tests while taking DUETACT should be managed as described under Warnings and Precautions . | ||
<h4>Concomitant Use with an Insulin Secretagogue or Insulin</h4> | <h4>Concomitant Use with an Insulin Secretagogue or Insulin</h4> | ||
| Line 46: | Line 46: | ||
<i>Concomitant Use with Strong CYP2C8 Inhibitors</i> | <i>Concomitant Use with Strong CYP2C8 Inhibitors</i> | ||
* Coadministration of pioglitazone and gemfibrozil, a strong CYP2C8 inhibitor, increases pioglitazone exposure approximately 3-fold. Therefore, the maximum recommended dose of pioglitazone is '''15 mg daily''' when used in combination with gemfibrozil or other strong CYP2C8 inhibitors. If gemfibrozil or other CYP2C8 inhibitors need to co-administered, patients should switch to individual components of DUETACT because the minimum dose of pioglitazone in DUETACT exceeds '''15 mg''' . | * Coadministration of pioglitazone and gemfibrozil, a strong [[CYP2C8]] inhibitor, increases [[pioglitazone]] exposure approximately 3-fold. Therefore, the maximum recommended dose of pioglitazone is '''15 mg daily''' when used in combination with [[gemfibrozil]] or other strong [[CYP2C8]] inhibitors. If [[gemfibrozil]] or other [[CYP2C8]] inhibitors need to co-administered, patients should switch to individual components of DUETACT because the minimum dose of [[pioglitazone]] in DUETACT exceeds '''15 mg''' . | ||
<i>Concomitant Use with | <i>Concomitant Use with Colesevelam</i> | ||
* When colesevelam is coadministered with glimepiride, maximum plasma concentration and total exposure to glimepiride is reduced. Therefore, DUETACT should be administered at least four hours prior to [[colesevelam]] . | * When [[colesevelam]] is coadministered with glimepiride, maximum plasma concentration and total exposure to glimepiride is reduced. Therefore, DUETACT should be administered at least four hours prior to [[colesevelam]] . | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Pioglitazone/Glimepiride in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Pioglitazone/Glimepiride in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Pioglitazone/Glimepiride in adult patients. | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Pioglitazone/Glimepiride in adult patients. | ||
| Line 56: | Line 56: | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Pioglitazone/Glimepiride in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Pioglitazone/Glimepiride in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Pioglitazone/Glimepiride in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Pioglitazone/Glimepiride in pediatric patients. | ||
|contraindications=* Initiation in patients with established NYHA Class III or IV heart failure . | |contraindications=* Initiation in patients with established NYHA Class III or IV [[heart failure]] . | ||
* Use in patients with known hypersensitivity to pioglitazone, glimepiride or any other component of DUETACT . | * Use in patients with known [[hypersensitivity]] to [[pioglitazone]], [[glimepiride]] or any other component of DUETACT . | ||
* Use in patients with known history of an allergic reaction to sulfonamide derivatives. | * Use in patients with known history of an allergic reaction to sulfonamide derivatives. | ||
|warnings====Congestive | |warnings====Congestive heart failure=== | ||
====Pioglitazone==== | ====Pioglitazone==== | ||
Pioglitazone, like other thiazolidinediones, can cause dose-related fluid retention when used alone or in combination with other antidiabetic medications and is most common when DUETACT is used in combination with insulin. Fluid retention may lead to or exacerbate | * Pioglitazone, like other [[thiazolidinediones]], can cause dose-related fluid retention when used alone or in combination with other antidiabetic medications and is most common when DUETACT is used in combination with [[insulin]]. Fluid retention may lead to or exacerbate [[Congestive heart failure]]. Patients should be observed for signs and symptoms of [[Congestive heart failure]]. If [[Congestive heart failure]] develops, it should be managed according to current standards of care and discontinuation or dose reduction of DUETACT must be considered . | ||
===Hypoglycemia=== | ===Hypoglycemia=== | ||
| Line 71: | Line 71: | ||
====Glimepiride==== | ====Glimepiride==== | ||
All sulfonylureas, including glimepiride, a component of DUETACT, can cause severe hypoglycemia | * All sulfonylureas, including glimepiride, a component of DUETACT, can cause severe [[hypoglycemia]] . The patient's ability to concentrate and react may be impaired as a result of [[hypoglycemia]]. These impairments may present a risk in situations where these abilities are especially important, such as driving or operating other machinery. Severe [[hypoglycemia]] can lead to unconsciousness or [[convulsions]] and may result in temporary or permanent impairment of brain function or death. | ||
Patients must be educated to recognize and manage hypoglycemia. Use caution when initiating and increasing DUETACT doses in patients who may be predisposed to hypoglycemia (e.g., the elderly, patients with renal impairment, patients on other antidiabetic medications). Debilitated or malnourished patients and those with adrenal, pituitary, or hepatic impairment are particularly susceptible to the hypoglycemic action of glucose-lowering medications. | * Patients must be educated to recognize and manage [[hypoglycemia]]. Use caution when initiating and increasing DUETACT doses in patients who may be predisposed to [[hypoglycemia]] (e.g., the elderly, patients with renal impairment, patients on other antidiabetic medications). Debilitated or malnourished patients and those with adrenal, pituitary, or hepatic impairment are particularly susceptible to the hypoglycemic action of glucose-lowering medications. [[hypoglycemia]] is also more likely to occur when caloric intake is deficient, after severe or prolonged exercise, or when alcohol is ingested. | ||
Early warning symptoms of hypoglycemia may be different or less pronounced in patients with autonomic neuropathy, the elderly, and in patients who are taking beta-adrenergic blocking medications or other sympatholytic agents. These situations may result in severe hypoglycemia before the patient is aware of the hypoglycemia. | Early warning symptoms of [[hypoglycemia]] may be different or less pronounced in patients with autonomic [[neuropathy]], the elderly, and in patients who are taking beta-adrenergic blocking medications or other sympatholytic agents. These situations may result in severe [[hypoglycemia]] before the patient is aware of the [[hypoglycemia]]. | ||
===Hypersensitivity Reactions=== | ===Hypersensitivity Reactions=== | ||
| Line 79: | Line 79: | ||
====Glimepiride==== | ====Glimepiride==== | ||
There have been postmarketing reports of hypersensitivity reactions in patients treated with glimepiride, a component of DUETACT, including serious reactions such as anaphylaxis, angioedema, and Stevens-Johnson Syndrome. If a hypersensitivity reaction is suspected, promptly discontinue DUETACT, assess for other potential causes for the reaction, and institute alternative treatment for diabetes. | * There have been postmarketing reports of hypersensitivity reactions in patients treated with [[glimepiride]], a component of DUETACT, including serious reactions such as [[anaphylaxis]], [[angioedema]], and [[Stevens-Johnson Syndrome]]. If a [[hypersensitivity reaction]] is suspected, promptly discontinue DUETACT, assess for other potential causes for the reaction, and institute alternative treatment for diabetes. | ||
===Potential Increased Risk of Cardiovascular Mortality with Sulfonylureas=== | ===Potential Increased Risk of Cardiovascular Mortality with Sulfonylureas=== | ||
| Line 85: | Line 85: | ||
====Glimepiride==== | ====Glimepiride==== | ||
The administration of oral hypoglycemic drugs has been reported to be associated with increased cardiovascular mortality as compared to treatment with diet alone or diet plus insulin. This warning is based on the study conducted by the University Group Diabetes Program (UGDP), a long-term, prospective clinical trial designed to evaluate the effectiveness of glucose-lowering drugs in preventing or delaying vascular complications in patients with non-insulin-dependent diabetes. The study involved 823 patients who were randomly assigned to one of four treatment groups. | * The administration of oral hypoglycemic drugs has been reported to be associated with increased cardiovascular mortality as compared to treatment with diet alone or diet plus insulin. This warning is based on the study conducted by the University Group Diabetes Program (UGDP), a long-term, prospective clinical trial designed to evaluate the effectiveness of glucose-lowering drugs in preventing or delaying vascular complications in patients with non-insulin-dependent diabetes. The study involved 823 patients who were randomly assigned to one of four treatment groups. | ||
UGDP reported that patients treated for 5 to 8 years with diet plus a fixed dose of tolbutamide (1.5 grams per day) had a rate of cardiovascular mortality approximately 2.5 times that of patients treated with diet alone. A significant increase in total mortality was not observed, but the use of tolbutamide was discontinued based on the increase in cardiovascular mortality, thus limiting the opportunity for the study to show an increase in overall mortality. Despite controversy regarding the interpretation of these results, the findings of the UGDP study provide an adequate basis for this warning. The patient should be informed of the potential risks and advantages of glimepiride tablets and of alternative modes of therapy. | * UGDP reported that patients treated for 5 to 8 years with diet plus a fixed dose of [[tolbutamide]] (1.5 grams per day) had a rate of cardiovascular mortality approximately 2.5 times that of patients treated with diet alone. A significant increase in total mortality was not observed, but the use of tolbutamide was discontinued based on the increase in cardiovascular mortality, thus limiting the opportunity for the study to show an increase in overall mortality. Despite controversy regarding the interpretation of these results, the findings of the UGDP study provide an adequate basis for this warning. The patient should be informed of the potential risks and advantages of [[glimepiride]] tablets and of alternative modes of therapy. | ||
Although only one drug in the sulfonylurea class (tolbutamide) was included in this study, it is prudent from a safety standpoint to consider that this warning may also apply to other oral hypoglycemic drugs in this class, in view of their close similarities in mode of action and chemical structure. | * Although only one drug in the [[sulfonylurea]] class ([[tolbutamide]]) was included in this study, it is prudent from a safety standpoint to consider that this warning may also apply to other oral hypoglycemic drugs in this class, in view of their close similarities in mode of action and chemical structure. | ||
===Hepatic Effects=== | ===Hepatic Effects=== | ||
| Line 93: | Line 93: | ||
====Pioglitazone==== | ====Pioglitazone==== | ||
There have been postmarketing reports of fatal and non-fatal hepatic failure in patients taking pioglitazone, although the reports contain insufficient information necessary to establish the probable cause. There has been no evidence of drug-induced hepatotoxicity in the pioglitazone-controlled clinical trial database to date . | * There have been postmarketing reports of fatal and non-fatal [[hepatic failure]] in patients taking pioglitazone, although the reports contain insufficient information necessary to establish the probable cause. There has been no evidence of drug-induced [[hepatotoxicity]] in the pioglitazone-controlled clinical trial database to date . | ||
Patients with type 2 diabetes may have fatty liver disease or cardiac disease with episodic | * Patients with type 2 diabetes may have fatty liver disease or cardiac disease with episodic [[Congestive heart failure]], both of which may cause liver test abnormalities, and they may also have other forms of liver disease, many of which can be treated or managed. Therefore, obtaining a liver test panel (serum [[alanine aminotransferase]] ([[ALT]]), [[aspartate aminotransferase]] ([[AST]]), [[alkaline phosphatase]], and total [[bilirubin]]) and assessing the patient is recommended before initiating DUETACT therapy. In patients with abnormal liver tests, DUETACT should be initiated with caution. | ||
Measure liver tests promptly in patients who report symptoms that may indicate liver injury, including fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice. In this clinical context, if the patient is found to have abnormal liver tests (ALT greater than 3 times the upper limit of the reference range), DUETACT treatment should be interrupted and investigation done to establish the probable cause. DUETACT should not be restarted in these patients without another explanation for the liver test abnormalities. | * Measure liver tests promptly in patients who report symptoms that may indicate liver injury, including [[fatigue]], [[anorexia]], right upper abdominal discomfort, [[dark urine]] or [[jaundice]]. In this clinical context, if the patient is found to have abnormal liver tests ([[ALT]] greater than 3 times the upper limit of the reference range), DUETACT treatment should be interrupted and investigation done to establish the probable cause. DUETACT should not be restarted in these patients without another explanation for the liver test abnormalities. | ||
Patients who have serum ALT greater than three times the reference range with serum total bilirubin greater than two times the reference range without alternative etiologies are at risk for severe drug-induced liver injury and should not be restarted on DUETACT. For patients with lesser elevations of serum ALT or bilirubin and with an alternate probable cause, treatment with DUETACT can be used with caution. | * Patients who have serum ALT greater than three times the reference range with serum total [[bilirubin]] greater than two times the reference range without alternative etiologies are at risk for severe drug-induced liver injury and should not be restarted on DUETACT. For patients with lesser elevations of serum ALT or [[bilirubin]] and with an alternate probable cause, treatment with DUETACT can be used with caution. | ||
===Urinary Bladder Tumors=== | ===Urinary Bladder Tumors=== | ||
| Line 102: | Line 102: | ||
====Pioglitazone==== | ====Pioglitazone==== | ||
Tumors were observed in the urinary bladder of male rats in the two-year carcinogenicity study | * Tumors were observed in the urinary bladder of male rats in the two-year carcinogenicity study . In two 3-year trials in which [[pioglitazone]] was compared to placebo or [[glyburide]], there were 16/3656 (0.44%) reports of [[bladder cancer]] in patients taking [[pioglitazone]] compared to 5/3679 (0.14%) in patients not taking pioglitazone. After excluding patients in whom exposure to study drug was less than one year at the time of diagnosis of [[bladder cancer]], there were six (0.16%) cases on pioglitazone and two (0.05%) cases on placebo. | ||
A five-year interim report of an ongoing 10-year observational cohort study found a non-significant increase in the risk for bladder cancer in subjects ever exposed to pioglitazone, compared to subjects never exposed to pioglitazone (HR 1.2 [95% CI 0.9 −1.5]). Compared to never exposure, a duration of pioglitazone therapy longer than 12 months was associated with an increase in risk (HR 1.4 [95% CI 0.9 −2.1]), which reached statistical significance after more than 24 months of pioglitazone use (HR 1.4 [95% CI 1.03 −2.0]). Interim results from this study suggested that taking pioglitazone longer than 12 months increased the relative risk of developing bladder cancer in any given year by 40% which equates to an absolute increase of three cases in 10,000 (from approximately seven in 10,000 [without pioglitazone] to approximately 10 in 10,000 [with pioglitazone]). | * A five-year interim report of an ongoing 10-year observational cohort study found a non-significant increase in the risk for [[bladder cancer]] in subjects ever exposed to pioglitazone, compared to subjects never exposed to [[pioglitazone]] (HR 1.2 [95% CI 0.9 −1.5]). Compared to never exposure, a duration of [[pioglitazone]] therapy longer than 12 months was associated with an increase in risk (HR 1.4 [95% CI 0.9 −2.1]), which reached statistical significance after more than 24 months of [[pioglitazone]] use (HR 1.4 [95% CI 1.03 −2.0]). Interim results from this study suggested that taking pioglitazone longer than 12 months increased the relative risk of developing [[bladder cancer]] in any given year by 40% which equates to an absolute increase of three cases in 10,000 (from approximately seven in 10,000 [without pioglitazone] to approximately 10 in 10,000 [with pioglitazone]). | ||
There are insufficient data to determine whether pioglitazone is a tumor promoter for urinary bladder tumors. Consequently, DUETACT should not be used in patients with active bladder cancer and the benefits of glycemic control versus unknown risks for cancer recurrence with DUETACT should be considered in patients with a prior history of bladder cancer. | There are insufficient data to determine whether [[pioglitazone]] is a tumor promoter for urinary bladder tumors. Consequently, DUETACT should not be used in patients with active [[bladder cancer]] and the benefits of glycemic control versus unknown risks for cancer recurrence with DUETACT should be considered in patients with a prior history of [[bladder cancer]]. | ||
===Edema=== | ===Edema=== | ||
| Line 110: | Line 110: | ||
====Pioglitazone==== | ====Pioglitazone==== | ||
In controlled clinical trials, edema was reported more frequently in patients treated with pioglitazone than in placebo-treated patients and is dose-related | * In controlled clinical trials, [[edema]] was reported more frequently in patients treated with pioglitazone than in placebo-treated patients and is dose-related * In postmarketing experience, reports of new onset or worsening [[edema]] have been received. | ||
DUETACT should be used with caution in patients with edema. Because thiazolidinediones, including pioglitazone, can cause fluid retention, which can exacerbate or lead to | * DUETACT should be used with caution in patients with [[edema]]. Because [[thiazolidinediones]], including pioglitazone, can cause fluid retention, which can exacerbate or lead to [[Congestive heart failure]], DUETACT should be used with caution in patients at risk for [[Congestive heart failure]]. Patients treated with DUETACT should be monitored for signs and symptoms of [[Congestive heart failure]] . | ||
===Fractures=== | ===Fractures=== | ||
| Line 117: | Line 117: | ||
====Pioglitazone==== | ====Pioglitazone==== | ||
In PROactive (the Prospective Pioglitazone Clinical Trial in Macrovascular Events), 5238 patients with type 2 diabetes and a history of macrovascular disease were randomized to pioglitazone (N=2605), force-titrated up to 45 mg daily or placebo (N=2633) in addition to standard of care. During a mean follow-up of 34.5 months, the incidence of bone fracture in females was 5.1% (44/870) for pioglitazone versus 2.5% (23/905) for placebo. This difference was noted after the first year of treatment and persisted during the course of the study. The majority of fractures observed in female patients were nonvertebral fractures including lower limb and distal upper limb. No increase in the incidence of fracture was observed in men treated with pioglitazone (1.7%) versus placebo (2.1%). The risk of fracture should be considered in the care of patients, especially female patients, treated with DUETACT and attention should be given to assessing and maintaining bone health according to current standards of care. | * In PROactive (the Prospective Pioglitazone Clinical Trial in Macrovascular Events), 5238 patients with type 2 diabetes and a history of macrovascular disease were randomized to [[pioglitazone]] (N=2605), force-titrated up to 45 mg daily or placebo (N=2633) in addition to standard of care. During a mean follow-up of 34.5 months, the incidence of bone fracture in females was 5.1% (44/870) for [[pioglitazone]] versus 2.5% (23/905) for placebo. This difference was noted after the first year of treatment and persisted during the course of the study. The majority of fractures observed in female patients were nonvertebral fractures including lower limb and distal upper limb. No increase in the incidence of fracture was observed in men treated with pioglitazone (1.7%) versus placebo (2.1%). The risk of fracture should be considered in the care of patients, especially female patients, treated with DUETACT and attention should be given to assessing and maintaining bone health according to current standards of care. | ||
===Hemolytic Anemia=== | ===Hemolytic Anemia=== | ||
| Line 123: | Line 123: | ||
====Glimepiride==== | ====Glimepiride==== | ||
Sulfonylureas can cause hemolytic anemia in patients with glucose 6-phosphate dehydrogenase (G6PD) deficiency. Because DUETACT contains glimepiride, which belongs to the class of sulfonylurea agents, use caution in patients with G6PD deficiency and consider the use of a nonsulfonylurea alternative. There are also postmarketing reports of hemolytic anemia in patients receiving glimepiride who did not have known G6PD deficiency . | * [[Sulfonylureas]] can cause [[hemolytic anemia]] in patients with glucose 6-phosphate dehydrogenase (G6PD) deficiency. Because DUETACT contains [[glimepiride]], which belongs to the class of [[sulfonylurea]] agents, use caution in patients with [[G6PD deficiency]] and consider the use of a nonsulfonylurea alternative. There are also postmarketing reports of [[hemolytic anemia]] in patients receiving [[glimepiride]] who did not have known [[G6PD deficiency]] | ||
. | |||
===Macular | ===Macular edema=== | ||
====Pioglitazone==== | ====Pioglitazone==== | ||
Macular edema has been reported in postmarketing experience in diabetic patients who were taking pioglitazone or another thiazolidinedione. Some patients presented with blurred vision or decreased visual acuity, but others were diagnosed on routine ophthalmologic examination. | * [[Macular edema]] has been reported in postmarketing experience in diabetic patients who were taking pioglitazone or another [[thiazolidinedione]]. Some patients presented with [[blurred vision]] or decreased visual acuity, but others were diagnosed on routine ophthalmologic examination. | ||
Most patients had peripheral edema at the time | * Most patients had peripheral [[edema]] at the time [[Macular edema]] was diagnosed. Some patients had improvement in their [[Macular edema]] after discontinuation of the [[thiazolidinedione]]. | ||
Patients with diabetes should have regular eye exams by an ophthalmologist according to current standards of care. Patients with diabetes who report any visual symptoms should be promptly referred to an ophthalmologist, regardless of the patient’s underlying medications or other physical findings . | * Patients with diabetes should have regular eye exams by an ophthalmologist according to current standards of care. Patients with diabetes who report any visual symptoms should be promptly referred to an ophthalmologist, regardless of the patient’s underlying medications or other physical findings . | ||
===Ovulation=== | ===Ovulation=== | ||
| Line 137: | Line 138: | ||
====Pioglitazone==== | ====Pioglitazone==== | ||
Therapy with pioglitazone, like other thiazolidinediones, may result in ovulation in some premenopausal anovulatory women. As a result, these patients may be at an increased risk for pregnancy while taking DUETACT. This effect has not been investigated in clinical trials, so the frequency of this occurrence is not known. Adequate contraception in all premenopausal women treated with DUETACT is recommended. | * Therapy with pioglitazone, like other [[thiazolidinediones]], may result in ovulation in some premenopausal anovulatory women. As a result, these patients may be at an increased risk for pregnancy while taking DUETACT. This effect has not been investigated in clinical trials, so the frequency of this occurrence is not known. Adequate contraception in all premenopausal women treated with DUETACT is recommended. | ||
===Macrovascular Outcomes=== | ===Macrovascular Outcomes=== | ||
There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with DUETACT or any other antidiabetic drug. | * There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with DUETACT or any other antidiabetic drug. | ||
|clinicalTrials=Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | |clinicalTrials=* Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | ||
The adverse events reported in at least 5% of patients in the controlled 16-week clinical studies between placebo plus a sulfonylurea and pioglitazone (15 mg and 30 mg combined) plus sulfonylurea treatment arms were upper respiratory tract infection (15.5% and 16.6%), accidental injury (8.6% and 3.5%), and combined edema/peripheral edema (2.1% and 7.2%), respectively. | The adverse events reported in at least 5% of patients in the controlled 16-week clinical studies between placebo plus a [[sulfonylurea]] and [[pioglitazone]] (15 mg and 30 mg combined) plus sulfonylurea treatment arms were [[upper respiratory tract infection]] (15.5% and 16.6%), accidental injury (8.6% and 3.5%), and combined [[edema]]/[[peripheral edema]] (2.1% and 7.2%), respectively. | ||
The incidence and type of adverse events reported in at least 5% of patients in any combined treatment group from the 24-week study comparing pioglitazone 30 mg plus a sulfonylurea and pioglitazone 45 mg plus a sulfonylurea are shown in Table 1; the rate of adverse events resulting in study discontinuation between the two treatment groups was 6% and 9.7%, respectively. | * The incidence and type of adverse events reported in at least 5% of patients in any combined treatment group from the 24-week study comparing pioglitazone 30 mg plus a sulfonylurea and pioglitazone 45 mg plus a [[sulfonylurea]] are shown in Table 1; the rate of adverse events resulting in study discontinuation between the two treatment groups was 6% and 9.7%, respectively. | ||
[[File:Duetact_adverse_01.png|thumb|none| | [[File:Duetact_adverse_01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
In US double-blind studies, anemia was reported in ≤2% of patients treated with pioglitazone plus a sulfonylurea . | * In US double-blind studies, [[anemia]] was reported in ≤2% of patients treated with [[pioglitazone]] plus a [[sulfonylurea]] . | ||
===Pioglitazone=== | ===Pioglitazone=== | ||
Over 8500 patients with type 2 diabetes have been treated with pioglitazone in randomized, double-blind, controlled clinical trials, including 2605 patients with type 2 diabetes and macrovascular disease treated with pioglitazone in the PROactive clinical trial. In these trials, over 6000 patients have been treated with pioglitazone for six months or longer, over 4500 patients have been treated with pioglitazone for one year or longer, and over 3000 patients have been treated with pioglitazone for at least two years. | * Over 8500 patients with [[type 2 diabetes]] have been treated with [[pioglitazone]] in randomized, double-blind, controlled clinical trials, including 2605 patients with [[type 2 diabetes]] and macrovascular disease treated with pioglitazone in the PROactive clinical trial. In these trials, over 6000 patients have been treated with pioglitazone for six months or longer, over 4500 patients have been treated with pioglitazone for one year or longer, and over 3000 patients have been treated with pioglitazone for at least two years. | ||
