Chlorpropamide
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Chlorpropamide is a 1st generation sulfonylurea and hypoglycemic agent that is FDA approved for the treatment of type 2 diabetes mellitus. Common adverse reactions include hypoglycemia, diarrhea, hunger, loss of appetite, nausea and vomiting.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- There is no fixed dosage regimen for the management of type 2 diabetes with chlorpropamide or any other hypoglycemic agent. The patient’s blood glucose must be monitored periodically to determine the minimum effective dose for the patient; to detect primary failure, i.e., inadequate lowering of blood glucose at the maximum recommended dose of medication; and to detect secondary failure, i.e., loss of an adequate blood glucose lowering response after an initial period of effectiveness. Glycosylated hemoglobin levels may also be of value in monitoring the patient’s response to therapy.
Short-term administration of chlorpropamide may be sufficient during periods of transient loss of control in patients usually controlled well on diet.
- The total daily dosage is generally taken at a single time each morning with breakfast. Occasionally cases of gastrointestinal intolerance may be relieved by dividing the daily dosage. A loading or priming dose is not necessary and should not be used.
Initial Therapy
- The mild to moderately severe, middle-aged, stable type 2 diabetes patient should be started on 250 mg daily. In elderly patients, debilitated or malnourished patients, and patients with impaired renal function or impaired hepatic function, the initial and maintenance dosing should be conservative to avoid hypoglycemic reactions. Older patients should be started on smaller amounts of chlorpropamide, in the range of 100 mg to 125 mg daily.
- No transition period is necessary when transferring patients from other oral hypoglycemic agents to chlorpropamide. *The other agent may be discontinued abruptly and chlorpropamide started at once. In prescribing chlorpropamide, due consideration must be given to its greater potency.
- Many mild to moderately severe, middle-aged, stable type 2 diabetes patients receiving insulin can be placed directly on the oral drug and their insulin abruptly discontinued. For patients requiring more than 40 units of insulin daily, therapy with chlorpropamide may be initiated with a 50% reduction in insulin for the first few days, with subsequent further reductions dependent upon the response.
- During the initial period of therapy with chlorpropamide, hypoglycemic reactions may occasionally occur, particularly during the transition from insulin to the oral drug. Hypoglycemia within 24 hours after withdrawal of the intermediate or long-acting types of insulin will usually prove to be the result of insulin carry-over and not primarily due to the effect of chlorpropamide.
- During the insulin withdrawal period, the patient should self-monitor glucose levels at least 3 times daily. If they are abnormal, the physician should be notified immediately. In some cases, it may be advisable to consider hospitalization during the transition period.
- Five to 7 days after the initial therapy, the blood level of chlorpropamide reaches a plateau. Dosage may subsequently be adjusted upward or downward by increments of not more than 50 mg to 125 mg at intervals of 3 to 5 days to obtain optimal control. More frequent adjustments are usually undesirable.
Maintenance Therapy
- Most moderately severe, middle-aged, stable type 2 diabetes patients are controlled by approximately 250 mg daily. Many investigators have found that some milder diabetics do well on daily doses of 100 mg or less. Many of the more severe diabetics may require 500 mg daily for adequate control. Patients who do not respond completely to 500 mg daily will usually not respond to higher doses. Maintenance doses above 750 mg daily should be avoided.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Chlorpropamide in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Chlorpropamide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Chlorpropamide FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Chlorpropamide in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Chlorpropamide in pediatric patients.
Contraindications
Chlorpropamide tablets are contraindicated in patients with:
- Known hypersensitivity to any component of this medicine.
- Type 1 diabetes mellitus, diabetic ketoacidosis, with or without coma. This condition should be treated with insulin.
Warnings
Special Warning on Increased Risk of Cardiovascular Mortality
- The administration of oral hypoglycemic drugs has been reported to be associated with increased cardiovascular mortality as compared to treatment with diet alone or diet plus insulin. This warning is based on the study conducted by the University Group Diabetes Program (UGDP), a long-term prospective clinical trial designed to evaluate the effectiveness of glucose-lowering drugs in preventing or delaying vascular complications in patients with non-insulin-dependent diabetes. The study involved 823 patients who were randomly assigned to one of four treatment groups (Diabetes, 19 [supp 2]:747-830, 1970).
- UGDP reported that patients treated for 5 to 8 years with diet plus a fixed dose of tolbutamide (1.5 grams per day) had a rate of cardiovascular mortality approximately 2½ times that of patients treated with diet alone. A significant increase in total mortality was not observed, but the use of tolbutamide was discontinued based on the increase in cardiovascular mortality, thus limiting the opportunity for the study to show an increase in overall mortality. Despite controversy regarding the interpretation of these results, the findings of the UGDP study provide an adequate basis for this warning. The patient should be informed of the potential risks and advantages of chlorpropamide and of alternative modes of therapy.