In six pooled 16- to 26-week placebo-controlled monotherapy and 16- to 24-week add-on combination therapy trials, the incidence of withdrawals due to adverse events was 4.5% for patients treated with pioglitazone and 5.8% for comparator-treated patients. The most common adverse events leading to withdrawal were related to inadequate glycemic control, although the incidence of these events was lower (1.5%) with pioglitazone than with placebo (3.0%). | * In six pooled 16- to 26-week placebo-controlled monotherapy and 16- to 24-week add-on combination therapy trials, the incidence of withdrawals due to adverse events was 4.5% for patients treated with [[pioglitazone]] and 5.8% for comparator-treated patients. The most common adverse events leading to withdrawal were related to inadequate glycemic control, although the incidence of these events was lower (1.5%) with pioglitazone than with placebo (3.0%). | ||
In the PROactive trial, the incidence of withdrawals due to adverse events was 9.0% for patients treated with pioglitazone and 7.7% for placebo-treated patients. Congestive heart failure was the most common serious adverse event leading to withdrawal occurring in 1.3% of patients treated with pioglitazone and 0.6% of patients treated with placebo. | * In the PROactive trial, the incidence of withdrawals due to adverse events was 9.0% for patients treated with pioglitazone and 7.7% for placebo-treated patients. [[Congestive heart failure]] was the most common serious adverse event leading to withdrawal occurring in 1.3% of patients treated with pioglitazone and 0.6% of patients treated with placebo. | ||
'''<i>Common Adverse Events: 16- to 26-Week Monotherapy Trials:</i>''' | '''<i>Common Adverse Events: 16- to 26-Week Monotherapy Trials:</i>''' | ||
A summary of the incidence and type of common adverse events reported in three pooled 16- to 26-week placebo-controlled monotherapy trials of pioglitazone is provided in Table 2. Terms that are reported represent those that occurred at an incidence of >5% and more commonly in patients treated with pioglitazone than in patients who received placebo. None of these adverse events were related to the pioglitazone dose. | * A summary of the incidence and type of common adverse events reported in three pooled 16- to 26-week placebo-controlled monotherapy trials of [[pioglitazone]] is provided in Table 2. Terms that are reported represent those that occurred at an incidence of >5% and more commonly in patients treated with [[pioglitazone]] than in patients who received placebo. None of these adverse events were related to the [[pioglitazone]] dose. | ||
[[File:Duetact_adverse_02.png|thumb|none| | [[File:Duetact_adverse_02.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
A summary of the overall incidence and types of common adverse events reported in the PROactive trial is provided in Table 3. Terms that are reported represent those that occurred at an incidence of >5% and more commonly in patients treated with pioglitazone than in patients who received placebo. | * A summary of the overall incidence and types of common adverse events reported in the PROactive trial is provided in Table 3. Terms that are reported represent those that occurred at an incidence of >5% and more commonly in patients treated with pioglitazone than in patients who received placebo. | ||
[[File:Duetact_adverse_03.png|thumb|none| | [[File:Duetact_adverse_03.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
'''<i>Congestive | '''<i>Congestive heart failure</i>''' | ||
A summary of the incidence of adverse events related to | * A summary of the incidence of adverse events related to [[Congestive heart failure]] is provided in Table 4 for the 16- to 24-week add-on to [[sulfonylurea]] trials, for the 16- to 24-week add-on to [[insulin]] trials, and for the 16- to 24-week add-on to [[metformin]] trials. None of the events were fatal. | ||
[[File:Duetact_adverse_04.png|thumb|none| | [[File:Duetact_adverse_04.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
Patients with type 2 diabetes and NYHA class II or early class III | * Patients with [[type 2 diabetes]] and [[NYHA class II]] or early class III [[Congestive heart failure]] were randomized to receive 24 weeks of double-blind treatment with either [[pioglitazone]] at daily doses of 30 mg to 45 mg (n=262) or [[glyburide]] at daily doses of 10 mg to 15 mg (n=256). A summary of the incidence of adverse events related to [[Congestive heart failure]] reported in this study is provided in Table 5. | ||
[[File:Duetact_adverse_05.png|thumb|none| | [[File:Duetact_adverse_05.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
Congestive heart failure events leading to hospitalization that occurred during the PROactive trial are summarized in Table 6. | * [[Congestive heart failure]] events leading to hospitalization that occurred during the PROactive trial are summarized in Table 6. | ||
[[File:Duetact_adverse_06.png|thumb|none| | [[File:Duetact_adverse_06.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
'''<i>Cardiovascular Safety</i>''' | '''<i>Cardiovascular Safety</i>''' | ||
In the PROactive trial, 5238 patients with type 2 diabetes and a history of macrovascular disease were randomized to pioglitazone (N=2605), force-titrated up to 45 mg daily or placebo (N=2633) in addition to standard of care. Almost all patients (95%) were receiving cardiovascular medications (beta blockers, ACE inhibitors, angiotensin II receptor blockers, calcium channel blockers, nitrates, diuretics, aspirin, statins, and fibrates). At baseline, patients had a mean age of 62 years, mean duration of diabetes of 9.5 years, and mean HbA1c of 8.1%. Mean duration of follow-up was 34.5 months. | * In the PROactive trial, 5238 patients with type 2 diabetes and a history of macrovascular disease were randomized to [[pioglitazone]] (N=2605), force-titrated up to 45 mg daily or placebo (N=2633) in addition to standard of care. Almost all patients (95%) were receiving cardiovascular medications ([[beta blockers]], [[ACE inhibitors]], [[angiotensin II receptor blockers]], [[calcium channel blockers]], [[nitrates]], [[diuretics]], [[aspirin]], [[statins]], and [[fibrates]]). At baseline, patients had a mean age of 62 years, mean duration of [[diabetes]] of 9.5 years, and mean [[HbA1c]] of 8.1%. Mean duration of follow-up was 34.5 months. | ||
The primary objective of this trial was to examine the effect of pioglitazone on mortality and macrovascular morbidity in patients with type 2 diabetes mellitus who were at high risk for macrovascular events. The primary efficacy variable was the time to the first occurrence of any event in a cardiovascular composite endpoint that included all-cause mortality, nonfatal myocardial infarction (MI) including silent MI, stroke, acute coronary syndrome, cardiac intervention including coronary artery bypass grafting or percutaneous intervention, major leg amputation above the ankle, and bypass surgery or revascularization in the leg. A total of 514 (19.7%) patients treated with pioglitazone and 572 (21.7%) placebo-treated patients experienced at least one event from the primary composite endpoint (hazard ratio 0.90; 95% Confidence Interval: 0.80, 1.02; p=0.10). | * The primary objective of this trial was to examine the effect of pioglitazone on mortality and macrovascular morbidity in patients with [[type 2 diabetes mellitus]] who were at high risk for macrovascular events. The primary efficacy variable was the time to the first occurrence of any event in a cardiovascular composite endpoint that included all-cause mortality, nonfatal [[myocardial infarction]] (MI) including silent MI, [[stroke]], [[acute coronary syndrome]], cardiac intervention including [[coronary artery bypass grafting]] or percutaneous intervention, major leg amputation above the ankle, and bypass surgery or revascularization in the leg. A total of 514 (19.7%) patients treated with pioglitazone and 572 (21.7%) placebo-treated patients experienced at least one event from the primary composite endpoint (hazard ratio 0.90; 95% Confidence Interval: 0.80, 1.02; p=0.10). | ||
Although there was no statistically significant difference between pioglitazone and placebo for the three-year incidence of a first event within this composite, there was no increase in mortality or in total macrovascular events with pioglitazone. The number of first occurrences and total individual events contributing to the primary composite endpoint is shown in Table 7. | * Although there was no statistically significant difference between [[pioglitazone]] and placebo for the three-year incidence of a first event within this composite, there was no increase in mortality or in total macrovascular events with pioglitazone. The number of first occurrences and total individual events contributing to the primary composite endpoint is shown in Table 7. | ||
[[File:Duetact_adverse_07.png|thumb|none| | [[File:Duetact_adverse_07.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
'''<i>Weight Gain</i>''' | '''<i>Weight Gain</i>''' | ||
Dose-related weight gain occurs when pioglitazone is used alone or in combination with other antidiabetic medications. The mechanism of weight gain is unclear but probably involves a combination of fluid retention and fat accumulation. | * Dose-related weight gain occurs when pioglitazone is used alone or in combination with other antidiabetic medications. The mechanism of weight gain is unclear but probably involves a combination of fluid retention and fat accumulation. | ||
Tables 8 and 9 summarize the changes in body weight with pioglitazone and placebo in the 16- to 26-week randomized, double-blind monotherapy and 16- to 24-week combination add-on therapy trials and in the PROactive trial. | * Tables 8 and 9 summarize the changes in body weight with pioglitazone and placebo in the 16- to 26-week randomized, double-blind monotherapy and 16- to 24-week combination add-on therapy trials and in the PROactive trial. | ||
[[File:Duetact_adverse_08.png|thumb|none| | [[File:Duetact_adverse_08.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
[[File:Duetact_adverse_09.png|thumb|none| | [[File:Duetact_adverse_09.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
'''<i>Edema</i>''' | '''<i>Edema</i>''' | ||
* [[edema]] induced from taking pioglitazone is reversible when pioglitazone is discontinued. The [[edema]] usually does not require hospitalization unless there is coexisting [[Congestive heart failure]]. A summary of the frequency and types of [[edema]] adverse events occurring in clinical investigations of pioglitazone is provided in Table 10. | |||
[[File:Duetact_adverse_10.png|thumb|none| | [[File:Duetact_adverse_10.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
'''<i>Hepatic Effects</i>''' | '''<i>Hepatic Effects</i>''' | ||
There has been no evidence of pioglitazone-induced hepatotoxicity in the pioglitazone-controlled clinical trial database to date. One randomized, double-blind, 3-year trial comparing pioglitazone to glyburide as add-on to metformin and insulin therapy was specifically designed to evaluate the incidence of serum ALT elevation to greater than three times the upper limit of the reference range, measured every eight weeks for the first 48 weeks of the trial then every 12 weeks thereafter. A total of 3/1051 (0.3%) patients treated with pioglitazone and 9/1046 (0.9%) patients treated with glyburide developed ALT values greater than three times the upper limit of the reference range. None of the patients treated with pioglitazone in the pioglitazone-controlled clinical trial database to date have had a serum ALT greater than three times the upper limit of the reference range and a corresponding total bilirubin greater than two times the upper limit of the reference range, a combination predictive of the potential for severe drug-induced liver injury. | * There has been no evidence of pioglitazone-induced [[hepatotoxicity]] in the pioglitazone-controlled clinical trial database to date. One randomized, double-blind, 3-year trial comparing [[pioglitazone]] to [[glyburide]] as add-on to [[metformin]] and [[insulin]] therapy was specifically designed to evaluate the incidence of serum ALT elevation to greater than three times the upper limit of the reference range, measured every eight weeks for the first 48 weeks of the trial then every 12 weeks thereafter. A total of 3/1051 (0.3%) patients treated with [[pioglitazone]] and 9/1046 (0.9%) patients treated with [[glyburide]] developed [[ALT]] values greater than three times the upper limit of the reference range. None of the patients treated with [[pioglitazone]] in the pioglitazone-controlled clinical trial database to date have had a serum ALT greater than three times the upper limit of the reference range and a corresponding total [[bilirubin]] greater than two times the upper limit of the reference range, a combination predictive of the potential for severe drug-induced liver injury. | ||
'''<i>Hypoglycemia</i>''' | '''<i>Hypoglycemia</i>''' | ||
In the pioglitazone clinical trials, adverse events of hypoglycemia were reported based on clinical judgment of the investigators and did not require confirmation with fingerstick glucose testing. | * In the pioglitazone clinical trials, adverse events of [[hypoglycemia]] were reported based on clinical judgment of the investigators and did not require confirmation with fingerstick glucose testing. | ||
In the 16-week add-on to sulfonylurea trial, the incidence of reported hypoglycemia was 3.7% with pioglitazone 30 mg and 0.5% with placebo. In the 16-week add-on to insulin trial, the incidence of reported hypoglycemia was 7.9% with pioglitazone 15 mg, 15.4% with pioglitazone 30 mg, and 4.8% with placebo. | * In the 16-week add-on to [[sulfonylurea]] trial, the incidence of reported [[hypoglycemia]] was 3.7% with [[pioglitazone]] 30 mg and 0.5% with placebo. In the 16-week add-on to insulin trial, the incidence of reported [[hypoglycemia]] was 7.9% with [[pioglitazone]] 15 mg, 15.4% with pioglitazone 30 mg, and 4.8% with placebo. | ||
The incidence of reported hypoglycemia was higher with pioglitazone 45 mg compared to pioglitazone 30 mg in both the 24-week add-on to sulfonylurea trial (15.7% versus 13.4%) and in the 24-week add-on to insulin trial (47.8% versus 43.5%). | The incidence of reported [[hypoglycemia]] was higher with pioglitazone 45 mg compared to pioglitazone 30 mg in both the 24-week add-on to [[sulfonylurea]] trial (15.7% versus 13.4%) and in the 24-week add-on to [[insulin]] trial (47.8% versus 43.5%). | ||
Three patients in these four trials were hospitalized due to hypoglycemia. All three patients were receiving pioglitazone 30 mg (0.9%) in the 24-week add-on to insulin trial. An additional 14 patients reported severe hypoglycemia (defined as causing considerable interference with patient’s usual activities) that did not require hospitalization. These patients were receiving pioglitazone 45 mg in combination with sulfonylurea (N=2) or pioglitazone 30 mg or 45 mg in combination with insulin (N=12). | * Three patients in these four trials were hospitalized due to [[hypoglycemia]]. All three patients were receiving pioglitazone 30 mg (0.9%) in the 24-week add-on to insulin trial. An additional 14 patients reported severe [[hypoglycemia]] (defined as causing considerable interference with patient’s usual activities) that did not require hospitalization. These patients were receiving [[pioglitazone]] 45 mg in combination with [[sulfonylurea]] (N=2) or pioglitazone 30 mg or 45 mg in combination with [[insulin]] (N=12). | ||
'''<i>Urinary Bladder Tumors</i>''' | '''<i>Urinary Bladder Tumors</i>''' | ||
Tumors were observed in the urinary bladder of male rats in the two-year carcinogenicity study | * Tumors were observed in the urinary bladder of male rats in the two-year carcinogenicity study. In two 3-year trials in which [[pioglitazone]] was compared to placebo or [[glyburide]], there were 16/3656 (0.44%) reports of [[bladder cancer]] in patients taking pioglitazone compared to 5/3679 (0.14%) in patients not taking pioglitazone. After excluding patients in whom exposure to study drug was less than one year at the time of diagnosis of [[bladder cancer]], there were six (0.16%) cases on [[pioglitazone]] and two (0.05%) cases on placebo. There are too few events of [[bladder cancer]] to establish causality. | ||
'''<i>Glimepiride</i>''' | '''<i>Glimepiride</i>''' | ||
Adverse events that occurred in controlled clinical trials with placebo and glimepiride monotherapy, other than hypoglycemia, included: headache (7.8% and 8.2%), accidental injury (3.4% and 5.8%), flu syndrome (4.4% and 5.4%), nausea (3.4% and 5.0%) and dizziness (2.4% and 5.0%), respectively. | * Adverse events that occurred in controlled clinical trials with placebo and [[glimepiride]] monotherapy, other than [[hypoglycemia]], included: [[headache]] (7.8% and 8.2%), [[accidental injury]] (3.4% and 5.8%), [[flu syndrome]] (4.4% and 5.4%), [[nausea]] (3.4% and 5.0%) and [[dizziness]] (2.4% and 5.0%), respectively. | ||
'''<i> | '''<i>hypoglycemia</i>''' | ||
In a randomized, double-blind, placebo-controlled monotherapy trial of 14 weeks duration, patients already on sulfonylurea therapy underwent a 3-week washout period then were randomized to glimepiride 1 mg, 4 mg, 8 mg or placebo. Patients randomized to glimepiride 4 mg or 8 mg underwent forced-titration from an initial dose of 1 mg to these final doses, as tolerated. The overall incidence of possible hypoglycemia (defined by the presence of at least one symptom that the investigator believed might be related to hypoglycemia; a concurrent glucose measurement was not required) was 4% for glimepiride 1 mg, 17% for glimepiride 4 mg, 16% for glimepiride 8 mg and 0% for placebo. All of these events were self-treated. | * In a randomized, double-blind, placebo-controlled monotherapy trial of 14 weeks duration, patients already on [[sulfonylurea]] therapy underwent a 3-week washout period then were randomized to [[glimepiride]] 1 mg, 4 mg, 8 mg or placebo. Patients randomized to glimepiride 4 mg or 8 mg underwent forced-titration from an initial dose of 1 mg to these final doses, as tolerated. The overall incidence of possible [[hypoglycemia]] (defined by the presence of at least one symptom that the investigator believed might be related to [[hypoglycemia]]; a concurrent glucose measurement was not required) was 4% for glimepiride 1 mg, 17% for glimepiride 4 mg, 16% for glimepiride 8 mg and 0% for placebo. All of these events were self-treated. | ||
In a randomized, double-blind, placebo-controlled monotherapy trial of 22 weeks duration, patients received a starting dose of either 1 mg glimepiride or placebo daily. The dose of glimepiride was titrated to a target fasting plasma glucose of 90 −150 mg/dL. Final daily doses of glimepiride were 1, 2, 3, 4, 6 or 8 mg. The overall incidence of possible hypoglycemia (as defined above for the 14-week trial) for glimepiride versus placebo was 19.7% vs. 3.2%. All of these events were self-treated. | * In a randomized, double-blind, placebo-controlled monotherapy trial of 22 weeks duration, patients received a starting dose of either 1 mg [[glimepiride]] or placebo daily. The dose of [[glimepiride]] was titrated to a target fasting plasma glucose of 90 −150 mg/dL. Final daily doses of [[glimepiride]] were 1, 2, 3, 4, 6 or 8 mg. The overall incidence of possible [[hypoglycemia]] (as defined above for the 14-week trial) for glimepiride versus placebo was 19.7% vs. 3.2%. All of these events were self-treated. | ||
'''<i>Weight Gain</i>''' | '''<i>Weight Gain</i>''' | ||
Glimepiride, like all sulfonylureas, can cause weight gain. | * [[Glimepiride]], like all [[sulfonylureas]], can cause [[weight gain]]. | ||
'''<i>Allergic Reactions</i>''' | '''<i>Allergic Reactions</i>''' | ||
In clinical trials, allergic reactions, such as pruritus, erythema, urticaria, and morbilliform or maculopapular eruptions, occurred in less than 1% of glimepiride-treated patients. These may resolve despite continued treatment with glimepiride. There are postmarketing reports of more serious allergic reactions (e.g., dyspnea, hypotension, shock) | * In clinical trials, allergic reactions, such as [[pruritus]], [[erythema]], [[urticaria]], and morbilliform or maculopapular eruptions, occurred in less than 1% of glimepiride-treated patients. These may resolve despite continued treatment with [[glimepiride]]. There are postmarketing reports of more serious allergic reactions (e.g., [[dyspnea]], [[hypotension]], [[shock]]). | ||
'''<i>Laboratory Tests</i>''' | '''<i>Laboratory Tests</i>''' | ||
| Line 237: | Line 238: | ||
'''<i>Elevated Serum Alanine Aminotransferase (ALT)</i>''' | '''<i>Elevated Serum Alanine Aminotransferase (ALT)</i>''' | ||
In 11 pooled placebo-controlled trials of glimepiride, 1.9% of glimepiride-treated patients and 0.8% of placebo-treated patients developed serum ALT greater than two times the upper limit of the reference range. | * In 11 pooled placebo-controlled trials of [[glimepiride]], 1.9% of glimepiride-treated patients and 0.8% of placebo-treated patients developed serum ALT greater than two times the upper limit of the reference range. | ||
'''<i>Laboratory Abnormalities</i>''' | '''<i>Laboratory Abnormalities</i>''' | ||
| Line 245: | Line 246: | ||
'''<i>Hematologic Effects</i>''' | '''<i>Hematologic Effects</i>''' | ||
Pioglitazone may cause decreases in hemoglobin and hematocrit. In placebo-controlled monotherapy trials, mean hemoglobin values declined by 2% to 4% in patients treated with pioglitazone compared with a mean change in hemoglobin of -1% to +1% in placebo-treated patients. These changes primarily occurred within the first 4 to 12 weeks of therapy and remained relatively constant thereafter. These changes may be related to increased plasma volume associated with pioglitazone therapy and are not likely to be associated with any clinically significant hematologic effects. | * Pioglitazone may cause decreases in [[hemoglobin]] and [[hematocrit]]. In placebo-controlled monotherapy trials, mean hemoglobin values declined by 2% to 4% in patients treated with pioglitazone compared with a mean change in [[hemoglobin]] of -1% to +1% in placebo-treated patients. These changes primarily occurred within the first 4 to 12 weeks of therapy and remained relatively constant thereafter. These changes may be related to increased plasma volume associated with [[pioglitazone]] therapy and are not likely to be associated with any clinically significant hematologic effects. | ||
'''<i>Creatine Phosphokinase</i>''' | '''<i>Creatine Phosphokinase</i>''' | ||
During protocol-specified measurement of serum creatine phosphokinase (CPK) in pioglitazone clinical trials, an isolated elevation in CPK to greater than 10 times the upper limit of the reference range was noted in nine (0.2%) patients treated with pioglitazone (values of 2150 to 11400 IU/L) and in no comparator-treated patients. Six of these nine patients continued to receive pioglitazone, two patients were noted to have the CPK elevation on the last day of dosing and one patient discontinued pioglitazone due to the elevation. These elevations resolved without any apparent clinical sequelae. The relationship of these events to pioglitazone therapy is unknown. | * During protocol-specified measurement of serum [[creatine phosphokinase]] (CPK) in [[pioglitazone]] clinical trials, an isolated elevation in [[CPK]] to greater than 10 times the upper limit of the reference range was noted in nine (0.2%) patients treated with [[pioglitazone]] (values of 2150 to 11400 IU/L) and in no comparator-treated patients. Six of these nine patients continued to receive [[pioglitazone]], two patients were noted to have the [[CPK]] elevation on the last day of dosing and one patient discontinued [[pioglitazone]] due to the elevation. These elevations resolved without any apparent clinical sequelae. The relationship of these events to [[pioglitazone]] therapy is unknown. | ||
|postmarketing=The following adverse reactions have been identified during post-approval use of pioglitazone and glimepiride. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | |postmarketing=* The following adverse reactions have been identified during post-approval use of [[pioglitazone]] and glimepiride. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | ||
'''Pioglitazone''' | '''Pioglitazone''' | ||
* New onset or worsening diabetic | * New onset or worsening [[diabetic Macular edema]] with decreased [[visual acuity]] . | ||
* Fatal and nonfatal hepatic failure. | * Fatal and nonfatal [[hepatic failure]]. | ||
Postmarketing reports of | * Postmarketing reports of [[Congestive heart failure]] have been reported in patients treated with [[pioglitazone]], both with and without previously known heart disease and both with and without concomitant insulin administration. | ||
In postmarketing experience, there have been reports of unusually rapid increases in weight and increases in excess of that generally observed in clinical trials. Patients who experience such increases should be assessed for fluid accumulation and volume-related events such as excessive edema and | * In postmarketing experience, there have been reports of unusually rapid increases in weight and increases in excess of that generally observed in clinical trials. Patients who experience such increases should be assessed for fluid accumulation and volume-related events such as excessive [[edema]] and [[Congestive heart failure]] . | ||
'''Glimepiride''' | '''Glimepiride''' | ||
* Serious hypersensitivity reactions, including anaphylaxis, angioedema, and Stevens-Johnson Syndrome | * Serious [[hypersensitivity reactions]], including [[anaphylaxis]], [[angioedema]], and [[Stevens-Johnson Syndrome]] | ||
* Hemolytic anemia in patients with and without G6PD deficiency | * [[Hemolytic anemia]] in patients with and without [[G6PD deficiency]] | ||
* Impairment of liver function (e.g. with cholestasis and jaundice), as well as hepatitis, which may progress to liver failure. | * Impairment of liver function (e.g. with [[cholestasis]] and [[jaundice]]), as well as [[hepatitis]], which may progress to [[liver failure]]. | ||
* Porphyria cutanea tarda, photosensitivity reactions and allergic vasculitis | * [[Porphyria cutanea tarda]], [[photosensitivity reactions]] and [[allergic vasculitis]] | ||
* Leukopenia, agranulocytosis, thrombocytopenia, aplastic anemia, and pancytopenia | * [[Leukopenia]], [[agranulocytosis]], [[thrombocytopenia]], [[aplastic anemia]], and [[pancytopenia]] | ||
* Hepatic porphyria reactions and disulfiram-like reactions | * [[Hepatic porphyria reactions]] and [[disulfiram-like reactions]] | ||
* Hyponatremia and syndrome of inappropriate antidiuretic hormone secretion (SIADH), most often in patients who are on other medications or who have medical conditions known to cause hyponatremia or increase release of antidiuretic hormone | * [[Hyponatremia]] and [[syndrome of inappropriate antidiuretic hormone secretion]] ([[SIADH]]), most often in patients who are on other medications or who have medical conditions known to cause [[hyponatremia]] or increase release of antidiuretic hormone | ||
|drugInteractions=====Strong CYP2C8 Inhibitors==== | |drugInteractions=====Strong CYP2C8 Inhibitors==== | ||
'''Pioglitazone''' | '''Pioglitazone''' | ||
An inhibitor of CYP2C8 (e.g., gemfibrozil) significantly increases the exposure (area under the serum concentration-time curve or AUC) and half-life (t½) of pioglitazone. Therefore, the maximum recommended dose of pioglitazone is 15 mg daily if used in combination with gemfibrozil or other strong CYP2C8 inhibitors. Since the minimum dose of pioglitazone in DUETACT exceeds 15 mg, patients taking concomitant strong CYP2C8 inhibitors should switch to individual components of DUETACT, unless the prescribing health care provider determines that the benefit of DUETACT clearly outweighs the risk of increased pioglitazone exposure . | * An inhibitor of [[CYP2C8]] (e.g., [[gemfibrozil]]) significantly increases the exposure (area under the serum concentration-time curve or [[AUC]]) and half-life (t½) of pioglitazone. Therefore, the maximum recommended dose of [[pioglitazone]] is 15 mg daily if used in combination with [[gemfibrozil]] or other strong CYP2C8 inhibitors. Since the minimum dose of [[pioglitazone]] in DUETACT exceeds 15 mg, patients taking concomitant strong [[CYP2C8]] inhibitors should switch to individual components of DUETACT, unless the prescribing health care provider determines that the benefit of DUETACT clearly outweighs the risk of increased [[pioglitazone]] exposure . | ||
====CYP2C8 Inducers==== | ====CYP2C8 Inducers==== | ||
| Line 286: | Line 287: | ||
'''Pioglitazone''' | '''Pioglitazone''' | ||
An inducer of CYP2C8 (e.g., rifampin) may significantly decrease the exposure (AUC) of pioglitazone. Therefore, if an inducer of CYP2C8 is started or stopped during treatment with pioglitazone, changes in diabetes treatment may be needed based on clinical response without exceeding the maximum recommended daily dose of 45 mg for pioglitazone . | * An inducer of [[CYP2C8]] (e.g., [[rifampin]]) may significantly decrease the exposure ([[AUC]]) of pioglitazone. Therefore, if an inducer of [[CYP2C8]] is started or stopped during treatment with pioglitazone, changes in diabetes treatment may be needed based on clinical response without exceeding the maximum recommended daily dose of 45 mg for pioglitazone . | ||
====Miconazole==== | ====Miconazole==== | ||
| Line 292: | Line 293: | ||
'''Glimepiride''' | '''Glimepiride''' | ||
A potential interaction between oral miconazole and sulfonylureas leading to severe hypoglycemia has been reported. Whether this interaction also occurs with other dosage forms of miconazole is not known. | * A potential interaction between oral miconazole and sulfonylureas leading to severe [[hypoglycemia]] has been reported. Whether this interaction also occurs with other dosage forms of miconazole is not known. | ||
====CYP2C9 Interactions==== | ====CYP2C9 Interactions==== | ||
| Line 298: | Line 299: | ||
'''Glimepiride''' | '''Glimepiride''' | ||
There may be an interaction between glimepiride and inhibitors (e.g., fluconazole) and inducers (e.g., rifampin) of CYP2C9. Fluconazole may inhibit the metabolism of glimepiride, causing increased plasma concentrations of glimepiride which may lead to hypoglycemia. Rifampin may induce the metabolism of | * There may be an interaction between [[glimepiride]] and inhibitors (e.g., [[fluconazole]]) and inducers (e.g., [[rifampin]]) of [[CYP2C9]]. [[Fluconazole]] may inhibit the metabolism of glimepiride, causing increased plasma concentrations of glimepiride which may lead to [[hypoglycemia]]. Rifampin may induce the metabolism of glimepiride, causing decreased plasma concentrations of glimepiride which may lead to worsening glycemic control. | ||
glimepiride, causing decreased plasma concentrations of glimepiride which may lead to worsening glycemic control. | |||
====Concomitant Administration of Colesevelam==== | ====Concomitant Administration of Colesevelam==== | ||
| Line 305: | Line 305: | ||
'''Glimepiride''' | '''Glimepiride''' | ||
Colesevelam can reduce the maximum plasma concentrations and total exposure of glimepiride when the two are coadministered. However, absorption is not reduced when glimepiride is administered four hours prior to colesevelam. Therefore, DUETACT should be administered at least four hours prior to colesevelam. | * Colesevelam can reduce the maximum plasma concentrations and total exposure of [[glimepiride]] when the two are coadministered. However, absorption is not reduced when glimepiride is administered four hours prior to [[colesevelam]]. Therefore, DUETACT should be administered at least four hours prior to colesevelam. | ||
|FDAPregCat=C | |FDAPregCat=C | ||
|useInPregnancyFDA='''<i>Pioglitazone</i>''' | |useInPregnancyFDA='''<i>Pioglitazone</i>''' | ||
There are no adequate and well-controlled studies of DUETACT in pregnant women. Animal studies show increased rates of postimplantation loss, delayed development, reduced fetal weights, and delayed parturition at doses 10 to 40 times the maximum recommended human dose. DUETACT should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. | * There are no adequate and well-controlled studies of DUETACT in pregnant women. Animal studies show increased rates of postimplantation loss, delayed development, reduced fetal weights, and delayed parturition at doses 10 to 40 times the maximum recommended human dose. DUETACT should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. | ||
'''<i><u>Clinical Considerations</u></i>''' | '''<i><u>Clinical Considerations</u></i>''' | ||
Abnormal blood glucose concentrations during pregnancy are associated with a higher incidence of congenital anomalies, as well as increased neonatal morbidity and mortality. Most experts recommend the use of insulin during pregnancy to maintain blood glucose concentrations as close to normal as possible for patients with diabetes. | * Abnormal blood [[glucose]] concentrations during pregnancy are associated with a higher incidence of congenital anomalies, as well as increased neonatal morbidity and mortality. Most experts recommend the use of insulin during pregnancy to maintain blood [[glucose]] concentrations as close to normal as possible for patients with diabetes. | ||
'''<i><u>Animal Data</u></i>''' | '''<i><u>Animal Data</u></i>''' | ||
In animal reproductive studies, pregnant rats and rabbits received pioglitazone at doses up to approximately 17 (rat) and 40 (rabbit) times the maximum recommended human oral dose (MRHD) based on body surface area (mg/m2); no teratogenicity was observed. Increases in embryotoxicity (increased postimplantation losses, delayed development, reduced fetal weights, and delayed parturition) occurred in rats that received oral doses approximately 10 or more times the MRHD (mg/m2 basis). No functional or behavioral toxicity was observed in rat offspring. When pregnant rats received pioglitazone during late gestation and lactation, delayed postnatal development, attributed to decreased body weight, occurred in rat offspring at oral maternal doses approximately two or more times the MRHD (mg/m2 basis). In rabbits, embryotoxicity occurred at oral doses approximately 40 times the MRHD (mg/m2 basis). | * In animal reproductive studies, pregnant rats and rabbits received pioglitazone at doses up to approximately 17 (rat) and 40 (rabbit) times the maximum recommended human oral dose (MRHD) based on body surface area (mg/m2); no teratogenicity was observed. Increases in embryotoxicity (increased postimplantation losses, delayed development, reduced fetal weights, and delayed parturition) occurred in rats that received oral doses approximately 10 or more times the [[MRHD]] (mg/m2 basis). No functional or behavioral toxicity was observed in rat offspring. When pregnant rats received pioglitazone during late gestation and lactation, delayed postnatal development, attributed to decreased body weight, occurred in rat offspring at oral maternal doses approximately two or more times the MRHD (mg/m2 basis). In rabbits, embryotoxicity occurred at oral doses approximately 40 times the MRHD (mg/m2 basis). | ||
'''<i>Glimepiride</i>''' | '''<i>Glimepiride</i>''' | ||
| Line 322: | Line 322: | ||
'''<i><u>Teratogenic Effects</u></i>''' | '''<i><u>Teratogenic Effects</u></i>''' | ||
In animal studies there was no increase in congenital anomalies, but an increase in fetal deaths occurred in rats and rabbits at glimepiride doses 50 times (rats) and 0.1 times (rabbits) the maximum recommended human dose (based on body surface area). This fetotoxicity, observed only at doses inducing maternal hypoglycemia, is believed to be directly related to the pharmacologic (hypoglycemic) action of glimepiride and has been similarly noted with other sulfonylureas. DUETACT should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Because data suggest that abnormal blood glucose during pregnancy is associated with a higher incidence of congenital abnormalities, diabetes treatment during pregnancy should maintain blood glucose as close to normal as possible. | * In animal studies there was no increase in congenital anomalies, but an increase in fetal deaths occurred in rats and rabbits at glimepiride doses 50 times (rats) and 0.1 times (rabbits) the maximum recommended human dose (based on body surface area). This fetotoxicity, observed only at doses inducing maternal [[hypoglycemia]], is believed to be directly related to the pharmacologic ([[hypoglycemic]]) action of [[glimepiride]] and has been similarly noted with other sulfonylureas. DUETACT should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Because data suggest that abnormal blood glucose during pregnancy is associated with a higher incidence of congenital abnormalities, [[diabetes]] treatment during pregnancy should maintain blood glucose as close to normal as possible. | ||
'''<i><u>Nonteratogenic Effects</u></i>''' | '''<i><u>Nonteratogenic Effects</u></i>''' | ||
Prolonged severe hypoglycemia (4 to 10 days) has been reported in neonates born to mothers receiving a sulfonylurea at the time of delivery. | * Prolonged severe [[hypoglycemia]] (4 to 10 days) has been reported in neonates born to mothers receiving a sulfonylurea at the time of delivery. | ||
|useInNursing=No studies have been conducted with the combined components of DUETACT. In studies performed with the individual components, pioglitazone was secreted in the milk of lactating rats and significant concentrations of glimepiride were observed in the serum and breast milk of the dams and serum of the pups. It is not known whether pioglitazone or glimepiride are secreted in human milk. However, other sulfonylureas are excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for DUETACT to cause serious adverse reactions in nursing infants, a decision should be made to discontinue nursing or discontinue DUETACT, taking into account the importance of DUETACT to the mother. | |useInNursing=No studies have been conducted with the combined components of DUETACT. In studies performed with the individual components, [[pioglitazone]] was secreted in the milk of lactating rats and significant concentrations of [[glimepiride]] were observed in the serum and breast milk of the dams and serum of the pups. It is not known whether [[pioglitazone]] or [[glimepiride]] are secreted in human milk. However, other [[sulfonylureas]] are excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for DUETACT to cause serious adverse reactions in nursing infants, a decision should be made to discontinue nursing or discontinue DUETACT, taking into account the importance of DUETACT to the mother. | ||
|useInPed=Safety and effectiveness of DUETACT in pediatric patients have not been established. | |useInPed=* Safety and effectiveness of DUETACT in pediatric patients have not been established. | ||
DUETACT is not recommended for use in pediatric patients based on adverse effects observed in adults, including fluid retention and | * DUETACT is not recommended for use in pediatric patients based on adverse effects observed in adults, including fluid retention and [[Congestive heart failure]], [[fractures]], and [[urinary bladder]] tumors. | ||
'''Glimepiride''' | '''Glimepiride''' | ||
The pharmacokinetics, efficacy and safety of glimepiride have been evaluated in pediatric patients with type 2 diabetes as described below. Glimepiride is not recommended in pediatric patients because of its adverse effects on body weight and hypoglycemia. | * The pharmacokinetics, efficacy and safety of glimepiride have been evaluated in pediatric patients with type 2 diabetes as described below. Glimepiride is not recommended in pediatric patients because of its adverse effects on body weight and [[hypoglycemia]]. | ||
The pharmacokinetics of a 1 mg single dose of glimepiride was evaluated in 30 patients with type 2 diabetes (male = 7; female = 23) between ages 10 and 17 years. The mean (±SD) AUC (0-last) (339±203 ng•hr/mL), Cmax (102±48 ng/mL) and t1/2 (3.1±1.7 hours) for glimepiride were comparable to historical data from adults (AUC (0-last) 315±96 ng•hr/mL, Cmax 103±34 ng/mL and t1/2 5.3±4.1 hours). | * The pharmacokinetics of a 1 mg single dose of glimepiride was evaluated in 30 patients with [[type 2 diabetes]] (male = 7; female = 23) between ages 10 and 17 years. The mean (±SD) [[AUC]] (0-last) (339±203 ng•hr/mL), [[Cmax]] (102±48 ng/mL) and t1/2 (3.1±1.7 hours) for glimepiride were comparable to historical data from adults (AUC (0-last) 315±96 ng•hr/mL, [[Cmax]] 103±34 ng/mL and t1/2 5.3±4.1 hours). | ||
The safety and efficacy of glimepiride in pediatric patients was evaluated in a single-blind, 24-week trial that randomized 272 patients (8 to 17 years of age) with type 2 diabetes to glimepiride (n=135) or metformin (n=137). Both treatment-naïve patients (those treated with only diet and exercise for at least two weeks prior to randomization) and previously treated patients (those previously treated or currently treated with other oral antidiabetic medications for at least three months) were eligible to participate. Patients who were receiving oral antidiabetic agents at the time of study entry discontinued these medications before randomization without a washout period. Glimepiride was initiated at 1 mg, and then titrated up to 2, 4 or 8 mg (mean last dose 4 mg) through Week 12, targeting a self monitored fasting fingerstick blood glucose <126 mg/dL. Metformin was initiated at 500 mg twice daily and titrated at Week 12 up to 1000 mg twice daily (mean last dose 1365 mg). | * The safety and efficacy of glimepiride in pediatric patients was evaluated in a single-blind, 24-week trial that randomized 272 patients (8 to 17 years of age) with type 2 diabetes to glimepiride (n=135) or [[metformin]] (n=137). Both treatment-naïve patients (those treated with only diet and exercise for at least two weeks prior to randomization) and previously treated patients (those previously treated or currently treated with other oral antidiabetic medications for at least three months) were eligible to participate. Patients who were receiving oral antidiabetic agents at the time of study entry discontinued these medications before randomization without a washout period. [[Glimepiride]] was initiated at 1 mg, and then titrated up to 2, 4 or 8 mg (mean last dose 4 mg) through Week 12, targeting a self monitored fasting fingerstick blood glucose <126 mg/dL. Metformin was initiated at 500 mg twice daily and titrated at Week 12 up to 1000 mg twice daily (mean last dose 1365 mg). | ||
After 24 weeks, the overall mean treatment difference in HbA1c between glimepiride and metformin was 0.2%, favoring metformin (95% confidence interval -0.3% to +0.6%). | * After 24 weeks, the overall mean treatment difference in [[HbA1c]] between [[glimepiride]] and [[metformin]] was 0.2%, favoring [[metformin]] (95% confidence interval -0.3% to +0.6%). | ||
Based on these results, the trial did not meet its primary objective of showing a similar reduction in HbA1c with glimepiride compared to metformin. | * Based on these results, the trial did not meet its primary objective of showing a similar reduction in [[HbA1c]] with [[glimepiride]] compared to [[metformin]]. | ||
The profile of adverse reactions in pediatric patients treated with glimepiride was similar to that observed in adults. | The profile of adverse reactions in pediatric patients treated with glimepiride was similar to that observed in adults. | ||
Hypoglycemic events documented by blood glucose values <36 mg/dL were observed in 4% of pediatric patients treated with glimepiride and in 1% of pediatric patients treated with metformin. One patient in each treatment group experienced a severe hypoglycemic episode (severity was determined by the investigator based on observed signs and symptoms). | * Hypoglycemic events documented by blood glucose values <36 mg/dL were observed in 4% of pediatric patients treated with [[glimepiride]] and in 1% of pediatric patients treated with [[metformin]]. One patient in each treatment group experienced a severe hypoglycemic episode (severity was determined by the investigator based on observed signs and symptoms). | ||
|useInGeri=To minimize the risk of hypoglycemia, the initial dosing, dose increments, and maintenance dosage of DUETACT should be conservative. During initiation of DUETACT therapy and any subsequent dose adjustments, geriatric patients should be observed carefully for hypoglycemia. | |useInGeri=* To minimize the risk of [[hypoglycemia]], the initial dosing, dose increments, and maintenance dosage of DUETACT should be conservative. During initiation of DUETACT therapy and any subsequent dose adjustments, geriatric patients should be observed carefully for [[hypoglycemia]]. | ||
'''Pioglitazone''' | '''Pioglitazone''' | ||
A total of 92 patients (15.2%) treated with pioglitazone in the three pooled 16- to 26-week double-blind, placebo-controlled, monotherapy trials were ≥65 years old and two patients (0.3%) were ≥75 years old. In the two pooled 16- to 24-week add-on to sulfonylurea trials, 201 patients (18.7%) treated with pioglitazone were ≥65 years old and 19 (1.8%) were ≥75 years old. In the two pooled 16- to 24-week add-on to metformin trials, 155 patients (15.5%) treated with pioglitazone were ≥65 years old and 19 (1.9%) were ≥75 years old. In the two pooled 16- to 24-week add-on to insulin trials, 272 patients (25.4%) treated with pioglitazone were ≥65 years old and 22 (2.1%) were ≥75 years old. | * A total of 92 patients (15.2%) treated with [[pioglitazone]] in the three pooled 16- to 26-week double-blind, placebo-controlled, monotherapy trials were ≥65 years old and two patients (0.3%) were ≥75 years old. In the two pooled 16- to 24-week add-on to [[sulfonylurea]] trials, 201 patients (18.7%) treated with pioglitazone were ≥65 years old and 19 (1.8%) were ≥75 years old. In the two pooled 16- to 24-week add-on to [[metformin]] trials, 155 patients (15.5%) treated with pioglitazone were ≥65 years old and 19 (1.9%) were ≥75 years old. In the two pooled 16- to 24-week add-on to insulin trials, 272 patients (25.4%) treated with pioglitazone were ≥65 years old and 22 (2.1%) were ≥75 years old. | ||
In PROactive, 1068 patients (41.0%) treated with pioglitazone were ≥65 years old and 42 (1.6%) were ≥75 years old. | * In PROactive, 1068 patients (41.0%) treated with [[pioglitazone]] were ≥65 years old and 42 (1.6%) were ≥75 years old. | ||
In pharmacokinetic studies with pioglitazone, no significant differences were observed in pharmacokinetic parameters between elderly and younger patients . | * In pharmacokinetic studies with [[pioglitazone]], no significant differences were observed in pharmacokinetic parameters between elderly and younger patients . | ||
Although clinical experiences have not identified differences in effectiveness and safety between the elderly (≥65 years) and younger patients, these conclusions are limited by small sample sizes for patients ≥75 years old. | Although clinical experiences have not identified differences in effectiveness and safety between the elderly (≥65 years) and younger patients, these conclusions are limited by small sample sizes for patients ≥75 years old. | ||
'''Glimepiride''' | '''Glimepiride''' | ||
In clinical trials of glimepiride, 1053 of 3491 patients (30%) were ≥65 years of age. No overall differences in safety or effectiveness were observed between these patients and younger patients, but greater sensitivity of some older individuals cannot be ruled out. | * In clinical trials of [[glimepiride]], 1053 of 3491 patients (30%) were ≥65 years of age. No overall differences in safety or effectiveness were observed between these patients and younger patients, but greater sensitivity of some older individuals cannot be ruled out. | ||
There were no significant differences in glimepiride pharmacokinetics between patients with type 2 diabetes ≤65 years (n=49) and those >65 years (n=42). | * There were no significant differences in glimepiride pharmacokinetics between patients with type 2 diabetes ≤65 years (n=49) and those >65 years (n=42). | ||
Glimepiride is substantially excreted by the kidney. Elderly patients are more likely to have renal impairment. In addition, hypoglycemia may be difficult to recognize in the elderly . Use caution when initiating DUETACT and increasing the dose of DUETACT in this patient population. | Glimepiride is substantially excreted by the kidney. Elderly patients are more likely to have renal impairment. In addition, [[hypoglycemia]] may be difficult to recognize in the elderly . Use caution when initiating DUETACT and increasing the dose of DUETACT in this patient population. | ||
|useInRenalImpair=To minimize the risk of hypoglycemia, the initial dosing, dose increments and maintenance dosage of DUETACT should be conservative. During initiation of DUETACT therapy and any subsequent dose adjustments, these patients should be observed carefully for hypoglycemia. | |useInRenalImpair=* To minimize the risk of [[hypoglycemia]], the initial dosing, dose increments and maintenance dosage of DUETACT should be conservative. During initiation of DUETACT therapy and any subsequent dose adjustments, these patients should be observed carefully for [[hypoglycemia]]. | ||
A multiple-dose titration study was conducted in 16 patients with type 2 diabetes and renal impairment using doses ranging from 1 mg to 8 mg daily for three months. Baseline creatinine clearance ranged from 10 to 60 mL/min. The pharmacokinetics of glimepiride were evaluated in the multiple-dose titration study and the results were consistent with those observed in patients enrolled in a single-dose study. In both studies, the relative total clearance of glimepiride increased when kidney function was impaired. Both studies also demonstrated that the elimination of the two major metabolites was reduced in patients with renal impairment | * A multiple-dose titration study was conducted in 16 patients with [[type 2 diabetes]] and [[renal impairment]] using doses ranging from 1 mg to 8 mg daily for three months. Baseline [[creatinine clearance]] ranged from 10 to 60 mL/min. The pharmacokinetics of [[glimepiride]] were evaluated in the multiple-dose titration study and the results were consistent with those observed in patients enrolled in a single-dose study. In both studies, the relative total clearance of glimepiride increased when kidney function was impaired. Both studies also demonstrated that the elimination of the two major metabolites was reduced in patients with renal impairment | ||
|administration=Oral | |administration=* Oral | ||
|monitoring=FDA Package Insert for Pioglitazone/Glimepiride contains no information regarding Drug Monitoring. | |monitoring=FDA Package Insert for Pioglitazone/Glimepiride contains no information regarding Drug Monitoring. | ||
|IVCompat=There is limited information about the IV Compabitility. | |IVCompat=There is limited information about the IV Compabitility. | ||
|overdose='''Pioglitazone''' | |overdose='''Pioglitazone''' | ||
During controlled clinical trials, one case of overdose with pioglitazone was reported. A male patient took 120 mg per day for four days, then 180 mg per day for seven days. The patient denied any clinical symptoms during this period. | * During controlled clinical trials, one case of overdose with pioglitazone was reported. A male patient took 120 mg per day for four days, then 180 mg per day for seven days. The patient denied any clinical symptoms during this period. | ||
In the event of overdosage, appropriate supportive treatment should be initiated according to the patient’s clinical signs and symptoms. | * In the event of overdosage, appropriate supportive treatment should be initiated according to the patient’s clinical signs and symptoms. | ||
'''Glimepiride''' | '''Glimepiride''' | ||
An overdosage of glimepiride, as with other sulfonylureas, can produce severe hypoglycemia. Mild episodes of hypoglycemia can be treated with oral glucose. Severe hypoglycemic reactions constitute medical emergencies requiring immediate treatment. Severe hypoglycemia with coma, seizure, or neurological impairment can be treated with glucagon or intravenous glucose. Continued observation and additional carbohydrate intake may be necessary because hypoglycemia may recur after apparent clinical recovery. | * An overdosage of [[glimepiride]], as with other [[sulfonylureas]], can produce severe [[hypoglycemia]]. Mild episodes of [[hypoglycemia]] can be treated with oral glucose. Severe hypoglycemic reactions constitute medical emergencies requiring immediate treatment. Severe [[hypoglycemia]] with coma, seizure, or neurological impairment can be treated with [[glucagon]] or [[intravenous]] [[glucose]]. Continued observation and additional carbohydrate intake may be necessary because [[hypoglycemia]] may recur after apparent clinical recovery. | ||
|mechAction=DUETACT combines 2 antihyperglycemic agents with different mechanisms of action to improve glycemic control in patients with type 2 diabetes: pioglitazone, a member of the thiazolidinedione class, and glimepiride, a member of the sulfonylurea class. | |mechAction=* DUETACT combines 2 antihyperglycemic agents with different mechanisms of action to improve glycemic control in patients with type 2 diabetes: pioglitazone, a member of the thiazolidinedione class, and [[glimepiride]], a member of the [[sulfonylurea]] class. [[thiazolidinediones]] are insulin-sensitizing agents that act primarily by enhancing peripheral glucose utilization, whereas [[sulfonylureas]] are insulin secretagogues that act primarily by stimulating release of [[insulin]] from functioning pancreatic beta cells. | ||
'''Pioglitazone''' | '''Pioglitazone''' | ||
Pioglitazone is a thiazolidinedione that depends on the presence of insulin for its mechanism of action. Pioglitazone decreases insulin resistance in the periphery and in the liver resulting in increased insulin-dependent glucose disposal and decreased hepatic glucose output. Pioglitazone is not an insulin secretagogue. Pioglitazone is an agonist for peroxisome proliferator-activated receptor-gamma (PPARγ). PPAR receptors are found in tissues important for insulin action such as adipose tissue, skeletal muscle, and liver. Activation of PPARγ nuclear receptors modulates the transcription of a number of insulin responsive genes involved in the control of glucose and lipid metabolism. | * [[Pioglitazone]] is a [[thiazolidinedione]] that depends on the presence of [[insulin]] for its mechanism of action. Pioglitazone decreases insulin resistance in the periphery and in the liver resulting in increased insulin-dependent glucose disposal and decreased hepatic glucose output. Pioglitazone is not an insulin secretagogue. [[Pioglitazone]] is an agonist for peroxisome proliferator-activated receptor-gamma (PPARγ). PPAR receptors are found in tissues important for insulin action such as adipose tissue, skeletal muscle, and liver. Activation of PPARγ nuclear receptors modulates the transcription of a number of insulin responsive genes involved in the control of glucose and lipid metabolism. | ||
In animal models of diabetes, pioglitazone reduces the hyperglycemia, hyperinsulinemia, and hypertriglyceridemia characteristic of insulin-resistant states such as type 2 diabetes. The metabolic changes produced by pioglitazone result in increased responsiveness of insulin-dependent tissues and are observed in numerous animal models of insulin resistance. | * In animal models of [[diabetes]], pioglitazone reduces the [[hyperglycemia]], [[hyperinsulinemia]], and [[hypertriglyceridemia]] characteristic of insulin-resistant states such as type 2 diabetes. The metabolic changes produced by pioglitazone result in increased responsiveness of insulin-dependent tissues and are observed in numerous animal models of insulin resistance. | ||