- Although only one drug in the sulfonylurea class (tolbutamide) was included in this study, it is prudent from a safety standpoint to consider that this warning may also apply to other oral hypoglycemic drugs in this class, in view of their close similarities in mode of action and chemical structure.
Adverse Reactions
Clinical Trials Experience
- Body as a Whole: Disulfiram-like reactions have rarely been reported with chlorpropamide
- Central and Peripheral Nervous System: Dizziness and headache.
- Hypoglycemia
- Gastrointestinal: Gastrointestinal disturbances are the most common reactions; nausea has been reported in less than 5% of patients, and diarrhea, vomiting, anorexia, and hunger in less than 2%. Other gastrointestinal disturbances have occurred in less than 1% of patients including proctocolitis. They tend to be dose related and may disappear when dosage is reduced.
- Liver/Biliary: Cholestatic jaundice may occur rarely; chlorpropamide should be discontinued if this occurs. Hepatic porphyria and disulfiram-like reactions have been reported with chlorpropamide.
- Skin/Appendages: Pruritus has been reported in less than 3% of patients. Other allergic skin reactions, e.g., urticaria and maculopapular eruptions have been reported in approximately 1% or less of patients. These may be transient and may disappear despite continued use of chlorpropamide; if skin reactions persist the drug should be discontinued.
- As with other sulfonylureas, porphyria cutanea tarda and photosensitivity reactions have been reported. Skin eruptions rarely progressing to erythema multiforme and exfoliative dermatitis have also been reported.
- Hematologic Reactions: Leukopenia, agranulocytosis, thrombocytopenia, hemolytic anemia, aplastic anemia, pancytopenia, and eosinophilia have been reported with sulfonylureas.
- Metabolic/Nutritional Reactions: Hypoglycemia. Hepatic porphyria and disulfiram-like reactions have been reported with chlorpropamide.
- Endocrine Reactions: On rare occasions, chlorpropamide has caused a reaction identical to the syndrome of inappropriate antidiuretic hormone (ADH) secretion. The features of this syndrome result from excessive water retention and include hyponatremia, low serum osmolality, and high urine osmolality. This reaction has also been reported for other sulfonylureas.
Postmarketing Experience
There is limited information regarding Chlorpropamide Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Chlorpropamide Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Animal reproductive studies have not been conducted with chlorpropamide. It is also not known whether chlorpropamide can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Chlorpropamide should be given to a pregnant woman only if the potential benefits justify the potential risk to the patient and fetus.
- Because data suggest that abnormal blood glucose levels during pregnancy are associated with a higher incidence of congenital abnormalities, many experts recommend that insulin be used during pregnancy to maintain blood glucose levels as close to normal as possible.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Chlorpropamide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Chlorpropamide during labor and delivery.
Nursing Mothers
- An analysis of a composite of two samples of human breast milk each taken 5 hours after ingestion of 500 mg of chlorpropamide by a patient revealed a concentration of 5 mcg/mL. For reference, the normal peak blood level of chlorpropamide after a single 250 mg dose is 30 mcg/mL. Therefore, it is not recommended that a woman breast-feed while taking this medication.
Pediatric Use
- Safety and effectiveness in children have not been established.
Geriatic Use
There is no FDA guidance on the use of Chlorpropamide in geriatric settings.
Gender
There is no FDA guidance on the use of Chlorpropamide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Chlorpropamide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Chlorpropamide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Chlorpropamide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Chlorpropamide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Chlorpropamide in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Chlorpropamide Administration in the drug label.
Monitoring
There is limited information regarding Chlorpropamide Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Chlorpropamide and IV administrations.
Overdosage
- Overdosage of sulfonylureas including chlorpropamide can produce hypoglycemia. Mild hypoglycemic symptoms without loss of consciousness or neurologic findings should be treated aggressively with oral glucose and adjustments in drug dosage and/or meal patterns. Close monitoring should continue until the physician is assured that the patient is out of danger. Severe hypoglycemic reactions with coma, seizure, or other neurological impairment occur infrequently, but constitute medical emergencies requiring immediate hospitalization. If hypoglycemic coma is diagnosed or suspected, the patient should be given a rapid intravenous injection of concentrated (50%) glucose solution. This should be followed by a continuous infusion of a more dilute (10%) glucose solution at a rate that will maintain the blood glucose at a level above 100 mg/dL. Patients should be closely monitored for a minimum of 24 to 48 hours since hypoglycemia may recur after apparent clinical recovery.
Pharmacology
Mechanism of Action
- Chlorpropamide appears to lower the blood glucose acutely by stimulating the release of insulin from the pancreas, an effect dependent upon functioning beta cells in the pancreatic islets. The mechanism by which chlorpropamide lowers blood glucose during long-term administration has not been clearly established. Extrapancreatic effects may play a part in the mechanism of action of oral sulfonylurea hypoglycemic drugs. While chlorpropamide is a sulfonamide derivative, it is devoid of antibacterial activity.