Because pioglitazone enhances the effects of circulating insulin (by decreasing insulin resistance), it does not lower blood glucose in animal models that lack endogenous insulin. | * Because pioglitazone enhances the effects of circulating [[insulin]] (by decreasing insulin resistance), it does not lower blood [[glucose]] in animal models that lack endogenous insulin. | ||
'''Glimepiride''' | '''Glimepiride''' | ||
Glimepiride primarily lowers blood glucose by stimulating the release of insulin from pancreatic beta cells. Sulfonylureas bind to the sulfonylurea receptor in the pancreatic beta cell plasma membrane, leading to closure of the ATP-sensitive potassium channel, thereby stimulating the release of insulin. | * Glimepiride primarily lowers blood glucose by stimulating the release of [[insulin]] from [[pancreatic beta cells]]. [[Sulfonylureas]] bind to the sulfonylurea receptor in the pancreatic beta cell plasma membrane, leading to closure of the ATP-sensitive potassium channel, thereby stimulating the release of insulin. | ||
|structure=DUETACT tablets are a thiazolidinedione and a sulfonylurea combination product that contains two oral antihyperglycemic agents: pioglitazone and glimepiride. The concomitant use of pioglitazone and a sulfonylurea, the class of drugs that includes glimepiride, has been previously approved based on clinical trials in patients with type 2 diabetes inadequately controlled on a sulfonylurea. Additional efficacy and safety information about pioglitazone and glimepiride monotherapies may be found in the prescribing information for each individual drug. | |structure=* DUETACT tablets are a [[thiazolidinedione]] and a [[sulfonylurea]] combination product that contains two oral antihyperglycemic agents: [[pioglitazone]] and [[glimepiride]]. The concomitant use of pioglitazone and a [[sulfonylurea]], the class of drugs that includes [[glimepiride]], has been previously approved based on clinical trials in patients with type 2 diabetes inadequately controlled on a [[sulfonylurea]]. Additional efficacy and safety information about pioglitazone and glimepiride monotherapies may be found in the prescribing information for each individual drug. | ||
Pioglitazone is an oral antidiabetic medication. | * Pioglitazone is an oral antidiabetic medication. | ||
Pioglitazone [(±)-5-[ [4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-] thiazolidinedione monohydrochloride contains one asymmetric carbon, and the compound is synthesized and used as the racemic mixture. The two enantiomers of pioglitazone interconvert in vivo. No differences were found in the pharmacologic activity between the two enantiomers. The structural formula is as shown: | * Pioglitazone [(±)-5-[ [4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-] thiazolidinedione monohydrochloride contains one asymmetric carbon, and the compound is synthesized and used as the racemic mixture. The two enantiomers of pioglitazone interconvert in vivo. No differences were found in the pharmacologic activity between the two enantiomers. The structural formula is as shown: | ||
[[File:Duetact_structure_01.png|thumb|none| | [[File:Duetact_structure_01.png|thumb|none|500px|This image is provided by the National Library of Medicine.]] | ||
Pioglitazone hydrochloride is an odorless, white crystalline powder that has a molecular formula of C19H20N2O3S•HCl and a molecular weight of 392.90 daltons. It is soluble in N,N‑dimethylformamide, slightly soluble in anhydrous ethanol, very slightly soluble in acetone and acetonitrile, practically insoluble in water, and insoluble in ether. | * Pioglitazone hydrochloride is an odorless, white crystalline powder that has a molecular formula of C19H20N2O3S•HCl and a molecular weight of 392.90 daltons. It is soluble in N,N‑dimethylformamide, slightly soluble in anhydrous ethanol, very slightly soluble in acetone and acetonitrile, practically insoluble in water, and insoluble in ether. | ||
Glimepiride is an oral sulfonylurea chemically identified as 1-[ [p-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1-carboxamido)ethyl]phenyl]sulfonyl]-3-(trans-4-methylcyclohexyl)-urea (C24H34N4O5S) with a molecular weight of 490.62. Glimepiride is a white to yellowish-white, crystalline, odorless to practically odorless powder and is practically insoluble in water. The structural formula is: | * Glimepiride is an oral sulfonylurea chemically identified as 1-[ [p-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1-carboxamido)ethyl]phenyl]sulfonyl]-3-(trans-4-methylcyclohexyl)-urea (C24H34N4O5S) with a molecular weight of 490.62. Glimepiride is a white to yellowish-white, crystalline, odorless to practically odorless powder and is practically insoluble in water. The structural formula is: | ||
[[File:Duetact_structure_02.png|thumb|none| | [[File:Duetact_structure_02.png|thumb|none|500px|This image is provided by the National Library of Medicine.]] | ||
DUETACT is available as a tablet for oral administration containing 30 mg pioglitazone (as the base) with 2 mg glimepiride (30 mg/2 mg) or 30 mg pioglitazone (as the base) with 4 mg glimepiride (30 mg/4 mg) formulated with the following excipients: croscarmellose sodium NF, lactose monohydrate NF, magnesium stearate NF, hydroxypropyl cellulose NF, polysorbate 80 NF, and microcrystalline cellulose NF. | * DUETACT is available as a tablet for oral administration containing 30 mg [[pioglitazone]] (as the base) with 2 mg [[glimepiride]] (30 mg/2 mg) or 30 mg pioglitazone (as the base) with 4 mg glimepiride (30 mg/4 mg) formulated with the following excipients: croscarmellose sodium NF, lactose monohydrate NF, magnesium stearate NF, hydroxypropyl cellulose NF, polysorbate 80 NF, and microcrystalline cellulose NF. | ||
|PD=Pioglitazone | |PD='''<I>Pioglitazone</I>''' | ||
Clinical studies demonstrate that pioglitazone improves insulin sensitivity in insulin-resistant patients. Pioglitazone enhances cellular responsiveness to insulin, increases insulin-dependent glucose disposal and improves hepatic sensitivity to insulin. In patients with type 2 diabetes, the decreased insulin resistance produced by pioglitazone results in lower plasma glucose concentrations, lower plasma insulin concentrations, and lower HbA1c values. In controlled clinical trials, pioglitazone had an additive effect on glycemic control when used in combination with a sulfonylurea, metformin, or insulin . | * Clinical studies demonstrate that [[pioglitazone]] improves [[insulin]] sensitivity in insulin-resistant patients. Pioglitazone enhances cellular responsiveness to insulin, increases insulin-dependent [[glucose]] disposal and improves hepatic sensitivity to [[insulin]]. In patients with type 2 diabetes, the decreased insulin resistance produced by pioglitazone results in lower plasma glucose concentrations, lower plasma [[insulin]] concentrations, and lower [[HbA1c]] values. In controlled clinical trials, pioglitazone had an additive effect on glycemic control when used in combination with a [[sulfonylurea]], [[metformin]], or [[insulin]] . | ||
Patients with lipid abnormalities were included in clinical trials with pioglitazone. Overall, patients treated with pioglitazone had mean decreases in serum triglycerides, mean increases in HDL cholesterol, and no consistent mean changes in LDL and total cholesterol. There is no conclusive evidence of macrovascular benefit with pioglitazone or any other antidiabetic medication . | * Patients with lipid abnormalities were included in clinical trials with [[pioglitazone]]. Overall, patients treated with pioglitazone had mean decreases in serum triglycerides, mean increases in [[HDL cholesterol]], and no consistent mean changes in [[LDL]] and total cholesterol. There is no conclusive evidence of macrovascular benefit with pioglitazone or any other antidiabetic medication . | ||
In a 26-week, placebo-controlled, dose-ranging monotherapy study, mean serum triglycerides decreased in the 15 mg, 30 mg, and 45 mg pioglitazone dose groups compared to a mean increase in the placebo group. Mean HDL cholesterol increased to a greater extent in patients treated with pioglitazone than in the placebo-treated patients. There were no consistent differences for LDL and total cholesterol in patients treated with pioglitazone compared to placebo (Table 12). | * In a 26-week, placebo-controlled, dose-ranging monotherapy study, mean serum triglycerides decreased in the 15 mg, 30 mg, and 45 mg pioglitazone dose groups compared to a mean increase in the placebo group. Mean [[HDL cholesterol]] increased to a greater extent in patients treated with pioglitazone than in the placebo-treated patients. There were no consistent differences for LDL and total cholesterol in patients treated with pioglitazone compared to placebo (Table 12). | ||
[[File:Duetact_PD_01.png|thumb|none| | [[File:Duetact_PD_01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
In the two other monotherapy studies (16 weeks and 24 weeks) and in combination therapy studies with sulfonylurea (16 weeks and 24 weeks), metformin (16 weeks and 24 weeks) or insulin (16 weeks and 24 weeks), the results were generally consistent with the data above. | * In the two other monotherapy studies (16 weeks and 24 weeks) and in combination therapy studies with [[sulfonylurea]] (16 weeks and 24 weeks), [[metformin]] (16 weeks and 24 weeks) or insulin (16 weeks and 24 weeks), the results were generally consistent with the data above. | ||
Glimepiride | =====Glimepiride===== | ||
In healthy subjects, the time to reach maximal effect (minimum blood glucose concentrations) was approximately by two to three hours after single oral doses of glimepiride. The effects of HbA1C, fasting plasma glucose, and post-prandial glucose have been assessed in clinical trials. | * In healthy subjects, the time to reach maximal effect (minimum blood glucose concentrations) was approximately by two to three hours after single oral doses of glimepiride. The effects of [[HbA1C]], fasting plasma glucose, and post-prandial glucose have been assessed in clinical trials. | ||
|PK=Absorption and Bioavailability: | |PK====Absorption and Bioavailability:=== | ||
DUETACT | =====DUETACT===== | ||
Bioequivalence studies were conducted following a single dose of the DUETACT 30 mg/2 mg and 30 mg/4 mg tablets and concomitant administration of pioglitazone (30 mg) and glimepiride (2 mg or 4 mg) under fasting conditions in healthy subjects. | * Bioequivalence studies were conducted following a single dose of the DUETACT 30 mg/2 mg and 30 mg/4 mg tablets and concomitant administration of [[pioglitazone]] (30 mg) and glimepiride (2 mg or 4 mg) under fasting conditions in healthy subjects. | ||
Based on the area under the curve (AUC) and maximum concentration (Cmax) of both pioglitazone and glimepiride, DUETACT 30 mg/2 mg and 30 mg/4 mg were bioequivalent to pioglitazone 30 mg concomitantly administered with glimepiride (2 mg or 4 mg, respectively). | * Based on the area under the curve ([[AUC]]) and maximum concentration ([[Cmax]]) of both pioglitazone and glimepiride, DUETACT 30 mg/2 mg and 30 mg/4 mg were bioequivalent to pioglitazone 30 mg concomitantly administered with [[glimepiride]] (2 mg or 4 mg, respectively). | ||
Food did not change the systemic exposures of glimepiride or pioglitazone following administration of DUETACT. The presence of food did not significantly alter the time to peak serum concentration (Tmax) of glimepiride or pioglitazone and Cmax of pioglitazone. However, for glimepiride, there was a 22% increase in Cmaxwhen DUETACT was administered with food. | * Food did not change the systemic exposures of [[glimepiride]] or [[pioglitazone]] following administration of DUETACT. The presence of food did not significantly alter the time to peak serum concentration ([[Tmax]]) of [[glimepiride]] or [[pioglitazone]] and [[Cmax]] of pioglitazone. However, for [[glimepiride]], there was a 22% increase in Cmaxwhen DUETACT was administered with food. | ||
Pioglitazone | '''<I>Pioglitazone</I>''' | ||
Following once-daily administration of pioglitazone, steady-state serum concentrations of both pioglitazone and its major active metabolites, M-III (keto derivative of pioglitazone) and M-IV (hydroxyl derivative of pioglitazone), are achieved within seven days. At steady-state, M-III and M-IV reach serum concentrations equal to or greater than that of pioglitazone. At steady-state, in both healthy volunteers and patients with type 2 diabetes, pioglitazone comprises approximately 30% to 50% of the peak total pioglitazone serum concentrations (pioglitazone plus active metabolites) and 20% to 25% of the total AUC. | * Following once-daily administration of [[pioglitazone]], steady-state serum concentrations of both pioglitazone and its major active metabolites, M-III (keto derivative of pioglitazone) and M-IV (hydroxyl derivative of pioglitazone), are achieved within seven days. At steady-state, M-III and M-IV reach serum concentrations equal to or greater than that of [[pioglitazone]]. At steady-state, in both healthy volunteers and patients with type 2 diabetes, pioglitazone comprises approximately 30% to 50% of the peak total pioglitazone serum concentrations (pioglitazone plus active metabolites) and 20% to 25% of the total [[AUC]]. | ||
Cmax, AUC, and trough serum concentrations (Cmin) for pioglitazone and M-III and M-IV, increased proportionally with administered doses of 15 mg and 30 mg per day. | * [[Cmax]], [[AUC]], and trough serum concentrations (Cmin) for [[pioglitazone]] and M-III and M-IV, increased proportionally with administered doses of 15 mg and 30 mg per day. | ||
Following oral administration of pioglitazone, Tmax of pioglitazone was within two hours. Food delays Tmax to three to four hours but does not alter the extent of absorption (AUC). | * Following oral administration of [[pioglitazone]], [[Tmax]] of pioglitazone was within two hours. Food delays Tmax to three to four hours but does not alter the extent of absorption (AUC). | ||
Glimepiride | '''<I>Glimepiride</I>''' | ||
Following single oral doses of glimepiride in healthy subjects and multiple oral doses in patients with type 2 diabetes Tmax was observed at two to three hours post-dose. When glimepiride was given with meals, the mean Cmax and AUC were decreased by 8% and 9%, respectively. | * Following single oral doses of glimepiride in healthy subjects and multiple oral doses in patients with type 2 diabetes [[Tmax]] was observed at two to three hours post-dose. When glimepiride was given with meals, the mean [[Cmax]] and [[AUC]] were decreased by 8% and 9%, respectively. | ||
Glimepiride does not accumulate in serum following multiple dosing. The pharmacokinetics of glimepiride does not differ between healthy subjects and patients with type 2 diabetes. Clearance (CL/F) of glimepiride after oral administration does not change over the 1 mg to 8 mg dose range, indicating linear pharmacokinetics. | * [[Glimepiride]] does not accumulate in serum following multiple dosing. The pharmacokinetics of glimepiride does not differ between healthy subjects and patients with [[type 2 diabetes]]. Clearance (CL/F) of [[glimepiride]] after oral administration does not change over the 1 mg to 8 mg dose range, indicating linear pharmacokinetics. | ||
In healthy subjects, the intra- and inter-individual variabilities of glimepiride pharmacokinetic parameters were 15% to 23% and 24% to 29%, respectively. | * In healthy subjects, the intra- and inter-individual variabilities of glimepiride pharmacokinetic parameters were 15% to 23% and 24% to 29%, respectively. | ||
Distribution | ====Distribution==== | ||
Pioglitazone | '''<I>Pioglitazone</I>''' | ||
The mean apparent volume of distribution (Vd/F) of pioglitazone following single-dose administration is 0.63 ± 0.41 (mean ± SD) L/kg of body weight. Pioglitazone is extensively protein bound (>99%) in human serum, principally to serum albumin. Pioglitazone also binds to other serum proteins, but with lower affinity. M-III and M-IV are also extensively bound (>98%) to serum albumin. | * The mean apparent volume of distribution (Vd/F) of [[pioglitazone]] following single-dose administration is 0.63 ± 0.41 (mean ± SD) L/kg of body weight. Pioglitazone is extensively protein bound (>99%) in human serum, principally to serum albumin. [[Pioglitazone]] also binds to other serum proteins, but with lower affinity. M-III and M-IV are also extensively bound (>98%) to serum [[albumin]]. | ||
Glimepiride | '''<I>Glimepiride</I>''' | ||
After intravenous (IV) dosing in healthy subjects, Vd/F was 8.8 L (113 mL/kg). Protein binding was greater than 99.5%. | * After [[intravenous]] (IV) dosing in healthy subjects, Vd/F was 8.8 L (113 mL/kg). Protein binding was greater than 99.5%. | ||
Metabolism | ====Metabolism==== | ||
Pioglitazone | '''<I>Pioglitazone</I>''' | ||
Pioglitazone is extensively metabolized by hydroxylation and oxidation; the metabolites also partly convert to glucuronide or sulfate conjugates. Metabolites M-III and M-IV are the major circulating active metabolites in humans. | * [[Pioglitazone]] is extensively metabolized by hydroxylation and oxidation; the metabolites also partly convert to [[glucuronide]] or sulfate conjugates. Metabolites M-III and M-IV are the major circulating active metabolites in humans. | ||
In vitro data demonstrate that multiple CYP isoforms are involved in the metabolism of pioglitazone which include CYP2C8 and, to a lesser degree, CYP3A4 with additional contributions from a variety of other isoforms including the mainly extrahepatic CYP1A1. In vivo study of pioglitazone in combination with gemfibrozil, a strong CYP2C8 inhibitor, showed that pioglitazone is a CYP2C8 substrate | * In vitro data demonstrate that multiple CYP isoforms are involved in the metabolism of [[pioglitazone]] which include [[CYP2C8]] and, to a lesser degree, [[CYP3A4]] with additional contributions from a variety of other isoforms including the mainly extrahepatic [[CYP1A1]]. In vivo study of pioglitazone in combination with gemfibrozil, a strong CYP2C8 inhibitor, showed that pioglitazone is a CYP2C8 substrate. Urinary 6ß-hydroxycortisol/cortisol ratios measured in patients treated with pioglitazone showed that pioglitazone is not a strong CYP3A4 enzyme inducer. | ||
Glimepiride | '''<I>Glimepiride</I>''' | ||
Glimepiride is completely metabolized by oxidative biotransformation after either an IV or oral dose. The major metabolites are the cyclohexyl hydroxy methyl derivative (M1) and the carboxyl derivative (M2). CYP2C9 is involved in the biotransformation of glimepiride to M1. M1 is further metabolized to M2 by one or several cytosolic enzymes. In animals, M1 possesses about one-third of the pharmacological activity of glimepiride, but it is unclear whether M1 results in clinically meaningful effects on blood glucose in humans. M2 is inactive. | * Glimepiride is completely metabolized by oxidative biotransformation after either an IV or oral dose. The major metabolites are the cyclohexyl hydroxy methyl derivative (M1) and the carboxyl derivative (M2). [[CYP2C9]] is involved in the biotransformation of [[glimepiride]] to M1. M1 is further metabolized to M2 by one or several cytosolic enzymes. In animals, M1 possesses about one-third of the pharmacological activity of [[glimepiride]], but it is unclear whether M1 results in clinically meaningful effects on blood glucose in humans. M2 is inactive. | ||
Excretion and Elimination | * Excretion and Elimination | ||
Pioglitazone | '''<I>Pioglitazone</I>''' | ||
Following oral administration, approximately 15% to 30% of the pioglitazone dose is recovered in the urine. Renal elimination of pioglitazone is negligible and the drug is excreted primarily as metabolites and their conjugates. It is presumed that most of the oral dose is excreted into the bile either unchanged or as metabolites and eliminated in the feces. | * Following oral administration, approximately 15% to 30% of the [[pioglitazone]] dose is recovered in the urine. Renal elimination of [[pioglitazone]] is negligible and the drug is excreted primarily as metabolites and their conjugates. It is presumed that most of the oral dose is excreted into the bile either unchanged or as metabolites and eliminated in the feces. | ||
The mean serum half-life (t1/2) of pioglitazone and its metabolites (M-III and M-IV) range from three to seven hours and 16 to 24 hours, respectively. Pioglitazone has an apparent clearance, CL/F, calculated to be five to seven L/hr. | * The mean serum half-life (t1/2) of pioglitazone and its metabolites (M-III and M-IV) range from three to seven hours and 16 to 24 hours, respectively. Pioglitazone has an apparent clearance, CL/F, calculated to be five to seven L/hr. | ||
Glimepiride | '''<I>Glimepiride</I>''' | ||
When 14C-glimepiride was given orally to three healthy male subjects, approximately 60% of the total radioactivity was recovered in the urine in seven days. M1 and M2 accounted for 80% to 90% of the radioactivity recovered in the urine. The ratio of M1 to M2 in the urine was approximately 3:2 in two subjects and 4:1 in one subject. Approximately 40% of the total radioactivity was recovered in feces. M1 and M2 accounted for approximately 70% (ratio of M1 to M2 was 1:3) of the radioactivity recovered in feces. No parent drug was recovered from urine or feces. After IV dosing in patients, no significant biliary excretion of glimepiride or its M1 metabolite was observed. Total body clearance (CL) after IV dosing was 47.8 mL/min. | * When 14C-glimepiride was given orally to three healthy male subjects, approximately 60% of the total radioactivity was recovered in the urine in seven days. M1 and M2 accounted for 80% to 90% of the radioactivity recovered in the urine. The ratio of M1 to M2 in the urine was approximately 3:2 in two subjects and 4:1 in one subject. Approximately 40% of the total radioactivity was recovered in feces. M1 and M2 accounted for approximately 70% (ratio of M1 to M2 was 1:3) of the radioactivity recovered in feces. No parent drug was recovered from urine or feces. After IV dosing in patients, no significant biliary excretion of glimepiride or its M1 metabolite was observed. Total body clearance (CL) after IV dosing was 47.8 mL/min. | ||
Renal Impairment | =====Renal Impairment===== | ||
Pioglitazone | =====Pioglitazone===== | ||
The serum elimination half-life of pioglitazone, M-III, and M-IV remains unchanged in patients with moderate [creatinine clearance (CLcr) 30 to 50 mL/min] and severe (CLcr <30 mL/min) renal impairment when compared to subjects with normal renal function. Therefore, no dose adjustment in patients with renal impairment is required. | * The serum elimination half-life of [[pioglitazone]], M-III, and M-IV remains unchanged in patients with moderate [creatinine clearance (CLcr) 30 to 50 mL/min] and severe (CLcr <30 mL/min) renal impairment when compared to subjects with normal renal function. Therefore, no dose adjustment in patients with renal impairment is required. | ||
Glimepiride | =====Glimepiride===== | ||
In a single-dose, open-label study glimepiride 3 mg was administered to patients with mild, moderate and severe renal impairment as estimated by CLcr: Group I consisted of five patients with mild renal impairment (CLcr >50 mL/min), Group II consisted of 3 patients with moderate renal impairment (CLcr = 20 to 50 mL/min) and Group III consisted of seven patients with severe renal impairment (CLcr <20 mL/min). Although, glimepiride serum concentrations decreased with decreasing renal function, Group III had a 2.3-fold higher mean AUC for M1 and an 8.6-fold higher mean AUC for M2 compared to corresponding mean AUCs in Group I. The t½ for glimepiride did not change, while the t½ for M1 and M2 increased as renal function decreased. Mean urinary excretion of M1 plus M2 as a percentage of dose decreased from 44.4% for Group I to 21.9% for Group II and 9.3% for Group III. | * In a single-dose, open-label study glimepiride 3 mg was administered to patients with mild, moderate and severe renal impairment as estimated by CLcr: Group I consisted of five patients with mild [[renal impairment]] (CLcr >50 mL/min), Group II consisted of 3 patients with moderate renal impairment (CLcr = 20 to 50 mL/min) and Group III consisted of seven patients with severe renal impairment (CLcr <20 mL/min). Although, glimepiride serum concentrations decreased with decreasing renal function, Group III had a 2.3-fold higher mean AUC for M1 and an 8.6-fold higher mean AUC for M2 compared to corresponding mean AUCs in Group I. The t½ for [[glimepiride]] did not change, while the t½ for M1 and M2 increased as renal function decreased. Mean urinary excretion of M1 plus M2 as a percentage of dose decreased from 44.4% for Group I to 21.9% for Group II and 9.3% for Group III. | ||
Hepatic Impairment | =====Hepatic Impairment===== | ||
Pioglitazone | '''<I>Pioglitazone</I>''' | ||
Compared with healthy controls, subjects with impaired hepatic function (Child-Turcotte-Pugh Grade B/C) have an approximate 45% reduction in pioglitazone and total pioglitazone (pioglitazone, M-III, and M-IV) mean Cmax but no change in the mean AUC values. Therefore, no dose adjustment in patients with hepatic impairment is required. | * Compared with healthy controls, subjects with impaired hepatic function ([[Child-Turcotte-Pugh]] Grade B/C) have an approximate 45% reduction in pioglitazone and total [[pioglitazone]] (pioglitazone, M-III, and M-IV) mean [[Cmax]] but no change in the mean [[AUC]] values. Therefore, no dose adjustment in patients with hepatic impairment is required. | ||
There are postmarketing reports of liver failure with pioglitazone and clinical trials have generally excluded patients with serum ALT >2.5 times the upper limit of the reference range. Use DUETACT with caution in patients with liver disease | * There are postmarketing reports of liver failure with pioglitazone and clinical trials have generally excluded patients with serum [[ALT]] >2.5 times the upper limit of the reference range. Use DUETACT with caution in patients with liver disease. | ||
Glimepiride | '''<I>Glimepiride</I>''' | ||
It is unknown whether there is an effect of hepatic impairment on glimepiride pharmacokinetics because the pharmacokinetics of glimepiride has not been adequately evaluated in patients with hepatic impairment. | * It is unknown whether there is an effect of hepatic impairment on glimepiride pharmacokinetics because the pharmacokinetics of [[glimepiride]] has not been adequately evaluated in patients with hepatic impairment. | ||
Geriatric Patients | ====Geriatric Patients==== | ||
Pioglitazone | '''<I>Pioglitazone</I>''' | ||
In healthy elderly subjects, Cmax of pioglitazone was not significantly different, but AUC values were approximately 21% higher than those achieved in younger subjects. The mean t½ of pioglitazone was also prolonged in elderly subjects (about 10 hours) as compared to younger subjects (about seven hours). These changes were not of a magnitude that would be considered clinically relevant. | * In healthy elderly subjects, [[Cmax]] of pioglitazone was not significantly different, but [[AUC]] values were approximately 21% higher than those achieved in younger subjects. The mean t½ of pioglitazone was also prolonged in elderly subjects (about 10 hours) as compared to younger subjects (about seven hours). These changes were not of a magnitude that would be considered clinically relevant. | ||
Glimepiride | '''<I>Glimepiride</I>''' | ||
Glimepiride pharmacokinetics in patients with type 2 diabetes ≤65 years and those >65 years was compared in a multiple-dose study using 6 mg daily dose. There were no significant differences in glimepiride pharmacokinetics between the two age groups. The mean AUC at steady state for the older patients was approximately 13% lower than that for the younger patients; the mean weight-adjusted clearance for the older patients was approximately 11% higher than that for the younger patients. | * Glimepiride pharmacokinetics in patients with [[type 2 diabetes]] ≤65 years and those >65 years was compared in a multiple-dose study using 6 mg daily dose. There were no significant differences in glimepiride pharmacokinetics between the two age groups. The mean [[AUC]] at steady state for the older patients was approximately 13% lower than that for the younger patients; the mean weight-adjusted clearance for the older patients was approximately 11% higher than that for the younger patients. | ||
Pediatric Patients | ====Pediatric Patients==== | ||
No pharmacokinetic studies of DUETACT were performed in pediatric patients. | * No pharmacokinetic studies of DUETACT were performed in pediatric patients. | ||
Pioglitazone | '''<I>Pioglitazone</I>''' | ||
Safety and efficacy of pioglitazone in pediatric patients have not been established. DUETACT is not recommended for use in pediatric patients | * Safety and efficacy of pioglitazone in pediatric patients have not been established. DUETACT is not recommended for use in pediatric patients. | ||
Gender | ====Gender==== | ||
Pioglitazone | '''<I>Pioglitazone</I>''' | ||
The mean Cmax and AUC values of pioglitazone were increased 20% to 60% in women compared to men. In controlled clinical trials, HbA1c decreases from baseline were generally greater for females than for males (average mean difference in HbA1c 0.5%). Because therapy should be individualized for each patient to achieve glycemic control, no dose adjustment is recommended based on gender alone. | * The mean [[Cmax]] and [[AUC]] values of pioglitazone were increased 20% to 60% in women compared to men. In controlled clinical trials, [[HbA1c]] decreases from baseline were generally greater for females than for males (average mean difference in [[HbA1c]] 0.5%). Because therapy should be individualized for each patient to achieve glycemic control, no dose adjustment is recommended based on gender alone. | ||
Glimepiride | '''<I>Glimepiride</I>''' | ||
There were no differences between males and females in the pharmacokinetics of glimepiride when adjustment was made for differences in body weight. | * There were no differences between males and females in the pharmacokinetics of glimepiride when adjustment was made for differences in body weight. | ||
Ethnicity | ====Ethnicity==== | ||
Pioglitazone | '''<I>Pioglitazone</I>''' | ||
Pharmacokinetic data among various ethnic groups are not available. | * Pharmacokinetic data among various ethnic groups are not available. | ||
Glimepiride | '''<I>Glimepiride</I>''' | ||
No studies have been conducted to assess the effects of race on glimepiride pharmacokinetics but in placebo-controlled trials of glimepiride in patients with type 2 diabetes, the reduction in HbA1c was comparable in Caucasians (n=536), blacks (n=63), and Hispanics (n=63). | * No studies have been conducted to assess the effects of race on glimepiride pharmacokinetics but in placebo-controlled trials of glimepiride in patients with [[type 2 diabetes]], the reduction in [[HbA1c]] was comparable in Caucasians (n=536), blacks (n=63), and Hispanics (n=63). | ||
Obese Patients | ====Obese Patients==== | ||
The pharmacokinetics of glimepiride and its metabolites were measured in a single-dose study involving 28 patients with type 2 diabetes who either had normal body weight or were morbidly obese. While the Tmax, CL/F, and Vd/F of glimepiride in the morbidly obese patients were similar to those in the normal weight group, the morbidly obese had lower Cmax and AUC than those of normal body weight. The mean Cmax, AUC0-24, AUC0-∞ values of glimepiride in normal vs. morbidly obese patients were 547 ± 218 ng/mL vs. 410 ± 124 ng/mL, 3210 ± 1030 hours·ng/mL vs. 2820 ± 1110 hours·ng/mL and 4000 ± 1320 hours·ng/mL versus 3280 ± 1360 hours·ng/mL, respectively. | * The pharmacokinetics of glimepiride and its metabolites were measured in a single-dose study involving 28 patients with type 2 diabetes who either had normal body weight or were morbidly obese. While the [[Tmax]], CL/F, and Vd/F of [[glimepiride]] in the morbidly obese patients were similar to those in the normal weight group, the morbidly obese had lower [[Cmax]] and [[AUC]] than those of normal body weight. The mean [[Cmax]], AUC0-24, AUC0-∞ values of glimepiride in normal vs. morbidly obese patients were 547 ± 218 ng/mL vs. 410 ± 124 ng/mL, 3210 ± 1030 hours·ng/mL vs. 2820 ± 1110 hours·ng/mL and 4000 ± 1320 hours·ng/mL versus 3280 ± 1360 hours·ng/mL, respectively. | ||
Other Populations | ====Other Populations==== | ||
Glimepiride | '''<I>Glimepiride</I>''' | ||
There were no important differences in glimepiride metabolism in subjects identified as phenotypically different drug-metabolizers by their metabolism of sparteine. The pharmacokinetics of glimepiride in morbidly obese patients were similar to those in the normal weight group, except for a lower Cmax and AUC. However, since neither Cmax nor AUC values were normalized for body surface area, the lower values of Cmax and AUC for the obese patients were likely the result of their excess weight and not due to a difference in the kinetics of glimepiride. | * There were no important differences in [[glimepiride]] metabolism in subjects identified as phenotypically different drug-metabolizers by their metabolism of sparteine. The pharmacokinetics of glimepiride in morbidly obese patients were similar to those in the normal weight group, except for a lower [[Cmax]] and [[AUC]]. However, since neither [[Cmax]] nor [[AUC]] values were normalized for body surface area, the lower values of Cmax and AUC for the obese patients were likely the result of their excess weight and not due to a difference in the kinetics of glimepiride. | ||
Drug-Drug Interactions | ====Drug-Drug Interactions==== | ||
Coadministration of pioglitazone (45 mg) and a sulfonylurea (5 mg glipizide) administered orally once daily for seven days did not alter the steady-state pharmacokinetics of glipizide. Glimepiride and glipizide have similar metabolic pathways and are mediated by CYP2C9; therefore, drug-drug interaction between pioglitazone and glimepiride is considered unlikely. Specific pharmacokinetic drug interaction studies with DUETACT have not been performed, although such studies have been conducted with the individual pioglitazone and glimepiride components. | * Coadministration of [[pioglitazone]] (45 mg) and a [[sulfonylurea]] (5 mg glipizide) administered orally once daily for seven days did not alter the steady-state pharmacokinetics of [[glipizide]]. [[Glimepiride]] and [[glipizide]] have similar metabolic pathways and are mediated by [[CYP2C9]]; therefore, drug-drug interaction between [[pioglitazone]] and glimepiride is considered unlikely. Specific pharmacokinetic drug interaction studies with DUETACT have not been performed, although such studies have been conducted with the individual pioglitazone and glimepiride components. | ||
Pioglitazone | '''<I>Pioglitazone</I>''' | ||
[[File:Duetact_PK_01.png|thumb|none| | [[File:Duetact_PK_01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
[[File:Duetact_PK_02.png|thumb|none| | [[File:Duetact_PK_02.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
Glimepiride | '''<I>Glimepiride</I>''' | ||
Aspirin | ====Aspirin==== | ||
In a randomized, double-blind, two-period, crossover study, healthy subjects were given either placebo or aspirin 1 gram three times daily for a total treatment period of 5 days. On Day 4 of each study period, a single 1 mg dose of glimepiride was administered. The glimepiride doses were separated by a 14-day washout period. Coadministration of aspirin and glimepiride resulted in a 34% decrease in the mean glimepiride AUC and a 4% decrease in the mean glimepiride Cmax. | * In a randomized, double-blind, two-period, crossover study, healthy subjects were given either placebo or aspirin 1 gram three times daily for a total treatment period of 5 days. On Day 4 of each study period, a single 1 mg dose of [[glimepiride]] was administered. The [[glimepiride]] doses were separated by a 14-day washout period. Coadministration of aspirin and glimepiride resulted in a 34% decrease in the mean glimepiride [[AUC]] and a 4% decrease in the mean glimepiride [[Cmax]]. | ||
Cimetidine and Ranitidine | ====Cimetidine and Ranitidine==== | ||
In a randomized, open-label, 3-way crossover study, healthy subjects received either a single 4 mg dose of glimepiride alone, glimepiride with ranitidine (150 mg twice daily for 4 days; glimepiride was administered on Day 3), or glimepiride with cimetidine (800 mg daily for 4 days; glimepiride was administered on Day 3). Coadministration of cimetidine or ranitidine with a single 4 mg oral dose of glimepiride did not significantly alter the absorption and disposition of glimepiride. | * In a randomized, open-label, 3-way crossover study, healthy subjects received either a single 4 mg dose of [[glimepiride]] alone, glimepiride with [[ranitidine]] (150 mg twice daily for 4 days; glimepiride was administered on Day 3), or glimepiride with [[cimetidine]] (800 mg daily for 4 days; glimepiride was administered on Day 3). Coadministration of cimetidine or [[ranitidine]] with a single 4 mg oral dose of glimepiride did not significantly alter the absorption and disposition of [[glimepiride]]. | ||
Propranolol | ====Propranolol==== | ||
In a randomized, double-blind, two-period, crossover study, healthy subjects were given either placebo or propranolol 40 mg three times daily for a total treatment period of five days. On Day 4 or each study period, a single 2 mg dose of glimepiride was administered. The glimepiride doses were separated by a 14-day washout period. Concomitant administration of propranolol and glimepiride significantly increased glimepiride Cmax, AUC, and t1/2 by 23%, 22%, and 15%, respectively, and decreased glimepiride CL/F by 18%. The recovery of M1 and M2 from urine was not changed. | * In a randomized, double-blind, two-period, crossover study, healthy subjects were given either placebo or [[propranolol]] 40 mg three times daily for a total treatment period of five days. On Day 4 or each study period, a single 2 mg dose of [[glimepiride]] was administered. The glimepiride doses were separated by a 14-day washout period. Concomitant administration of [[propranolol]] and glimepiride significantly increased glimepiride [[Cmax]], [[AUC]], and t1/2 by 23%, 22%, and 15%, respectively, and decreased glimepiride CL/F by 18%. The recovery of M1 and M2 from urine was not changed. | ||
Warfarin | ====Warfarin==== | ||
In an open-label, two-way, crossover study, healthy subjects received 4 mg of glimepiride daily for 10 days. Single 25 mg doses of warfarin were administered six days before starting glimepiride and on Day 4 of glimepiride administration. The concomitant administration of glimepiride did not alter the pharmacokinetics of R- and S-warfarin enantiomers. No changes were observed in warfarin plasma protein binding. Glimepiride resulted in a statistically significant decrease in the pharmacodynamic response to warfarin. The reductions in mean area under the prothrombin time (PT) curve and maximum PT values during glimepiride treatment were 3.3% and 9.9%, respectively, and are unlikely to be clinically relevant. | * In an open-label, two-way, crossover study, healthy subjects received 4 mg of glimepiride daily for 10 days. Single 25 mg doses of warfarin were administered six days before starting [[glimepiride]] and on Day 4 of [[glimepiride]] administration. The concomitant administration of glimepiride did not alter the pharmacokinetics of R- and S-warfarin enantiomers. No changes were observed in [[warfarin]] plasma protein binding. Glimepiride resulted in a statistically significant decrease in the pharmacodynamic response to [[warfarin]]. The reductions in mean area under the [[prothrombin time]] (PT) curve and maximum PT values during [[glimepiride]] treatment were 3.3% and 9.9%, respectively, and are unlikely to be clinically relevant. | ||
Colesevelam | ====[[Colesevelam]]==== | ||
Concomitant administration of colesevelam and glimepiride resulted in reductions in glimepiride AUC0-∞ and Cmax of 18% and 8%, respectively. When glimepiride was administered 4 hours prior to colesevelam, there was not significant change in glimepiride AUC0-∞ and Cmax, -6% and 3%, respectively | * Concomitant administration of [[colesevelam]] and [[glimepiride]] resulted in reductions in glimepiride AUC0-∞ and [[Cmax]] of 18% and 8%, respectively. When glimepiride was administered 4 hours prior to colesevelam, there was not significant change in glimepiride AUC0-∞ and [[Cmax]], -6% and 3%, respectively | ||
|nonClinToxic====Carcinogenesis, Mutagenesis, Impairment of Fertility=== | |nonClinToxic====Carcinogenesis, Mutagenesis, Impairment of Fertility=== | ||
No animal studies have been conducted with DUETACT. The following data are based on findings in studies performed with pioglitazone or glimepiride individually. | * No animal studies have been conducted with DUETACT. The following data are based on findings in studies performed with [[pioglitazone]] or [[glimepiride]] individually. | ||
Pioglitazone | '''<I>Pioglitazone</I>''' | ||
A two-year carcinogenicity study was conducted in male and female rats at oral doses up to 63 mg/kg (approximately 14 times the maximum recommended human oral dose of 45 mg based on mg/m2). Drug-induced tumors were not observed in any organ except for the urinary bladder of male rats. Benign and/or malignant transitional cell neoplasms were observed in male rats at 4 mg/kg/day and above (approximately equal to the maximum recommended human oral dose based on mg/m2). Urinary calculi with subsequent irritation and hyperplasia were postulated as the mechanism for bladder tumors observed in male rats. A two-year mechanistic study in male rats utilizing dietary acidification to reduce calculi formation was completed in 2009. Dietary acidification decreased but did not abolish the hyperplastic changes in the bladder. The presence of calculi exacerbated the hyperplastic response to pioglitazone but was not considered the primary cause of the hyperplastic changes. | * A two-year carcinogenicity study was conducted in male and female rats at oral doses up to 63 mg/kg (approximately 14 times the maximum recommended human oral dose of 45 mg based on mg/m2). Drug-induced tumors were not observed in any organ except for the urinary bladder of male rats. Benign and/or malignant transitional cell neoplasms were observed in male rats at 4 mg/kg/day and above (approximately equal to the maximum recommended human oral dose based on mg/m2). Urinary calculi with subsequent irritation and [[hyperplasia]] were postulated as the mechanism for bladder tumors observed in male rats. A two-year mechanistic study in male rats utilizing dietary acidification to reduce calculi formation was completed in 2009. Dietary acidification decreased but did not abolish the hyperplastic changes in the bladder. The presence of calculi exacerbated the hyperplastic response to [[pioglitazone]] but was not considered the primary cause of the hyperplastic changes. | ||
The relevance to humans of the bladder findings in the male rat cannot be excluded. | * The relevance to humans of the bladder findings in the male rat cannot be excluded. | ||
A two-year carcinogenicity study was also conducted in male and female mice at oral doses up to 100 mg/kg/day (approximately 11 times the maximum recommended human oral dose based on mg/m2). No drug-induced tumors were observed in any organ. | * A two-year carcinogenicity study was also conducted in male and female mice at oral doses up to 100 mg/kg/day (approximately 11 times the maximum recommended human oral dose based on mg/m2). No drug-induced tumors were observed in any organ. | ||
Pioglitazone hydrochloride was not mutagenic in a battery of genetic toxicology studies, including the Ames bacterial assay, a mammalian cell forward gene mutation assay (CHO/HPRT and AS52/XPRT), an in vitro cytogenetics assay using CHL cells, an unscheduled DNA synthesis assay, and an in vivo micronucleus assay. | * Pioglitazone hydrochloride was not mutagenic in a battery of genetic toxicology studies, including the Ames bacterial assay, a mammalian cell forward gene mutation assay (CHO/HPRT and AS52/XPRT), an in vitro cytogenetics assay using CHL cells, an unscheduled DNA synthesis assay, and an in vivo micronucleus assay. | ||
No adverse effects upon fertility were observed in male and female rats at oral doses up to 40 mg/kg pioglitazone hydrochloride daily prior to and throughout mating and gestation (approximately nine times the maximum recommended human oral dose based on mg/m2). | * No adverse effects upon fertility were observed in male and female rats at oral doses up to 40 mg/kg pioglitazone hydrochloride daily prior to and throughout mating and gestation (approximately nine times the maximum recommended human oral dose based on mg/m2). | ||
Glimepiride | '''<I>Glimepiride</I>''' | ||
Studies in rats at doses of up to 5000 parts per million (ppm) in complete feed (approximately 340 times the maximum recommended human dose, based on surface area) for 30 months showed no evidence of carcinogenesis. In mice, administration of glimepiride for 24 months resulted in an increase in benign pancreatic adenoma formation that was dose-related and was thought to be the result of chronic pancreatic stimulation. No adenoma formation in mice was observed at a dose of 320 ppm in complete feed, or 46 − 54 mg/kg body weight/day. This is about 35 times the maximum human recommended dose of 8 mg once daily based on surface area. | * Studies in rats at doses of up to 5000 parts per million (ppm) in complete feed (approximately 340 times the maximum recommended human dose, based on surface area) for 30 months showed no evidence of [[carcinogenesis]]. In mice, administration of glimepiride for 24 months resulted in an increase in benign [[pancreatic adenoma]] formation that was dose-related and was thought to be the result of chronic pancreatic stimulation. No [[adenoma]] formation in mice was observed at a dose of 320 ppm in complete feed, or 46 − 54 mg/kg body weight/day. This is about 35 times the maximum human recommended dose of 8 mg once daily based on surface area. | ||
Glimepiride was non-mutagenic in a battery of in vitro and in vivo mutagenicity studies (Ames test, somatic cell mutation, chromosomal aberration, unscheduled DNA synthesis and mouse micronucleus test). | * Glimepiride was non-mutagenic in a battery of in vitro and in vivo mutagenicity studies (Ames test, somatic cell mutation, chromosomal aberration, unscheduled DNA synthesis and mouse micronucleus test). | ||
There was no effect of glimepiride on male mouse fertility in animals exposed up to 2500 mg/kg body weight (>1,700 times the maximum recommended human dose based on surface area). Glimepiride had no effect on the fertility of male and female rats administered up to 4000 mg/kg body weight (approximately 4,000 times the maximum recommended human dose based on surface area). | * There was no effect of [[glimepiride]] on male mouse fertility in animals exposed up to 2500 mg/kg body weight (>1,700 times the maximum recommended human dose based on surface area). Glimepiride had no effect on the fertility of male and female rats administered up to 4000 mg/kg body weight (approximately 4,000 times the maximum recommended human dose based on surface area). | ||
===Animal Toxicology and/or Pharmacology=== | ===Animal Toxicology and/or Pharmacology=== | ||
Pioglitazone | '''<I>Pioglitazone</I>''' | ||
Heart enlargement has been observed in mice (100 mg/kg), rats (4 mg/kg and above), and dogs (3 mg/kg) treated orally with the pioglitazone hydrochloride component of DUETACT (approximately 11, one, and two times the maximum recommended human oral dose for mice, rats, and dogs, respectively, based on mg/m2). In a one-year rat study, drug-related early death due to apparent heart dysfunction occurred at an oral dose of 160 mg/kg/day (approximately 35 times the maximum recommended human oral dose based on mg/m2). Heart enlargement was seen in a 13-week study in monkeys at oral doses of 8.9 mg/kg and above (approximately four times the maximum recommended human oral dose based on mg/m2), but not in a 52-week study at oral doses up to 32 mg/kg (approximately 13 times the maximum recommended human oral dose based on mg/m2). | * Heart enlargement has been observed in mice (100 mg/kg), rats (4 mg/kg and above), and dogs (3 mg/kg) treated orally with the pioglitazone hydrochloride component of DUETACT (approximately 11, one, and two times the maximum recommended human oral dose for mice, rats, and dogs, respectively, based on mg/m2). In a one-year rat study, drug-related early death due to apparent heart dysfunction occurred at an oral dose of 160 mg/kg/day (approximately 35 times the maximum recommended human oral dose based on mg/m2). Heart enlargement was seen in a 13-week study in monkeys at oral doses of 8.9 mg/kg and above (approximately four times the maximum recommended human oral dose based on mg/m2), but not in a 52-week study at oral doses up to 32 mg/kg (approximately 13 times the maximum recommended human oral dose based on mg/m2). | ||
|clinicalStudies=There have been no clinical efficacy studies conducted with DUETACT. However, the efficacy and safety of the separate components have been previously established. The coadministration of pioglitazone and a sulfonylurea, including glimepiride, has been evaluated for efficacy and safety in two clinical studies. These clinical studies established an added benefit of pioglitazone in glycemic control of patients with inadequately controlled type 2 diabetes while on sulfonylurea therapy. Bioequivalence of DUETACT with coadministered pioglitazone and glimepiride tablets was demonstrated at the 30 mg/2 mg and 30 mg/4 mg dosage strengths . | |clinicalStudies=* There have been no clinical efficacy studies conducted with DUETACT. However, the efficacy and safety of the separate components have been previously established. The coadministration of [[pioglitazone]] and a [[sulfonylurea]], including [[glimepiride]], has been evaluated for efficacy and safety in two clinical studies. These clinical studies established an added benefit of pioglitazone in glycemic control of patients with inadequately controlled type 2 diabetes while on [[sulfonylurea]] therapy. Bioequivalence of DUETACT with coadministered pioglitazone and [[glimepiride]] tablets was demonstrated at the 30 mg/2 mg and 30 mg/4 mg dosage strengths . | ||
Two clinical trials were conducted with pioglitazone in combination with a sulfonylurea. Both studies included patients with type 2 diabetes on any dose of a sulfonylurea, either alone or in combination with another antidiabetic agent. All other antidiabetic agents were withdrawn at least three weeks prior to starting study treatment. | * Two clinical trials were conducted with [[pioglitazone]] in combination with a [[sulfonylurea]]. Both studies included patients with type 2 diabetes on any dose of a [[sulfonylurea]], either alone or in combination with another [[antidiabetic]] agent. All other antidiabetic agents were withdrawn at least three weeks prior to starting study treatment. | ||
In the first study, 560 patients were randomized to receive 15 mg or 30 mg of pioglitazone or placebo once daily for 16 weeks in addition to their current sulfonylurea regimen. Treatment with pioglitazone as add-on to sulfonylurea produced statistically significant improvements in HbA1c and FGP at endpoint compared to placebo add-on to sulfonylurea (Table 15). | * In the first study, 560 patients were randomized to receive 15 mg or 30 mg of [[pioglitazone]] or placebo once daily for 16 weeks in addition to their current [[sulfonylurea]] regimen. Treatment with pioglitazone as add-on to sulfonylurea produced statistically significant improvements in HbA1c and FGP at endpoint compared to placebo add-on to [[sulfonylurea]] (Table 15). | ||
[[File:Duetact_clinical studies_01.png|thumb|none| | [[File:Duetact_clinical studies_01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
In the second trial, 702 patients were randomized to receive 30 mg or 45 mg of pioglitazone once daily for 24 weeks in addition to their current sulfonylurea regimen. The mean reduction from baseline at Week 24 in HbA1c was 1.6% for the 30 mg dose and 1.7% for the 45 mg dose (see Table 16). The mean reduction from baseline at Week 24 in FPG was 52 mg/dL for the 30 mg dose and 56 mg/dL for the 45 mg dose. | * In the second trial, 702 patients were randomized to receive 30 mg or 45 mg of [[pioglitazone]] once daily for 24 weeks in addition to their current [[sulfonylurea]] regimen. The mean reduction from baseline at Week 24 in HbA1c was 1.6% for the 30 mg dose and 1.7% for the 45 mg dose (see Table 16). The mean reduction from baseline at Week 24 in FPG was 52 mg/dL for the 30 mg dose and 56 mg/dL for the 45 mg dose. | ||
The therapeutic effect of pioglitazone in combination with sulfonylurea was observed in patients regardless of the sulfonylurea dose. | * The therapeutic effect of [[pioglitazone]] in combination with [[sulfonylurea]] was observed in patients regardless of the sulfonylurea dose. | ||
[[File:Duetact_clinical studies_02.png|thumb|none| | [[File:Duetact_clinical studies_02.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
|howSupplied=DUETACT is available in 30 mg pioglitazone plus 2 mg glimepiride or 30 mg pioglitazone plus 4 mg glimepiride tablets as follows: | |howSupplied=* DUETACT is available in 30 mg pioglitazone plus 2 mg glimepiride or 30 mg pioglitazone plus 4 mg glimepiride tablets as follows: | ||
30 mg/2 mg tablet: white to off-white, round, convex tablets, debossed with 4833G on one side and 30/2 on the other, available in: | 30 mg/2 mg tablet: white to off-white, round, convex tablets, debossed with 4833G on one side and 30/2 on the other, available in: | ||
NDC 64764-302-30 Bottles of 30 | : NDC 64764-302-30 Bottles of 30 | ||
NDC 64764-302-90 Bottles of 90 | : NDC 64764-302-90 Bottles of 90 | ||
30 mg/4 mg tablet: white to off-white, round, convex tablets, debossed with 4833G on one side and 30/4 on the other, available in: | 30 mg/4 mg tablet: white to off-white, round, convex tablets, debossed with 4833G on one side and 30/4 on the other, available in: | ||
NDC 64764-304-30 Bottles of 30 | : NDC 64764-304-30 Bottles of 30 | ||
NDC 64764-304-90 Bottles of 90 | : NDC 64764-304-90 Bottles of 90 | ||
|fdaPatientInfo=Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Keep container tightly closed and protect from moisture and humidity. | |fdaPatientInfo=* Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Keep container tightly closed and protect from moisture and humidity. | ||
|alcohol=Alcohol-Pioglitazone/Glimepiride interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Pioglitazone/Glimepiride interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
|brandNames=Duetact | |brandNames=Duetact | ||
Latest revision as of 13:22, 27 March 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2], Rabin Bista, M.B.B.S. [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: CONGESTIVE HEART FAILURE
See full prescribing information for complete Boxed Warning.
Thiazolidinediones, including pioglitazone, which is a component of DUETACT, cause or exacerbate Congestive heart failure in some patients.
|
Overview
Pioglitazone/Glimepiride is a Thiazolidinedione that is FDA approved for the treatment of glycemic control in adults with type 2 diabetes mellitus who are already treated with a thiazolidinedione and sulfonylurea or who have inadequate glycemic control on a thiazolidinedione alone or a sulfonylurea alone. There is a Black Box Warning for this drug as shown here. Common adverse reactions include edema, edema of lower extremity, hypoglycemia, weight increased, diarrhea, nausea, backache, myalgia, pain in limb, headache, urinary tract infectious disease, pharyngitis, sinusitis, upper respiratory infection, accidental injury, influenza.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Type 2 Diabetes Mellitus
- Recommendations for All Patients
- DUETACT should be taken once daily with the first main meal.