Structure
There is limited information regarding Chlorpropamide Structure in the drug label.
Pharmacodynamics
- Chlorpropamide exerts a hypoglycemic effect in healthy subjects within one hour, becoming maximal at 3 to 6 hours and persisting for at least 24 hours. The potency of chlorpropamide is approximately 6 times that of tolbutamide. Some experimental results suggest that its increased duration of action may be the result of slower excretion and absence of significant deactivation.
Pharmacokinetics
- Chlorpropamide is absorbed rapidly from the gastrointestinal tract. Within one hour after a single oral dose, it is readily detectable in the blood, and the level reaches a maximum within 2 to 4 hours. It undergoes metabolism in humans and it is excreted in the urine as unchanged drug and as hydroxylated or hydrolyzed metabolites. The biological half-life of chlorpropamide averages about 36 hours. Within 96 hours, 80% to 90% of a single oral dose is excreted in the urine. However, long-term administration of therapeutic doses does not result in undue accumulation in the blood, since absorption and excretion rates become stabilized in about 5 to 7 days after the initiation of therapy.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies with chlorpropamide have not been conducted to evaluate carcinogenic or mutagenic potential.
- Rats treated with continuous chlorpropamide therapy for 6 to 12 months showed varying degrees of suppression of spermatogenesis at a dose level of 250 mg/kg (five times the human dose based on body surface area). The extent of suppression seemed to follow that of growth retardation associated with chronic administration of high-dose chlorpropamide in rats. The human dose of chlorpropamide is 500 mg/day (300 mg/M2). Six- and twelve-month toxicity work in the dog and rat, respectively, indicates the 150 mg/kg is well tolerated. Therefore, the safety margins based upon body-surface-area comparisons are 3 times human exposure in the rat and 10 times human exposure in the dog.
Clinical Studies
There is limited information regarding Chlorpropamide Clinical Studies in the drug label.
How Supplied
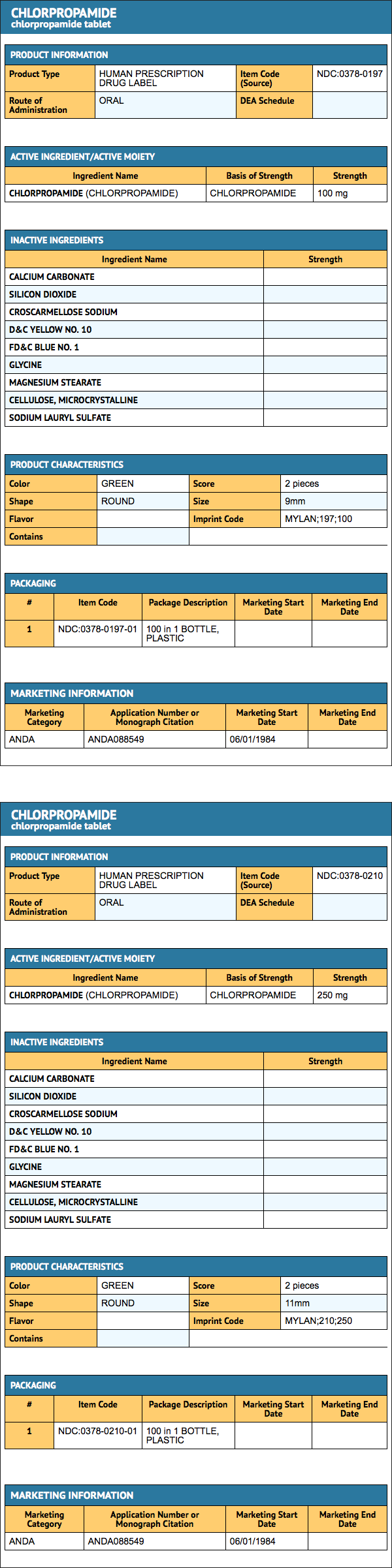
Chlorpropamide Tablets, USP are available containing either 100 mg or 250 mg of Chlorpropamide, USP.
The 100 mg tablets are green round, scored tablets debossed with MYLAN above the score and 197 below the score on one side of the tablet and 100 on the other side. They are available as follows:
- NDC 0378-0197-01: bottles of 100 tablets
- NDC 0378-0197-05: bottles of 500 tablets
The 250 mg tablets are green round, scored tablets debossed with MYLAN above the score and 210 below the score on one side of the tablet and 250 on the other side. They are available as follows:
- NDC 0378-0210-01: bottles of 100 tablets
- NDC 0378-0210-10: bottles of 1000 tablets
Storage
Store at 20° to 25°C (68° to 77°F). Protect from light.
Images
Drug Images
{{#ask: Page Name::Chlorpropamide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Chlorpropamide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Chlorpropamide Patient Counseling Information in the drug label.
Precautions with Alcohol
- Alcohol-Chlorpropamide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Chlorpropamide Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.