- DUETACT tablets are available as a 30 mg pioglitazone plus 2 mg glimepiride or a 30 mg pioglitazone plus 4 mg glimepiride tablet. If therapy with a combination tablet containing pioglitazone and glimepiride is considered appropriate the recommended starting dose is:
- 30 mg/2 mg or 30 mg/4 mg once daily and gradually titrated, as needed, after assessing adequacy of therapeutic response and tolerability,
- for patients inadequately controlled on glimepiride monotherapy: 30 mg/2 mg or 30 mg/4 mg once daily and gradually titrated, as needed, after assessing adequacy of therapeutic response and tolerability,
- for patients inadequately controlled on pioglitazone monotherapy: 30 mg/2 mg once daily and gradually titrated, as needed, after assessing adequacy of therapeutic response and tolerability,
- for patients who are changing from combination therapy of pioglitazone plus glimepiride as separate tablets: DUETACT should be taken at doses that are as close as possible to the dose of pioglitazone and glimepiride already being taken,
- for patients currently on a different sulfonylurea monotherapy or switching from combination therapy of pioglitazone plus a different sulfonylurea (e.g., glyburide, glipizide, chlorpropamide, tolbutamide, acetohexamide): 30 mg/2 mg once daily and adjusted after assessing adequacy of therapeutic response. Observe for hypoglycemia for one to two weeks due to the potential overlapping drug effect.
- for patients with systolic dysfunction, the lowest approved dose of DUETACT should be prescribed only after titration from 15 mg to 30 mg of pioglitazone has been safely tolerated.
- After initiation of DUETACT or with dose increase, monitor patients carefully for hypoglycemia and adverse reactions related to fluid retention such as weight gain, edema, and signs and symptoms of congestive heart failure .
- Liver tests (serum alanine and aspartate aminotransferases, alkaline phosphatase, and total bilirubin) should be obtained prior to initiating DUETACT. Routine periodic monitoring of liver tests during treatment with DUETACT is not recommended in patients without liver disease. Patients who have liver test abnormalities prior to initiation of DUETACT or who are found to have abnormal liver tests while taking DUETACT should be managed as described under Warnings and Precautions .
Concomitant Use with an Insulin Secretagogue or Insulin
- If hypoglycemia occurs in a patient coadministered DUETACT and an insulin secretagogue, the dose of the insulin secretagogue should be reduced.
- If hypoglycemia occurs in a patient coadministered DUETACT and insulin, the dose of insulin should be decreased by 10% to 25%. Further adjustments to the insulin dose should be individualized based on glycemic response.
Concomitant Use with Strong CYP2C8 Inhibitors
- Coadministration of pioglitazone and gemfibrozil, a strong CYP2C8 inhibitor, increases pioglitazone exposure approximately 3-fold. Therefore, the maximum recommended dose of pioglitazone is 15 mg daily when used in combination with gemfibrozil or other strong CYP2C8 inhibitors. If gemfibrozil or other CYP2C8 inhibitors need to co-administered, patients should switch to individual components of DUETACT because the minimum dose of pioglitazone in DUETACT exceeds 15 mg .
Concomitant Use with Colesevelam
- When colesevelam is coadministered with glimepiride, maximum plasma concentration and total exposure to glimepiride is reduced. Therefore, DUETACT should be administered at least four hours prior to colesevelam .
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pioglitazone/Glimepiride in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pioglitazone/Glimepiride in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness of DUETACT in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pioglitazone/Glimepiride in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pioglitazone/Glimepiride in pediatric patients.
Contraindications
- Initiation in patients with established NYHA Class III or IV heart failure .
- Use in patients with known hypersensitivity to pioglitazone, glimepiride or any other component of DUETACT .
- Use in patients with known history of an allergic reaction to sulfonamide derivatives.
Warnings
|
WARNING: CONGESTIVE HEART FAILURE
See full prescribing information for complete Boxed Warning.
Thiazolidinediones, including pioglitazone, which is a component of DUETACT, cause or exacerbate Congestive heart failure in some patients.
|
Congestive heart failure
Pioglitazone
- Pioglitazone, like other thiazolidinediones, can cause dose-related fluid retention when used alone or in combination with other antidiabetic medications and is most common when DUETACT is used in combination with insulin. Fluid retention may lead to or exacerbate Congestive heart failure. Patients should be observed for signs and symptoms of Congestive heart failure. If Congestive heart failure develops, it should be managed according to current standards of care and discontinuation or dose reduction of DUETACT must be considered .
Hypoglycemia
Glimepiride
- All sulfonylureas, including glimepiride, a component of DUETACT, can cause severe hypoglycemia . The patient's ability to concentrate and react may be impaired as a result of hypoglycemia. These impairments may present a risk in situations where these abilities are especially important, such as driving or operating other machinery. Severe hypoglycemia can lead to unconsciousness or convulsions and may result in temporary or permanent impairment of brain function or death.
- Patients must be educated to recognize and manage hypoglycemia. Use caution when initiating and increasing DUETACT doses in patients who may be predisposed to hypoglycemia (e.g., the elderly, patients with renal impairment, patients on other antidiabetic medications). Debilitated or malnourished patients and those with adrenal, pituitary, or hepatic impairment are particularly susceptible to the hypoglycemic action of glucose-lowering medications. hypoglycemia is also more likely to occur when caloric intake is deficient, after severe or prolonged exercise, or when alcohol is ingested.
Early warning symptoms of hypoglycemia may be different or less pronounced in patients with autonomic neuropathy, the elderly, and in patients who are taking beta-adrenergic blocking medications or other sympatholytic agents. These situations may result in severe hypoglycemia before the patient is aware of the hypoglycemia.
Hypersensitivity Reactions
Glimepiride
- There have been postmarketing reports of hypersensitivity reactions in patients treated with glimepiride, a component of DUETACT, including serious reactions such as anaphylaxis, angioedema, and Stevens-Johnson Syndrome. If a hypersensitivity reaction is suspected, promptly discontinue DUETACT, assess for other potential causes for the reaction, and institute alternative treatment for diabetes.
Potential Increased Risk of Cardiovascular Mortality with Sulfonylureas
Glimepiride
- The administration of oral hypoglycemic drugs has been reported to be associated with increased cardiovascular mortality as compared to treatment with diet alone or diet plus insulin. This warning is based on the study conducted by the University Group Diabetes Program (UGDP), a long-term, prospective clinical trial designed to evaluate the effectiveness of glucose-lowering drugs in preventing or delaying vascular complications in patients with non-insulin-dependent diabetes. The study involved 823 patients who were randomly assigned to one of four treatment groups.
- UGDP reported that patients treated for 5 to 8 years with diet plus a fixed dose of tolbutamide (1.5 grams per day) had a rate of cardiovascular mortality approximately 2.5 times that of patients treated with diet alone. A significant increase in total mortality was not observed, but the use of tolbutamide was discontinued based on the increase in cardiovascular mortality, thus limiting the opportunity for the study to show an increase in overall mortality. Despite controversy regarding the interpretation of these results, the findings of the UGDP study provide an adequate basis for this warning. The patient should be informed of the potential risks and advantages of glimepiride tablets and of alternative modes of therapy.
- Although only one drug in the sulfonylurea class (tolbutamide) was included in this study, it is prudent from a safety standpoint to consider that this warning may also apply to other oral hypoglycemic drugs in this class, in view of their close similarities in mode of action and chemical structure.
Hepatic Effects
Pioglitazone
- There have been postmarketing reports of fatal and non-fatal hepatic failure in patients taking pioglitazone, although the reports contain insufficient information necessary to establish the probable cause. There has been no evidence of drug-induced hepatotoxicity in the pioglitazone-controlled clinical trial database to date .
- Patients with type 2 diabetes may have fatty liver disease or cardiac disease with episodic Congestive heart failure, both of which may cause liver test abnormalities, and they may also have other forms of liver disease, many of which can be treated or managed. Therefore, obtaining a liver test panel (serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, and total bilirubin) and assessing the patient is recommended before initiating DUETACT therapy. In patients with abnormal liver tests, DUETACT should be initiated with caution.
- Measure liver tests promptly in patients who report symptoms that may indicate liver injury, including fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice. In this clinical context, if the patient is found to have abnormal liver tests (ALT greater than 3 times the upper limit of the reference range), DUETACT treatment should be interrupted and investigation done to establish the probable cause. DUETACT should not be restarted in these patients without another explanation for the liver test abnormalities.
- Patients who have serum ALT greater than three times the reference range with serum total bilirubin greater than two times the reference range without alternative etiologies are at risk for severe drug-induced liver injury and should not be restarted on DUETACT. For patients with lesser elevations of serum ALT or bilirubin and with an alternate probable cause, treatment with DUETACT can be used with caution.
Urinary Bladder Tumors
Pioglitazone
- Tumors were observed in the urinary bladder of male rats in the two-year carcinogenicity study . In two 3-year trials in which pioglitazone was compared to placebo or glyburide, there were 16/3656 (0.44%) reports of bladder cancer in patients taking pioglitazone compared to 5/3679 (0.14%) in patients not taking pioglitazone. After excluding patients in whom exposure to study drug was less than one year at the time of diagnosis of bladder cancer, there were six (0.16%) cases on pioglitazone and two (0.05%) cases on placebo.
- A five-year interim report of an ongoing 10-year observational cohort study found a non-significant increase in the risk for bladder cancer in subjects ever exposed to pioglitazone, compared to subjects never exposed to pioglitazone (HR 1.2 [95% CI 0.9 −1.5]). Compared to never exposure, a duration of pioglitazone therapy longer than 12 months was associated with an increase in risk (HR 1.4 [95% CI 0.9 −2.1]), which reached statistical significance after more than 24 months of pioglitazone use (HR 1.4 [95% CI 1.03 −2.0]). Interim results from this study suggested that taking pioglitazone longer than 12 months increased the relative risk of developing bladder cancer in any given year by 40% which equates to an absolute increase of three cases in 10,000 (from approximately seven in 10,000 [without pioglitazone] to approximately 10 in 10,000 [with pioglitazone]).
There are insufficient data to determine whether pioglitazone is a tumor promoter for urinary bladder tumors. Consequently, DUETACT should not be used in patients with active bladder cancer and the benefits of glycemic control versus unknown risks for cancer recurrence with DUETACT should be considered in patients with a prior history of bladder cancer.
Edema
Pioglitazone
- In controlled clinical trials, edema was reported more frequently in patients treated with pioglitazone than in placebo-treated patients and is dose-related * In postmarketing experience, reports of new onset or worsening edema have been received.
- DUETACT should be used with caution in patients with edema. Because thiazolidinediones, including pioglitazone, can cause fluid retention, which can exacerbate or lead to Congestive heart failure, DUETACT should be used with caution in patients at risk for Congestive heart failure. Patients treated with DUETACT should be monitored for signs and symptoms of Congestive heart failure .
Fractures
Pioglitazone
- In PROactive (the Prospective Pioglitazone Clinical Trial in Macrovascular Events), 5238 patients with type 2 diabetes and a history of macrovascular disease were randomized to pioglitazone (N=2605), force-titrated up to 45 mg daily or placebo (N=2633) in addition to standard of care. During a mean follow-up of 34.5 months, the incidence of bone fracture in females was 5.1% (44/870) for pioglitazone versus 2.5% (23/905) for placebo. This difference was noted after the first year of treatment and persisted during the course of the study. The majority of fractures observed in female patients were nonvertebral fractures including lower limb and distal upper limb. No increase in the incidence of fracture was observed in men treated with pioglitazone (1.7%) versus placebo (2.1%). The risk of fracture should be considered in the care of patients, especially female patients, treated with DUETACT and attention should be given to assessing and maintaining bone health according to current standards of care.
Hemolytic Anemia
Glimepiride
- Sulfonylureas can cause hemolytic anemia in patients with glucose 6-phosphate dehydrogenase (G6PD) deficiency. Because DUETACT contains glimepiride, which belongs to the class of sulfonylurea agents, use caution in patients with G6PD deficiency and consider the use of a nonsulfonylurea alternative. There are also postmarketing reports of hemolytic anemia in patients receiving glimepiride who did not have known G6PD deficiency
.
Macular edema
Pioglitazone
- Macular edema has been reported in postmarketing experience in diabetic patients who were taking pioglitazone or another thiazolidinedione. Some patients presented with blurred vision or decreased visual acuity, but others were diagnosed on routine ophthalmologic examination.
- Most patients had peripheral edema at the time Macular edema was diagnosed. Some patients had improvement in their Macular edema after discontinuation of the thiazolidinedione.
- Patients with diabetes should have regular eye exams by an ophthalmologist according to current standards of care. Patients with diabetes who report any visual symptoms should be promptly referred to an ophthalmologist, regardless of the patient’s underlying medications or other physical findings .
Ovulation
Pioglitazone
- Therapy with pioglitazone, like other thiazolidinediones, may result in ovulation in some premenopausal anovulatory women. As a result, these patients may be at an increased risk for pregnancy while taking DUETACT. This effect has not been investigated in clinical trials, so the frequency of this occurrence is not known. Adequate contraception in all premenopausal women treated with DUETACT is recommended.
Macrovascular Outcomes
- There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with DUETACT or any other antidiabetic drug.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The adverse events reported in at least 5% of patients in the controlled 16-week clinical studies between placebo plus a sulfonylurea and pioglitazone (15 mg and 30 mg combined) plus sulfonylurea treatment arms were upper respiratory tract infection (15.5% and 16.6%), accidental injury (8.6% and 3.5%), and combined edema/peripheral edema (2.1% and 7.2%), respectively.
- The incidence and type of adverse events reported in at least 5% of patients in any combined treatment group from the 24-week study comparing pioglitazone 30 mg plus a sulfonylurea and pioglitazone 45 mg plus a sulfonylurea are shown in Table 1; the rate of adverse events resulting in study discontinuation between the two treatment groups was 6% and 9.7%, respectively.
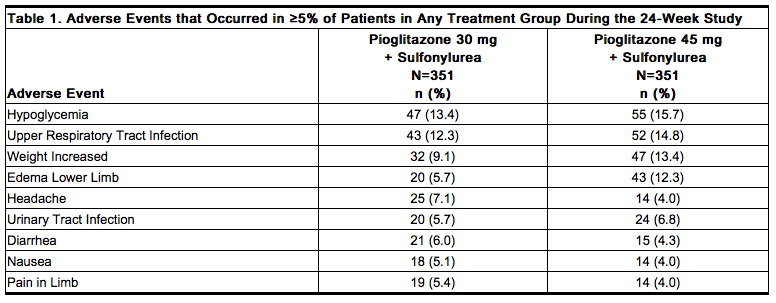
- In US double-blind studies, anemia was reported in ≤2% of patients treated with pioglitazone plus a sulfonylurea .
Pioglitazone
- Over 8500 patients with type 2 diabetes have been treated with pioglitazone in randomized, double-blind, controlled clinical trials, including 2605 patients with type 2 diabetes and macrovascular disease treated with pioglitazone in the PROactive clinical trial. In these trials, over 6000 patients have been treated with pioglitazone for six months or longer, over 4500 patients have been treated with pioglitazone for one year or longer, and over 3000 patients have been treated with pioglitazone for at least two years.
- In six pooled 16- to 26-week placebo-controlled monotherapy and 16- to 24-week add-on combination therapy trials, the incidence of withdrawals due to adverse events was 4.5% for patients treated with pioglitazone and 5.8% for comparator-treated patients. The most common adverse events leading to withdrawal were related to inadequate glycemic control, although the incidence of these events was lower (1.5%) with pioglitazone than with placebo (3.0%).
- In the PROactive trial, the incidence of withdrawals due to adverse events was 9.0% for patients treated with pioglitazone and 7.7% for placebo-treated patients. Congestive heart failure was the most common serious adverse event leading to withdrawal occurring in 1.3% of patients treated with pioglitazone and 0.6% of patients treated with placebo.
Common Adverse Events: 16- to 26-Week Monotherapy Trials:
- A summary of the incidence and type of common adverse events reported in three pooled 16- to 26-week placebo-controlled monotherapy trials of pioglitazone is provided in Table 2. Terms that are reported represent those that occurred at an incidence of >5% and more commonly in patients treated with pioglitazone than in patients who received placebo. None of these adverse events were related to the pioglitazone dose.
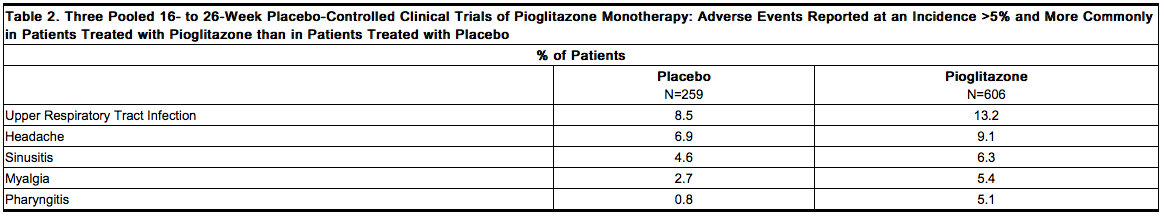
- A summary of the overall incidence and types of common adverse events reported in the PROactive trial is provided in Table 3. Terms that are reported represent those that occurred at an incidence of >5% and more commonly in patients treated with pioglitazone than in patients who received placebo.
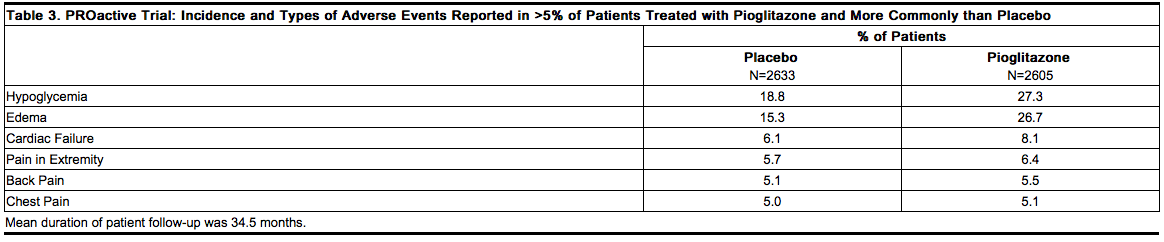
Congestive heart failure
- A summary of the incidence of adverse events related to Congestive heart failure is provided in Table 4 for the 16- to 24-week add-on to sulfonylurea trials, for the 16- to 24-week add-on to insulin trials, and for the 16- to 24-week add-on to metformin trials. None of the events were fatal.
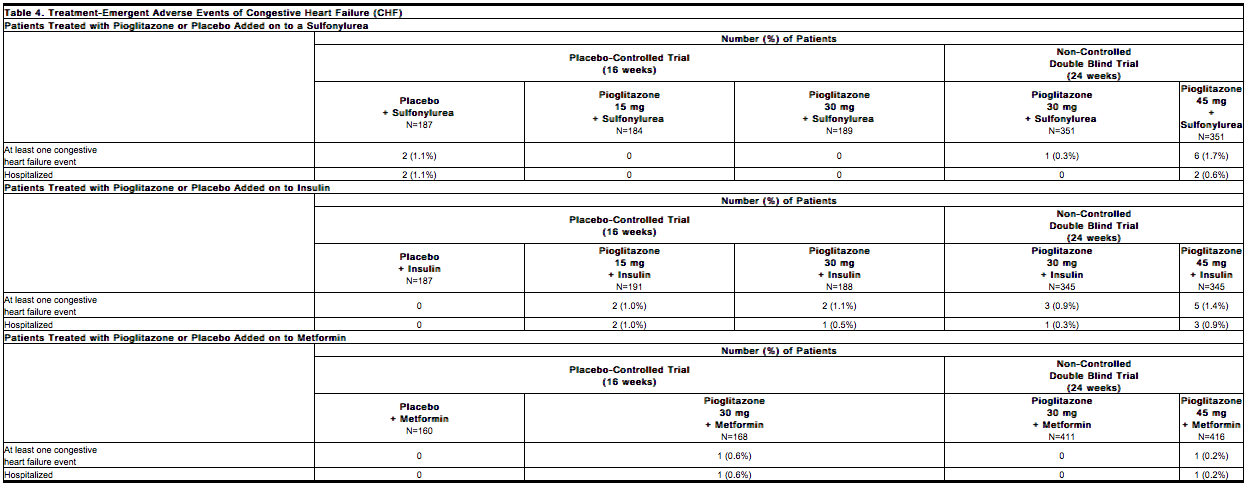
- Patients with type 2 diabetes and NYHA class II or early class III Congestive heart failure were randomized to receive 24 weeks of double-blind treatment with either pioglitazone at daily doses of 30 mg to 45 mg (n=262) or glyburide at daily doses of 10 mg to 15 mg (n=256). A summary of the incidence of adverse events related to Congestive heart failure reported in this study is provided in Table 5.

- Congestive heart failure events leading to hospitalization that occurred during the PROactive trial are summarized in Table 6.

Cardiovascular Safety
- In the PROactive trial, 5238 patients with type 2 diabetes and a history of macrovascular disease were randomized to pioglitazone (N=2605), force-titrated up to 45 mg daily or placebo (N=2633) in addition to standard of care. Almost all patients (95%) were receiving cardiovascular medications (beta blockers, ACE inhibitors, angiotensin II receptor blockers, calcium channel blockers, nitrates, diuretics, aspirin, statins, and fibrates). At baseline, patients had a mean age of 62 years, mean duration of diabetes of 9.5 years, and mean HbA1c of 8.1%. Mean duration of follow-up was 34.5 months.
- The primary objective of this trial was to examine the effect of pioglitazone on mortality and macrovascular morbidity in patients with type 2 diabetes mellitus who were at high risk for macrovascular events. The primary efficacy variable was the time to the first occurrence of any event in a cardiovascular composite endpoint that included all-cause mortality, nonfatal myocardial infarction (MI) including silent MI, stroke, acute coronary syndrome, cardiac intervention including coronary artery bypass grafting or percutaneous intervention, major leg amputation above the ankle, and bypass surgery or revascularization in the leg. A total of 514 (19.7%) patients treated with pioglitazone and 572 (21.7%) placebo-treated patients experienced at least one event from the primary composite endpoint (hazard ratio 0.90; 95% Confidence Interval: 0.80, 1.02; p=0.10).
- Although there was no statistically significant difference between pioglitazone and placebo for the three-year incidence of a first event within this composite, there was no increase in mortality or in total macrovascular events with pioglitazone. The number of first occurrences and total individual events contributing to the primary composite endpoint is shown in Table 7.
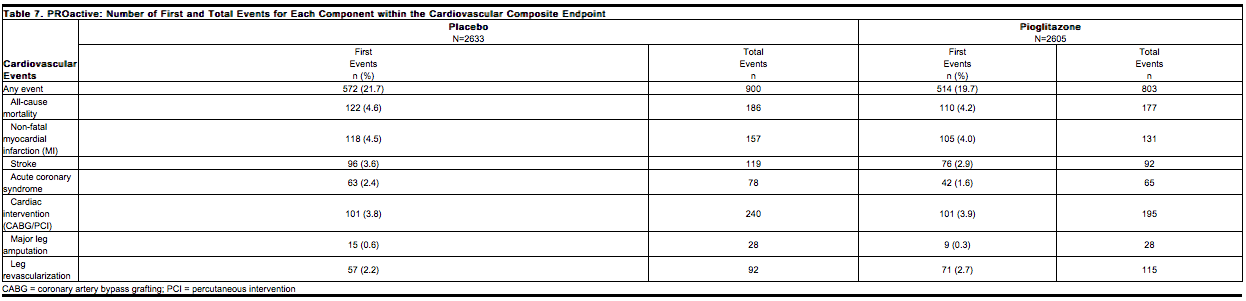
Weight Gain
- Dose-related weight gain occurs when pioglitazone is used alone or in combination with other antidiabetic medications. The mechanism of weight gain is unclear but probably involves a combination of fluid retention and fat accumulation.
- Tables 8 and 9 summarize the changes in body weight with pioglitazone and placebo in the 16- to 26-week randomized, double-blind monotherapy and 16- to 24-week combination add-on therapy trials and in the PROactive trial.
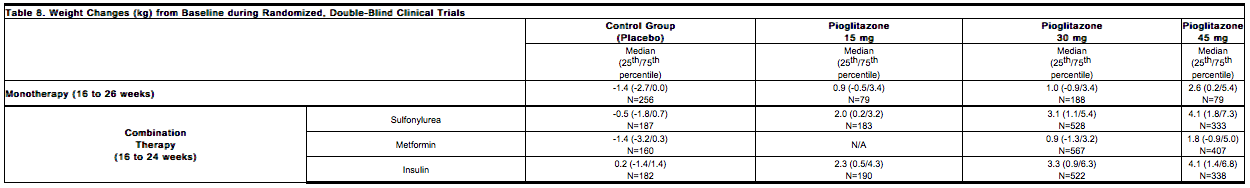

Edema
- edema induced from taking pioglitazone is reversible when pioglitazone is discontinued. The edema usually does not require hospitalization unless there is coexisting Congestive heart failure. A summary of the frequency and types of edema adverse events occurring in clinical investigations of pioglitazone is provided in Table 10.
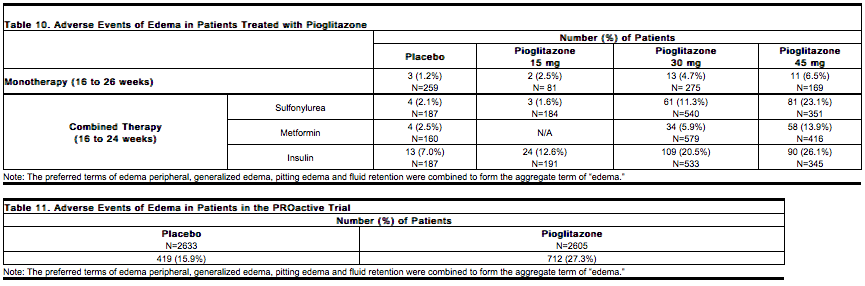
Hepatic Effects
- There has been no evidence of pioglitazone-induced hepatotoxicity in the pioglitazone-controlled clinical trial database to date. One randomized, double-blind, 3-year trial comparing pioglitazone to glyburide as add-on to metformin and insulin therapy was specifically designed to evaluate the incidence of serum ALT elevation to greater than three times the upper limit of the reference range, measured every eight weeks for the first 48 weeks of the trial then every 12 weeks thereafter. A total of 3/1051 (0.3%) patients treated with pioglitazone and 9/1046 (0.9%) patients treated with glyburide developed ALT values greater than three times the upper limit of the reference range. None of the patients treated with pioglitazone in the pioglitazone-controlled clinical trial database to date have had a serum ALT greater than three times the upper limit of the reference range and a corresponding total bilirubin greater than two times the upper limit of the reference range, a combination predictive of the potential for severe drug-induced liver injury.
Hypoglycemia
- In the pioglitazone clinical trials, adverse events of hypoglycemia were reported based on clinical judgment of the investigators and did not require confirmation with fingerstick glucose testing.
- In the 16-week add-on to sulfonylurea trial, the incidence of reported hypoglycemia was 3.7% with pioglitazone 30 mg and 0.5% with placebo. In the 16-week add-on to insulin trial, the incidence of reported hypoglycemia was 7.9% with pioglitazone 15 mg, 15.4% with pioglitazone 30 mg, and 4.8% with placebo.
The incidence of reported hypoglycemia was higher with pioglitazone 45 mg compared to pioglitazone 30 mg in both the 24-week add-on to sulfonylurea trial (15.7% versus 13.4%) and in the 24-week add-on to insulin trial (47.8% versus 43.5%).
- Three patients in these four trials were hospitalized due to hypoglycemia. All three patients were receiving pioglitazone 30 mg (0.9%) in the 24-week add-on to insulin trial. An additional 14 patients reported severe hypoglycemia (defined as causing considerable interference with patient’s usual activities) that did not require hospitalization. These patients were receiving pioglitazone 45 mg in combination with sulfonylurea (N=2) or pioglitazone 30 mg or 45 mg in combination with insulin (N=12).
Urinary Bladder Tumors
- Tumors were observed in the urinary bladder of male rats in the two-year carcinogenicity study. In two 3-year trials in which pioglitazone was compared to placebo or glyburide, there were 16/3656 (0.44%) reports of bladder cancer in patients taking pioglitazone compared to 5/3679 (0.14%) in patients not taking pioglitazone. After excluding patients in whom exposure to study drug was less than one year at the time of diagnosis of bladder cancer, there were six (0.16%) cases on pioglitazone and two (0.05%) cases on placebo. There are too few events of bladder cancer to establish causality.
Glimepiride
- Adverse events that occurred in controlled clinical trials with placebo and glimepiride monotherapy, other than hypoglycemia, included: headache (7.8% and 8.2%), accidental injury (3.4% and 5.8%), flu syndrome (4.4% and 5.4%), nausea (3.4% and 5.0%) and dizziness (2.4% and 5.0%), respectively.
hypoglycemia
- In a randomized, double-blind, placebo-controlled monotherapy trial of 14 weeks duration, patients already on sulfonylurea therapy underwent a 3-week washout period then were randomized to glimepiride 1 mg, 4 mg, 8 mg or placebo. Patients randomized to glimepiride 4 mg or 8 mg underwent forced-titration from an initial dose of 1 mg to these final doses, as tolerated. The overall incidence of possible hypoglycemia (defined by the presence of at least one symptom that the investigator believed might be related to hypoglycemia; a concurrent glucose measurement was not required) was 4% for glimepiride 1 mg, 17% for glimepiride 4 mg, 16% for glimepiride 8 mg and 0% for placebo. All of these events were self-treated.
- In a randomized, double-blind, placebo-controlled monotherapy trial of 22 weeks duration, patients received a starting dose of either 1 mg glimepiride or placebo daily. The dose of glimepiride was titrated to a target fasting plasma glucose of 90 −150 mg/dL. Final daily doses of glimepiride were 1, 2, 3, 4, 6 or 8 mg. The overall incidence of possible hypoglycemia (as defined above for the 14-week trial) for glimepiride versus placebo was 19.7% vs. 3.2%. All of these events were self-treated.
Weight Gain
- Glimepiride, like all sulfonylureas, can cause weight gain.
Allergic Reactions
- In clinical trials, allergic reactions, such as pruritus, erythema, urticaria, and morbilliform or maculopapular eruptions, occurred in less than 1% of glimepiride-treated patients. These may resolve despite continued treatment with glimepiride. There are postmarketing reports of more serious allergic reactions (e.g., dyspnea, hypotension, shock).
Laboratory Tests
Elevated Serum Alanine Aminotransferase (ALT)
- In 11 pooled placebo-controlled trials of glimepiride, 1.9% of glimepiride-treated patients and 0.8% of placebo-treated patients developed serum ALT greater than two times the upper limit of the reference range.
Laboratory Abnormalities
Pioglitazone
Hematologic Effects
- Pioglitazone may cause decreases in hemoglobin and hematocrit. In placebo-controlled monotherapy trials, mean hemoglobin values declined by 2% to 4% in patients treated with pioglitazone compared with a mean change in hemoglobin of -1% to +1% in placebo-treated patients. These changes primarily occurred within the first 4 to 12 weeks of therapy and remained relatively constant thereafter. These changes may be related to increased plasma volume associated with pioglitazone therapy and are not likely to be associated with any clinically significant hematologic effects.
Creatine Phosphokinase
- During protocol-specified measurement of serum creatine phosphokinase (CPK) in pioglitazone clinical trials, an isolated elevation in CPK to greater than 10 times the upper limit of the reference range was noted in nine (0.2%) patients treated with pioglitazone (values of 2150 to 11400 IU/L) and in no comparator-treated patients. Six of these nine patients continued to receive pioglitazone, two patients were noted to have the CPK elevation on the last day of dosing and one patient discontinued pioglitazone due to the elevation. These elevations resolved without any apparent clinical sequelae. The relationship of these events to pioglitazone therapy is unknown.
Postmarketing Experience
- The following adverse reactions have been identified during post-approval use of pioglitazone and glimepiride. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Pioglitazone
- New onset or worsening diabetic Macular edema with decreased visual acuity .
- Fatal and nonfatal hepatic failure.
- Postmarketing reports of Congestive heart failure have been reported in patients treated with pioglitazone, both with and without previously known heart disease and both with and without concomitant insulin administration.
- In postmarketing experience, there have been reports of unusually rapid increases in weight and increases in excess of that generally observed in clinical trials. Patients who experience such increases should be assessed for fluid accumulation and volume-related events such as excessive edema and Congestive heart failure .
Glimepiride
- Serious hypersensitivity reactions, including anaphylaxis, angioedema, and Stevens-Johnson Syndrome
- Hemolytic anemia in patients with and without G6PD deficiency
- Impairment of liver function (e.g. with cholestasis and jaundice), as well as hepatitis, which may progress to liver failure.
- Hyponatremia and syndrome of inappropriate antidiuretic hormone secretion (SIADH), most often in patients who are on other medications or who have medical conditions known to cause hyponatremia or increase release of antidiuretic hormone
Drug Interactions
Strong CYP2C8 Inhibitors
Pioglitazone
- An inhibitor of CYP2C8 (e.g., gemfibrozil) significantly increases the exposure (area under the serum concentration-time curve or AUC) and half-life (t½) of pioglitazone. Therefore, the maximum recommended dose of pioglitazone is 15 mg daily if used in combination with gemfibrozil or other strong CYP2C8 inhibitors. Since the minimum dose of pioglitazone in DUETACT exceeds 15 mg, patients taking concomitant strong CYP2C8 inhibitors should switch to individual components of DUETACT, unless the prescribing health care provider determines that the benefit of DUETACT clearly outweighs the risk of increased pioglitazone exposure .
CYP2C8 Inducers
Pioglitazone
- An inducer of CYP2C8 (e.g., rifampin) may significantly decrease the exposure (AUC) of pioglitazone. Therefore, if an inducer of CYP2C8 is started or stopped during treatment with pioglitazone, changes in diabetes treatment may be needed based on clinical response without exceeding the maximum recommended daily dose of 45 mg for pioglitazone .
Miconazole
Glimepiride
- A potential interaction between oral miconazole and sulfonylureas leading to severe hypoglycemia has been reported. Whether this interaction also occurs with other dosage forms of miconazole is not known.
CYP2C9 Interactions
Glimepiride
- There may be an interaction between glimepiride and inhibitors (e.g., fluconazole) and inducers (e.g., rifampin) of CYP2C9. Fluconazole may inhibit the metabolism of glimepiride, causing increased plasma concentrations of glimepiride which may lead to hypoglycemia. Rifampin may induce the metabolism of glimepiride, causing decreased plasma concentrations of glimepiride which may lead to worsening glycemic control.
Concomitant Administration of Colesevelam
Glimepiride
- Colesevelam can reduce the maximum plasma concentrations and total exposure of glimepiride when the two are coadministered. However, absorption is not reduced when glimepiride is administered four hours prior to colesevelam. Therefore, DUETACT should be administered at least four hours prior to colesevelam.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C Pioglitazone
- There are no adequate and well-controlled studies of DUETACT in pregnant women. Animal studies show increased rates of postimplantation loss, delayed development, reduced fetal weights, and delayed parturition at doses 10 to 40 times the maximum recommended human dose. DUETACT should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Clinical Considerations
- Abnormal blood glucose concentrations during pregnancy are associated with a higher incidence of congenital anomalies, as well as increased neonatal morbidity and mortality. Most experts recommend the use of insulin during pregnancy to maintain blood glucose concentrations as close to normal as possible for patients with diabetes.
Animal Data
- In animal reproductive studies, pregnant rats and rabbits received pioglitazone at doses up to approximately 17 (rat) and 40 (rabbit) times the maximum recommended human oral dose (MRHD) based on body surface area (mg/m2); no teratogenicity was observed. Increases in embryotoxicity (increased postimplantation losses, delayed development, reduced fetal weights, and delayed parturition) occurred in rats that received oral doses approximately 10 or more times the MRHD (mg/m2 basis). No functional or behavioral toxicity was observed in rat offspring. When pregnant rats received pioglitazone during late gestation and lactation, delayed postnatal development, attributed to decreased body weight, occurred in rat offspring at oral maternal doses approximately two or more times the MRHD (mg/m2 basis). In rabbits, embryotoxicity occurred at oral doses approximately 40 times the MRHD (mg/m2 basis).
Glimepiride
Teratogenic Effects
- In animal studies there was no increase in congenital anomalies, but an increase in fetal deaths occurred in rats and rabbits at glimepiride doses 50 times (rats) and 0.1 times (rabbits) the maximum recommended human dose (based on body surface area). This fetotoxicity, observed only at doses inducing maternal hypoglycemia, is believed to be directly related to the pharmacologic (hypoglycemic) action of glimepiride and has been similarly noted with other sulfonylureas. DUETACT should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Because data suggest that abnormal blood glucose during pregnancy is associated with a higher incidence of congenital abnormalities, diabetes treatment during pregnancy should maintain blood glucose as close to normal as possible.
Nonteratogenic Effects
- Prolonged severe hypoglycemia (4 to 10 days) has been reported in neonates born to mothers receiving a sulfonylurea at the time of delivery.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Pioglitazone/Glimepiride in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Pioglitazone/Glimepiride during labor and delivery.
Nursing Mothers
No studies have been conducted with the combined components of DUETACT. In studies performed with the individual components, pioglitazone was secreted in the milk of lactating rats and significant concentrations of glimepiride were observed in the serum and breast milk of the dams and serum of the pups. It is not known whether pioglitazone or glimepiride are secreted in human milk. However, other sulfonylureas are excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for DUETACT to cause serious adverse reactions in nursing infants, a decision should be made to discontinue nursing or discontinue DUETACT, taking into account the importance of DUETACT to the mother.
Pediatric Use
- Safety and effectiveness of DUETACT in pediatric patients have not been established.
- DUETACT is not recommended for use in pediatric patients based on adverse effects observed in adults, including fluid retention and Congestive heart failure, fractures, and urinary bladder tumors.
Glimepiride
- The pharmacokinetics, efficacy and safety of glimepiride have been evaluated in pediatric patients with type 2 diabetes as described below. Glimepiride is not recommended in pediatric patients because of its adverse effects on body weight and hypoglycemia.
- The pharmacokinetics of a 1 mg single dose of glimepiride was evaluated in 30 patients with type 2 diabetes (male = 7; female = 23) between ages 10 and 17 years. The mean (±SD) AUC (0-last) (339±203 ng•hr/mL), Cmax (102±48 ng/mL) and t1/2 (3.1±1.7 hours) for glimepiride were comparable to historical data from adults (AUC (0-last) 315±96 ng•hr/mL, Cmax 103±34 ng/mL and t1/2 5.3±4.1 hours).
- The safety and efficacy of glimepiride in pediatric patients was evaluated in a single-blind, 24-week trial that randomized 272 patients (8 to 17 years of age) with type 2 diabetes to glimepiride (n=135) or metformin (n=137). Both treatment-naïve patients (those treated with only diet and exercise for at least two weeks prior to randomization) and previously treated patients (those previously treated or currently treated with other oral antidiabetic medications for at least three months) were eligible to participate. Patients who were receiving oral antidiabetic agents at the time of study entry discontinued these medications before randomization without a washout period. Glimepiride was initiated at 1 mg, and then titrated up to 2, 4 or 8 mg (mean last dose 4 mg) through Week 12, targeting a self monitored fasting fingerstick blood glucose <126 mg/dL. Metformin was initiated at 500 mg twice daily and titrated at Week 12 up to 1000 mg twice daily (mean last dose 1365 mg).
- After 24 weeks, the overall mean treatment difference in HbA1c between glimepiride and metformin was 0.2%, favoring metformin (95% confidence interval -0.3% to +0.6%).
- Based on these results, the trial did not meet its primary objective of showing a similar reduction in HbA1c with glimepiride compared to metformin.
The profile of adverse reactions in pediatric patients treated with glimepiride was similar to that observed in adults.
- Hypoglycemic events documented by blood glucose values <36 mg/dL were observed in 4% of pediatric patients treated with glimepiride and in 1% of pediatric patients treated with metformin. One patient in each treatment group experienced a severe hypoglycemic episode (severity was determined by the investigator based on observed signs and symptoms).
Geriatic Use
- To minimize the risk of hypoglycemia, the initial dosing, dose increments, and maintenance dosage of DUETACT should be conservative. During initiation of DUETACT therapy and any subsequent dose adjustments, geriatric patients should be observed carefully for hypoglycemia.
Pioglitazone
- A total of 92 patients (15.2%) treated with pioglitazone in the three pooled 16- to 26-week double-blind, placebo-controlled, monotherapy trials were ≥65 years old and two patients (0.3%) were ≥75 years old. In the two pooled 16- to 24-week add-on to sulfonylurea trials, 201 patients (18.7%) treated with pioglitazone were ≥65 years old and 19 (1.8%) were ≥75 years old. In the two pooled 16- to 24-week add-on to metformin trials, 155 patients (15.5%) treated with pioglitazone were ≥65 years old and 19 (1.9%) were ≥75 years old. In the two pooled 16- to 24-week add-on to insulin trials, 272 patients (25.4%) treated with pioglitazone were ≥65 years old and 22 (2.1%) were ≥75 years old.
- In PROactive, 1068 patients (41.0%) treated with pioglitazone were ≥65 years old and 42 (1.6%) were ≥75 years old.
- In pharmacokinetic studies with pioglitazone, no significant differences were observed in pharmacokinetic parameters between elderly and younger patients .
Although clinical experiences have not identified differences in effectiveness and safety between the elderly (≥65 years) and younger patients, these conclusions are limited by small sample sizes for patients ≥75 years old.
Glimepiride
- In clinical trials of glimepiride, 1053 of 3491 patients (30%) were ≥65 years of age. No overall differences in safety or effectiveness were observed between these patients and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
- There were no significant differences in glimepiride pharmacokinetics between patients with type 2 diabetes ≤65 years (n=49) and those >65 years (n=42).
Glimepiride is substantially excreted by the kidney. Elderly patients are more likely to have renal impairment. In addition, hypoglycemia may be difficult to recognize in the elderly . Use caution when initiating DUETACT and increasing the dose of DUETACT in this patient population.
Gender
There is no FDA guidance on the use of Pioglitazone/Glimepiride with respect to specific gender populations.
Race
There is no FDA guidance on the use of Pioglitazone/Glimepiride with respect to specific racial populations.
Renal Impairment
- To minimize the risk of hypoglycemia, the initial dosing, dose increments and maintenance dosage of DUETACT should be conservative. During initiation of DUETACT therapy and any subsequent dose adjustments, these patients should be observed carefully for hypoglycemia.
- A multiple-dose titration study was conducted in 16 patients with type 2 diabetes and renal impairment using doses ranging from 1 mg to 8 mg daily for three months. Baseline creatinine clearance ranged from 10 to 60 mL/min. The pharmacokinetics of glimepiride were evaluated in the multiple-dose titration study and the results were consistent with those observed in patients enrolled in a single-dose study. In both studies, the relative total clearance of glimepiride increased when kidney function was impaired. Both studies also demonstrated that the elimination of the two major metabolites was reduced in patients with renal impairment
Hepatic Impairment
There is no FDA guidance on the use of Pioglitazone/Glimepiride in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Pioglitazone/Glimepiride in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Pioglitazone/Glimepiride in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
FDA Package Insert for Pioglitazone/Glimepiride contains no information regarding Drug Monitoring.
IV Compatibility
There is limited information about the IV Compabitility.
Overdosage
Pioglitazone
- During controlled clinical trials, one case of overdose with pioglitazone was reported. A male patient took 120 mg per day for four days, then 180 mg per day for seven days. The patient denied any clinical symptoms during this period.
- In the event of overdosage, appropriate supportive treatment should be initiated according to the patient’s clinical signs and symptoms.
Glimepiride
- An overdosage of glimepiride, as with other sulfonylureas, can produce severe hypoglycemia. Mild episodes of hypoglycemia can be treated with oral glucose. Severe hypoglycemic reactions constitute medical emergencies requiring immediate treatment. Severe hypoglycemia with coma, seizure, or neurological impairment can be treated with glucagon or intravenous glucose. Continued observation and additional carbohydrate intake may be necessary because hypoglycemia may recur after apparent clinical recovery.
Pharmacology
There is limited information regarding Pioglitazone/Glimepiride Pharmacology in the drug label.
Mechanism of Action
- DUETACT combines 2 antihyperglycemic agents with different mechanisms of action to improve glycemic control in patients with type 2 diabetes: pioglitazone, a member of the thiazolidinedione class, and glimepiride, a member of the sulfonylurea class. thiazolidinediones are insulin-sensitizing agents that act primarily by enhancing peripheral glucose utilization, whereas sulfonylureas are insulin secretagogues that act primarily by stimulating release of insulin from functioning pancreatic beta cells.
Pioglitazone
- Pioglitazone is a thiazolidinedione that depends on the presence of insulin for its mechanism of action. Pioglitazone decreases insulin resistance in the periphery and in the liver resulting in increased insulin-dependent glucose disposal and decreased hepatic glucose output. Pioglitazone is not an insulin secretagogue. Pioglitazone is an agonist for peroxisome proliferator-activated receptor-gamma (PPARγ). PPAR receptors are found in tissues important for insulin action such as adipose tissue, skeletal muscle, and liver. Activation of PPARγ nuclear receptors modulates the transcription of a number of insulin responsive genes involved in the control of glucose and lipid metabolism.
- In animal models of diabetes, pioglitazone reduces the hyperglycemia, hyperinsulinemia, and hypertriglyceridemia characteristic of insulin-resistant states such as type 2 diabetes. The metabolic changes produced by pioglitazone result in increased responsiveness of insulin-dependent tissues and are observed in numerous animal models of insulin resistance.
- Because pioglitazone enhances the effects of circulating insulin (by decreasing insulin resistance), it does not lower blood glucose in animal models that lack endogenous insulin.
Glimepiride
- Glimepiride primarily lowers blood glucose by stimulating the release of insulin from pancreatic beta cells. Sulfonylureas bind to the sulfonylurea receptor in the pancreatic beta cell plasma membrane, leading to closure of the ATP-sensitive potassium channel, thereby stimulating the release of insulin.
Structure
- DUETACT tablets are a thiazolidinedione and a sulfonylurea combination product that contains two oral antihyperglycemic agents: pioglitazone and glimepiride. The concomitant use of pioglitazone and a sulfonylurea, the class of drugs that includes glimepiride, has been previously approved based on clinical trials in patients with type 2 diabetes inadequately controlled on a sulfonylurea. Additional efficacy and safety information about pioglitazone and glimepiride monotherapies may be found in the prescribing information for each individual drug.
- Pioglitazone is an oral antidiabetic medication.
- Pioglitazone [(±)-5-[ [4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-] thiazolidinedione monohydrochloride contains one asymmetric carbon, and the compound is synthesized and used as the racemic mixture. The two enantiomers of pioglitazone interconvert in vivo. No differences were found in the pharmacologic activity between the two enantiomers. The structural formula is as shown:
- Pioglitazone hydrochloride is an odorless, white crystalline powder that has a molecular formula of C19H20N2O3S•HCl and a molecular weight of 392.90 daltons. It is soluble in N,N‑dimethylformamide, slightly soluble in anhydrous ethanol, very slightly soluble in acetone and acetonitrile, practically insoluble in water, and insoluble in ether.
- Glimepiride is an oral sulfonylurea chemically identified as 1-[ [p-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1-carboxamido)ethyl]phenyl]sulfonyl]-3-(trans-4-methylcyclohexyl)-urea (C24H34N4O5S) with a molecular weight of 490.62. Glimepiride is a white to yellowish-white, crystalline, odorless to practically odorless powder and is practically insoluble in water. The structural formula is:
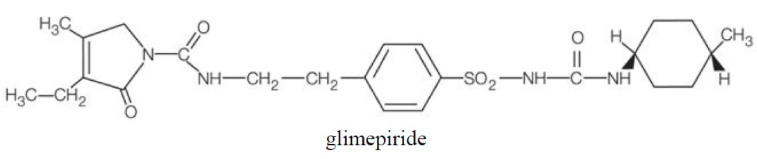
- DUETACT is available as a tablet for oral administration containing 30 mg pioglitazone (as the base) with 2 mg glimepiride (30 mg/2 mg) or 30 mg pioglitazone (as the base) with 4 mg glimepiride (30 mg/4 mg) formulated with the following excipients: croscarmellose sodium NF, lactose monohydrate NF, magnesium stearate NF, hydroxypropyl cellulose NF, polysorbate 80 NF, and microcrystalline cellulose NF.
Pharmacodynamics
Pioglitazone
- Clinical studies demonstrate that pioglitazone improves insulin sensitivity in insulin-resistant patients. Pioglitazone enhances cellular responsiveness to insulin, increases insulin-dependent glucose disposal and improves hepatic sensitivity to insulin. In patients with type 2 diabetes, the decreased insulin resistance produced by pioglitazone results in lower plasma glucose concentrations, lower plasma insulin concentrations, and lower HbA1c values. In controlled clinical trials, pioglitazone had an additive effect on glycemic control when used in combination with a sulfonylurea, metformin, or insulin .
- Patients with lipid abnormalities were included in clinical trials with pioglitazone. Overall, patients treated with pioglitazone had mean decreases in serum triglycerides, mean increases in HDL cholesterol, and no consistent mean changes in LDL and total cholesterol. There is no conclusive evidence of macrovascular benefit with pioglitazone or any other antidiabetic medication .
- In a 26-week, placebo-controlled, dose-ranging monotherapy study, mean serum triglycerides decreased in the 15 mg, 30 mg, and 45 mg pioglitazone dose groups compared to a mean increase in the placebo group. Mean HDL cholesterol increased to a greater extent in patients treated with pioglitazone than in the placebo-treated patients. There were no consistent differences for LDL and total cholesterol in patients treated with pioglitazone compared to placebo (Table 12).
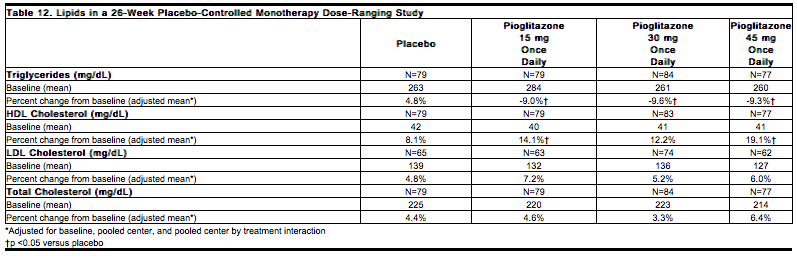
- In the two other monotherapy studies (16 weeks and 24 weeks) and in combination therapy studies with sulfonylurea (16 weeks and 24 weeks), metformin (16 weeks and 24 weeks) or insulin (16 weeks and 24 weeks), the results were generally consistent with the data above.
Glimepiride
- In healthy subjects, the time to reach maximal effect (minimum blood glucose concentrations) was approximately by two to three hours after single oral doses of glimepiride. The effects of HbA1C, fasting plasma glucose, and post-prandial glucose have been assessed in clinical trials.
Pharmacokinetics
Absorption and Bioavailability:
DUETACT
- Bioequivalence studies were conducted following a single dose of the DUETACT 30 mg/2 mg and 30 mg/4 mg tablets and concomitant administration of pioglitazone (30 mg) and glimepiride (2 mg or 4 mg) under fasting conditions in healthy subjects.
- Based on the area under the curve (AUC) and maximum concentration (Cmax) of both pioglitazone and glimepiride, DUETACT 30 mg/2 mg and 30 mg/4 mg were bioequivalent to pioglitazone 30 mg concomitantly administered with glimepiride (2 mg or 4 mg, respectively).
- Food did not change the systemic exposures of glimepiride or pioglitazone following administration of DUETACT. The presence of food did not significantly alter the time to peak serum concentration (Tmax) of glimepiride or pioglitazone and Cmax of pioglitazone. However, for glimepiride, there was a 22% increase in Cmaxwhen DUETACT was administered with food.
Pioglitazone
- Following once-daily administration of pioglitazone, steady-state serum concentrations of both pioglitazone and its major active metabolites, M-III (keto derivative of pioglitazone) and M-IV (hydroxyl derivative of pioglitazone), are achieved within seven days. At steady-state, M-III and M-IV reach serum concentrations equal to or greater than that of pioglitazone. At steady-state, in both healthy volunteers and patients with type 2 diabetes, pioglitazone comprises approximately 30% to 50% of the peak total pioglitazone serum concentrations (pioglitazone plus active metabolites) and 20% to 25% of the total AUC.
- Cmax, AUC, and trough serum concentrations (Cmin) for pioglitazone and M-III and M-IV, increased proportionally with administered doses of 15 mg and 30 mg per day.
- Following oral administration of pioglitazone, Tmax of pioglitazone was within two hours. Food delays Tmax to three to four hours but does not alter the extent of absorption (AUC).
Glimepiride
- Following single oral doses of glimepiride in healthy subjects and multiple oral doses in patients with type 2 diabetes Tmax was observed at two to three hours post-dose. When glimepiride was given with meals, the mean Cmax and AUC were decreased by 8% and 9%, respectively.
- Glimepiride does not accumulate in serum following multiple dosing. The pharmacokinetics of glimepiride does not differ between healthy subjects and patients with type 2 diabetes. Clearance (CL/F) of glimepiride after oral administration does not change over the 1 mg to 8 mg dose range, indicating linear pharmacokinetics.
- In healthy subjects, the intra- and inter-individual variabilities of glimepiride pharmacokinetic parameters were 15% to 23% and 24% to 29%, respectively.
Distribution
Pioglitazone
- The mean apparent volume of distribution (Vd/F) of pioglitazone following single-dose administration is 0.63 ± 0.41 (mean ± SD) L/kg of body weight. Pioglitazone is extensively protein bound (>99%) in human serum, principally to serum albumin. Pioglitazone also binds to other serum proteins, but with lower affinity. M-III and M-IV are also extensively bound (>98%) to serum albumin.
Glimepiride
- After intravenous (IV) dosing in healthy subjects, Vd/F was 8.8 L (113 mL/kg). Protein binding was greater than 99.5%.
Metabolism
Pioglitazone
- Pioglitazone is extensively metabolized by hydroxylation and oxidation; the metabolites also partly convert to glucuronide or sulfate conjugates. Metabolites M-III and M-IV are the major circulating active metabolites in humans.
- In vitro data demonstrate that multiple CYP isoforms are involved in the metabolism of pioglitazone which include CYP2C8 and, to a lesser degree, CYP3A4 with additional contributions from a variety of other isoforms including the mainly extrahepatic CYP1A1. In vivo study of pioglitazone in combination with gemfibrozil, a strong CYP2C8 inhibitor, showed that pioglitazone is a CYP2C8 substrate. Urinary 6ß-hydroxycortisol/cortisol ratios measured in patients treated with pioglitazone showed that pioglitazone is not a strong CYP3A4 enzyme inducer.
Glimepiride
- Glimepiride is completely metabolized by oxidative biotransformation after either an IV or oral dose. The major metabolites are the cyclohexyl hydroxy methyl derivative (M1) and the carboxyl derivative (M2). CYP2C9 is involved in the biotransformation of glimepiride to M1. M1 is further metabolized to M2 by one or several cytosolic enzymes. In animals, M1 possesses about one-third of the pharmacological activity of glimepiride, but it is unclear whether M1 results in clinically meaningful effects on blood glucose in humans. M2 is inactive.
- Excretion and Elimination
Pioglitazone
- Following oral administration, approximately 15% to 30% of the pioglitazone dose is recovered in the urine. Renal elimination of pioglitazone is negligible and the drug is excreted primarily as metabolites and their conjugates. It is presumed that most of the oral dose is excreted into the bile either unchanged or as metabolites and eliminated in the feces.
- The mean serum half-life (t1/2) of pioglitazone and its metabolites (M-III and M-IV) range from three to seven hours and 16 to 24 hours, respectively. Pioglitazone has an apparent clearance, CL/F, calculated to be five to seven L/hr.
Glimepiride
- When 14C-glimepiride was given orally to three healthy male subjects, approximately 60% of the total radioactivity was recovered in the urine in seven days. M1 and M2 accounted for 80% to 90% of the radioactivity recovered in the urine. The ratio of M1 to M2 in the urine was approximately 3:2 in two subjects and 4:1 in one subject. Approximately 40% of the total radioactivity was recovered in feces. M1 and M2 accounted for approximately 70% (ratio of M1 to M2 was 1:3) of the radioactivity recovered in feces. No parent drug was recovered from urine or feces. After IV dosing in patients, no significant biliary excretion of glimepiride or its M1 metabolite was observed. Total body clearance (CL) after IV dosing was 47.8 mL/min.
Renal Impairment
Pioglitazone
- The serum elimination half-life of pioglitazone, M-III, and M-IV remains unchanged in patients with moderate [creatinine clearance (CLcr) 30 to 50 mL/min] and severe (CLcr <30 mL/min) renal impairment when compared to subjects with normal renal function. Therefore, no dose adjustment in patients with renal impairment is required.
Glimepiride
- In a single-dose, open-label study glimepiride 3 mg was administered to patients with mild, moderate and severe renal impairment as estimated by CLcr: Group I consisted of five patients with mild renal impairment (CLcr >50 mL/min), Group II consisted of 3 patients with moderate renal impairment (CLcr = 20 to 50 mL/min) and Group III consisted of seven patients with severe renal impairment (CLcr <20 mL/min). Although, glimepiride serum concentrations decreased with decreasing renal function, Group III had a 2.3-fold higher mean AUC for M1 and an 8.6-fold higher mean AUC for M2 compared to corresponding mean AUCs in Group I. The t½ for glimepiride did not change, while the t½ for M1 and M2 increased as renal function decreased. Mean urinary excretion of M1 plus M2 as a percentage of dose decreased from 44.4% for Group I to 21.9% for Group II and 9.3% for Group III.
Hepatic Impairment
Pioglitazone
- Compared with healthy controls, subjects with impaired hepatic function (Child-Turcotte-Pugh Grade B/C) have an approximate 45% reduction in pioglitazone and total pioglitazone (pioglitazone, M-III, and M-IV) mean Cmax but no change in the mean AUC values. Therefore, no dose adjustment in patients with hepatic impairment is required.
- There are postmarketing reports of liver failure with pioglitazone and clinical trials have generally excluded patients with serum ALT >2.5 times the upper limit of the reference range. Use DUETACT with caution in patients with liver disease.
Glimepiride
- It is unknown whether there is an effect of hepatic impairment on glimepiride pharmacokinetics because the pharmacokinetics of glimepiride has not been adequately evaluated in patients with hepatic impairment.
Geriatric Patients
Pioglitazone
- In healthy elderly subjects, Cmax of pioglitazone was not significantly different, but AUC values were approximately 21% higher than those achieved in younger subjects. The mean t½ of pioglitazone was also prolonged in elderly subjects (about 10 hours) as compared to younger subjects (about seven hours). These changes were not of a magnitude that would be considered clinically relevant.
Glimepiride
- Glimepiride pharmacokinetics in patients with type 2 diabetes ≤65 years and those >65 years was compared in a multiple-dose study using 6 mg daily dose. There were no significant differences in glimepiride pharmacokinetics between the two age groups. The mean AUC at steady state for the older patients was approximately 13% lower than that for the younger patients; the mean weight-adjusted clearance for the older patients was approximately 11% higher than that for the younger patients.
Pediatric Patients
- No pharmacokinetic studies of DUETACT were performed in pediatric patients.
Pioglitazone
- Safety and efficacy of pioglitazone in pediatric patients have not been established. DUETACT is not recommended for use in pediatric patients.
Gender
Pioglitazone
- The mean Cmax and AUC values of pioglitazone were increased 20% to 60% in women compared to men. In controlled clinical trials, HbA1c decreases from baseline were generally greater for females than for males (average mean difference in HbA1c 0.5%). Because therapy should be individualized for each patient to achieve glycemic control, no dose adjustment is recommended based on gender alone.
Glimepiride
- There were no differences between males and females in the pharmacokinetics of glimepiride when adjustment was made for differences in body weight.
Ethnicity
Pioglitazone
- Pharmacokinetic data among various ethnic groups are not available.
Glimepiride
- No studies have been conducted to assess the effects of race on glimepiride pharmacokinetics but in placebo-controlled trials of glimepiride in patients with type 2 diabetes, the reduction in HbA1c was comparable in Caucasians (n=536), blacks (n=63), and Hispanics (n=63).
Obese Patients
- The pharmacokinetics of glimepiride and its metabolites were measured in a single-dose study involving 28 patients with type 2 diabetes who either had normal body weight or were morbidly obese. While the Tmax, CL/F, and Vd/F of glimepiride in the morbidly obese patients were similar to those in the normal weight group, the morbidly obese had lower Cmax and AUC than those of normal body weight. The mean Cmax, AUC0-24, AUC0-∞ values of glimepiride in normal vs. morbidly obese patients were 547 ± 218 ng/mL vs. 410 ± 124 ng/mL, 3210 ± 1030 hours·ng/mL vs. 2820 ± 1110 hours·ng/mL and 4000 ± 1320 hours·ng/mL versus 3280 ± 1360 hours·ng/mL, respectively.
Other Populations
Glimepiride
- There were no important differences in glimepiride metabolism in subjects identified as phenotypically different drug-metabolizers by their metabolism of sparteine. The pharmacokinetics of glimepiride in morbidly obese patients were similar to those in the normal weight group, except for a lower Cmax and AUC. However, since neither Cmax nor AUC values were normalized for body surface area, the lower values of Cmax and AUC for the obese patients were likely the result of their excess weight and not due to a difference in the kinetics of glimepiride.
Drug-Drug Interactions
- Coadministration of pioglitazone (45 mg) and a sulfonylurea (5 mg glipizide) administered orally once daily for seven days did not alter the steady-state pharmacokinetics of glipizide. Glimepiride and glipizide have similar metabolic pathways and are mediated by CYP2C9; therefore, drug-drug interaction between pioglitazone and glimepiride is considered unlikely. Specific pharmacokinetic drug interaction studies with DUETACT have not been performed, although such studies have been conducted with the individual pioglitazone and glimepiride components.
Pioglitazone
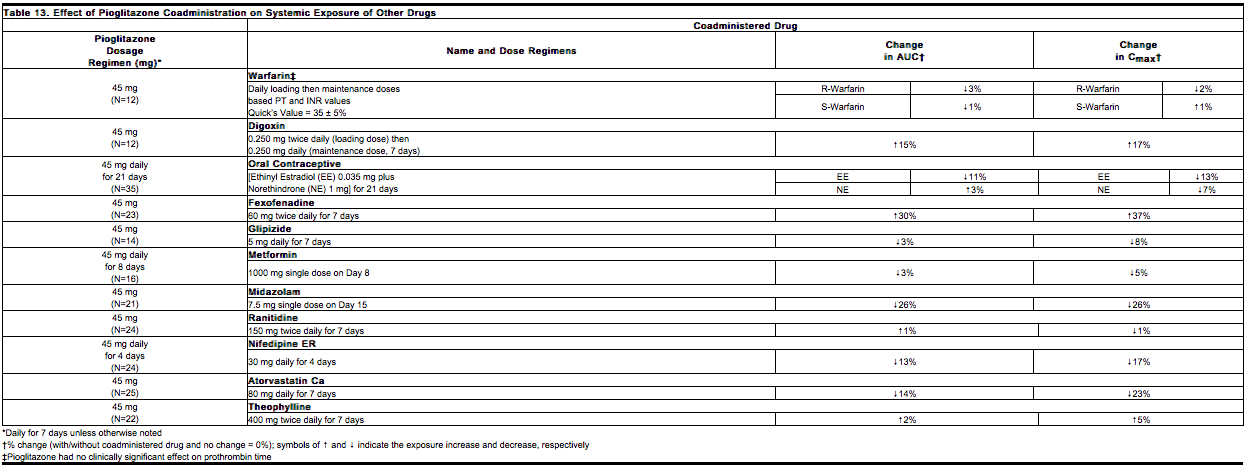
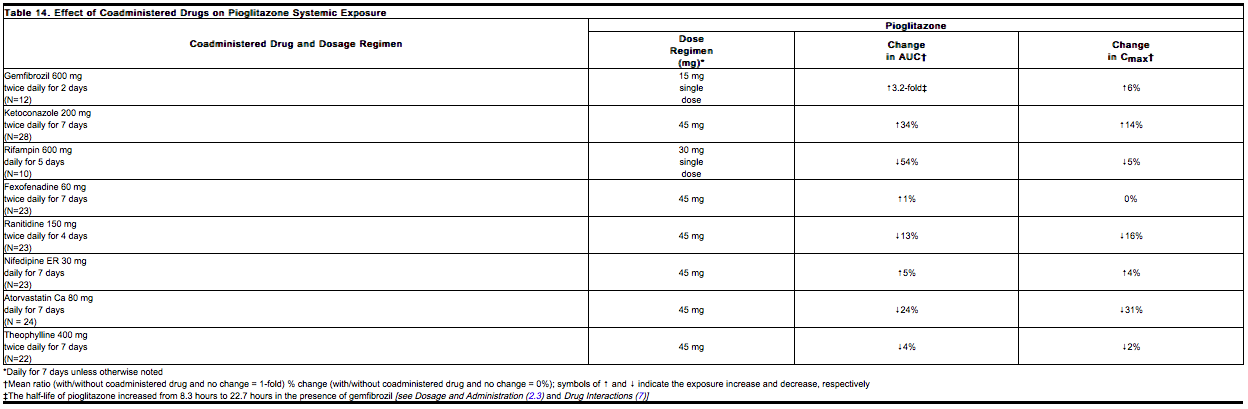
Glimepiride
Aspirin
- In a randomized, double-blind, two-period, crossover study, healthy subjects were given either placebo or aspirin 1 gram three times daily for a total treatment period of 5 days. On Day 4 of each study period, a single 1 mg dose of glimepiride was administered. The glimepiride doses were separated by a 14-day washout period. Coadministration of aspirin and glimepiride resulted in a 34% decrease in the mean glimepiride AUC and a 4% decrease in the mean glimepiride Cmax.
Cimetidine and Ranitidine
- In a randomized, open-label, 3-way crossover study, healthy subjects received either a single 4 mg dose of glimepiride alone, glimepiride with ranitidine (150 mg twice daily for 4 days; glimepiride was administered on Day 3), or glimepiride with cimetidine (800 mg daily for 4 days; glimepiride was administered on Day 3). Coadministration of cimetidine or ranitidine with a single 4 mg oral dose of glimepiride did not significantly alter the absorption and disposition of glimepiride.
Propranolol
- In a randomized, double-blind, two-period, crossover study, healthy subjects were given either placebo or propranolol 40 mg three times daily for a total treatment period of five days. On Day 4 or each study period, a single 2 mg dose of glimepiride was administered. The glimepiride doses were separated by a 14-day washout period. Concomitant administration of propranolol and glimepiride significantly increased glimepiride Cmax, AUC, and t1/2 by 23%, 22%, and 15%, respectively, and decreased glimepiride CL/F by 18%. The recovery of M1 and M2 from urine was not changed.
Warfarin
- In an open-label, two-way, crossover study, healthy subjects received 4 mg of glimepiride daily for 10 days. Single 25 mg doses of warfarin were administered six days before starting glimepiride and on Day 4 of glimepiride administration. The concomitant administration of glimepiride did not alter the pharmacokinetics of R- and S-warfarin enantiomers. No changes were observed in warfarin plasma protein binding. Glimepiride resulted in a statistically significant decrease in the pharmacodynamic response to warfarin. The reductions in mean area under the prothrombin time (PT) curve and maximum PT values during glimepiride treatment were 3.3% and 9.9%, respectively, and are unlikely to be clinically relevant.
Colesevelam
- Concomitant administration of colesevelam and glimepiride resulted in reductions in glimepiride AUC0-∞ and Cmax of 18% and 8%, respectively. When glimepiride was administered 4 hours prior to colesevelam, there was not significant change in glimepiride AUC0-∞ and Cmax, -6% and 3%, respectively
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- No animal studies have been conducted with DUETACT. The following data are based on findings in studies performed with pioglitazone or glimepiride individually.
Pioglitazone
- A two-year carcinogenicity study was conducted in male and female rats at oral doses up to 63 mg/kg (approximately 14 times the maximum recommended human oral dose of 45 mg based on mg/m2). Drug-induced tumors were not observed in any organ except for the urinary bladder of male rats. Benign and/or malignant transitional cell neoplasms were observed in male rats at 4 mg/kg/day and above (approximately equal to the maximum recommended human oral dose based on mg/m2). Urinary calculi with subsequent irritation and hyperplasia were postulated as the mechanism for bladder tumors observed in male rats. A two-year mechanistic study in male rats utilizing dietary acidification to reduce calculi formation was completed in 2009. Dietary acidification decreased but did not abolish the hyperplastic changes in the bladder. The presence of calculi exacerbated the hyperplastic response to pioglitazone but was not considered the primary cause of the hyperplastic changes.
- The relevance to humans of the bladder findings in the male rat cannot be excluded.
- A two-year carcinogenicity study was also conducted in male and female mice at oral doses up to 100 mg/kg/day (approximately 11 times the maximum recommended human oral dose based on mg/m2). No drug-induced tumors were observed in any organ.
- Pioglitazone hydrochloride was not mutagenic in a battery of genetic toxicology studies, including the Ames bacterial assay, a mammalian cell forward gene mutation assay (CHO/HPRT and AS52/XPRT), an in vitro cytogenetics assay using CHL cells, an unscheduled DNA synthesis assay, and an in vivo micronucleus assay.
- No adverse effects upon fertility were observed in male and female rats at oral doses up to 40 mg/kg pioglitazone hydrochloride daily prior to and throughout mating and gestation (approximately nine times the maximum recommended human oral dose based on mg/m2).
Glimepiride
- Studies in rats at doses of up to 5000 parts per million (ppm) in complete feed (approximately 340 times the maximum recommended human dose, based on surface area) for 30 months showed no evidence of carcinogenesis. In mice, administration of glimepiride for 24 months resulted in an increase in benign pancreatic adenoma formation that was dose-related and was thought to be the result of chronic pancreatic stimulation. No adenoma formation in mice was observed at a dose of 320 ppm in complete feed, or 46 − 54 mg/kg body weight/day. This is about 35 times the maximum human recommended dose of 8 mg once daily based on surface area.
- Glimepiride was non-mutagenic in a battery of in vitro and in vivo mutagenicity studies (Ames test, somatic cell mutation, chromosomal aberration, unscheduled DNA synthesis and mouse micronucleus test).
- There was no effect of glimepiride on male mouse fertility in animals exposed up to 2500 mg/kg body weight (>1,700 times the maximum recommended human dose based on surface area). Glimepiride had no effect on the fertility of male and female rats administered up to 4000 mg/kg body weight (approximately 4,000 times the maximum recommended human dose based on surface area).
Animal Toxicology and/or Pharmacology
Pioglitazone
- Heart enlargement has been observed in mice (100 mg/kg), rats (4 mg/kg and above), and dogs (3 mg/kg) treated orally with the pioglitazone hydrochloride component of DUETACT (approximately 11, one, and two times the maximum recommended human oral dose for mice, rats, and dogs, respectively, based on mg/m2). In a one-year rat study, drug-related early death due to apparent heart dysfunction occurred at an oral dose of 160 mg/kg/day (approximately 35 times the maximum recommended human oral dose based on mg/m2). Heart enlargement was seen in a 13-week study in monkeys at oral doses of 8.9 mg/kg and above (approximately four times the maximum recommended human oral dose based on mg/m2), but not in a 52-week study at oral doses up to 32 mg/kg (approximately 13 times the maximum recommended human oral dose based on mg/m2).
Clinical Studies
- There have been no clinical efficacy studies conducted with DUETACT. However, the efficacy and safety of the separate components have been previously established. The coadministration of pioglitazone and a sulfonylurea, including glimepiride, has been evaluated for efficacy and safety in two clinical studies. These clinical studies established an added benefit of pioglitazone in glycemic control of patients with inadequately controlled type 2 diabetes while on sulfonylurea therapy. Bioequivalence of DUETACT with coadministered pioglitazone and glimepiride tablets was demonstrated at the 30 mg/2 mg and 30 mg/4 mg dosage strengths .
- Two clinical trials were conducted with pioglitazone in combination with a sulfonylurea. Both studies included patients with type 2 diabetes on any dose of a sulfonylurea, either alone or in combination with another antidiabetic agent. All other antidiabetic agents were withdrawn at least three weeks prior to starting study treatment.
- In the first study, 560 patients were randomized to receive 15 mg or 30 mg of pioglitazone or placebo once daily for 16 weeks in addition to their current sulfonylurea regimen. Treatment with pioglitazone as add-on to sulfonylurea produced statistically significant improvements in HbA1c and FGP at endpoint compared to placebo add-on to sulfonylurea (Table 15).
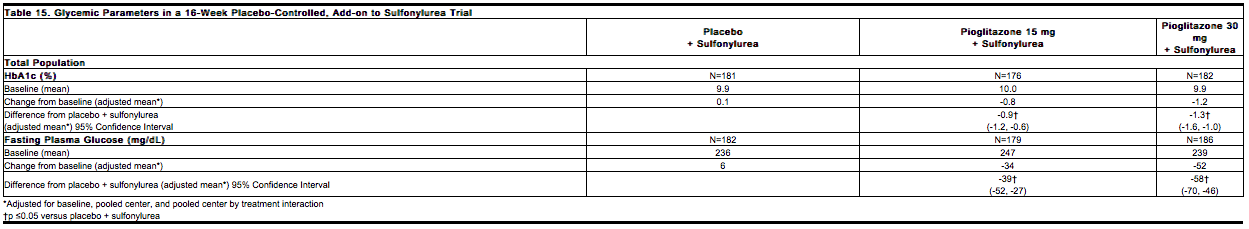
- In the second trial, 702 patients were randomized to receive 30 mg or 45 mg of pioglitazone once daily for 24 weeks in addition to their current sulfonylurea regimen. The mean reduction from baseline at Week 24 in HbA1c was 1.6% for the 30 mg dose and 1.7% for the 45 mg dose (see Table 16). The mean reduction from baseline at Week 24 in FPG was 52 mg/dL for the 30 mg dose and 56 mg/dL for the 45 mg dose.
- The therapeutic effect of pioglitazone in combination with sulfonylurea was observed in patients regardless of the sulfonylurea dose.
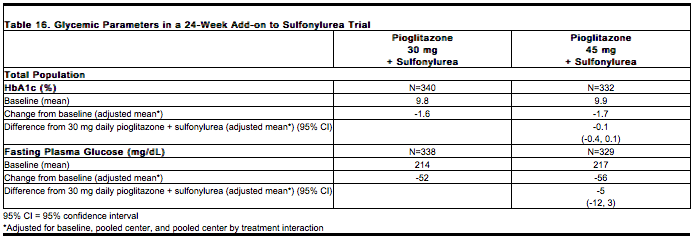
How Supplied
- DUETACT is available in 30 mg pioglitazone plus 2 mg glimepiride or 30 mg pioglitazone plus 4 mg glimepiride tablets as follows:
30 mg/2 mg tablet: white to off-white, round, convex tablets, debossed with 4833G on one side and 30/2 on the other, available in:
- NDC 64764-302-30 Bottles of 30
- NDC 64764-302-90 Bottles of 90
30 mg/4 mg tablet: white to off-white, round, convex tablets, debossed with 4833G on one side and 30/4 on the other, available in:
- NDC 64764-304-30 Bottles of 30
- NDC 64764-304-90 Bottles of 90
Storage
There is limited information regarding Pioglitazone/Glimepiride Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Pioglitazone/Glimepiride |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Pioglitazone/Glimepiride |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Keep container tightly closed and protect from moisture and humidity.
Precautions with Alcohol
Alcohol-Pioglitazone/Glimepiride interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Duetact
Look-Alike Drug Names
There is limited information about the look alike drug names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Pioglitazone/Glimepiride |Pill Name=Dueract_30 2 mg_NDC 64764-302.jpg |Drug Name=Duetact 30/2 MG Oral Tablet |Pill Ingred=croscarmellose sodium, lactose monohydrate, magnesium stearate, hydroxypropyl cellulose (type h), polysorbate 80, cellulose, microcrystalline|+sep=; |Pill Imprint=30;2;4833G |Pill Dosage=30/2 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=8.00 |Pill Scoring=1 |Pill Image= |Drug Author=Takeda Pharmaceuticals America, Inc. |NDC=64764-302
}}
{{#subobject:
|Page Name=Pioglitazone/Glimepiride |Pill Name=Dueract_30 4 mg_NDC 64764-304.jpg |Drug Name=Duetact 30/4 MG Oral Tablet |Pill Ingred=croscarmellose sodium, lactose monohydrate, magnesium stearate, hydroxypropyl cellulose (type h), polysorbate 80, cellulose, microcrystalline|+sep=; |Pill Imprint=30;4;4833G |Pill Dosage=30/4 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=9.00 |Pill Scoring=1 |Pill Image= |Drug Author=Takeda Pharmaceuticals America, Inc. |NDC=64764-304
}}
{{#subobject:
|Label Page=Pioglitazone/Glimepiride |Label Name=Duetact_label_01.jpg
}}
{{#subobject:
|Label Page=Pioglitazone/Glimepiride |Label Name=Duetact_label_02.jpg
}}
{{#subobject:
|Label Page=Pioglitazone/Glimepiride |Label Name=Duetact_panel_01.png
}}
{{#subobject:
|Label Page=Pioglitazone/Glimepiride |Label Name=Duetact_panel_02.png
}}