Microtubule
|
WikiDoc Resources for Microtubule |
|
Articles |
|---|
|
Most recent articles on Microtubule Most cited articles on Microtubule |
|
Media |
|
Powerpoint slides on Microtubule |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Microtubule at Clinical Trials.gov Clinical Trials on Microtubule at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Microtubule
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Microtubule Discussion groups on Microtubule Patient Handouts on Microtubule Directions to Hospitals Treating Microtubule Risk calculators and risk factors for Microtubule
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Microtubule |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Microtubules are one of the components of the cytoskeleton. They have diameter of ~ 24 nm and length varying from several micrometers to possibly millimeters in axons of nerve cells. Microtubules serve as structural components within cells and are involved in many cellular processes including mitosis, cytokinesis, and vesicular transport.
Structure
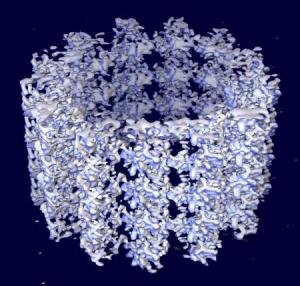
Microtubules are polymers of α- and β-tubulin dimers. The tubulin dimers polymerize end to end in protofilaments. The protofilaments then bundle in hollow cylindrical filaments. Typically, the protofilaments arrange themselves in an imperfect helix with one turn of the helix containing 13 tubulin dimers each from a different protofilament. The image above illustrates a small section of microtubule, a few αβ dimers in length.
Another important feature of microtubule structure is polarity. Tubulin polymerizes end to end with the α subunit of one tubulin dimer contacting the β subunit of the next. Therefore, in a protofilament, one end will have the α subunit exposed while the other end will have the β subunit exposed. These ends are designated (−) and (+) respectively. The protofilaments bundle parallel to one another, so in a microtubule, there is one end, the (+) end, with only β subunits exposed while the other end, the (−) end, only has α subunits exposed.
Organization within cells
Microtubules are nucleated and organized by the microtubule organizing centers (MTOCs), such as centrosomes and basal bodies. They are part of a structural network (the cytoskeleton) within the cell's cytoplasm, but, in addition to structural support, microtubules take part in many other processes, as well. They are capable of growing and shrinking in order to generate force, and there are also motor proteins that move along the microtubule. A notable structure involving microtubules is the mitotic spindle used by eukaryotic cells to segregate their chromosomes correctly during cell division. Microtubules are also part of the cilia and flagella of eukaryotic cells (prokaryote flagella are entirely different). Microtubules also move organelles and cell structures to new locations.
Nucleation and growth
Polymerization of microtubules is nucleated in a microtubule organizing center. Contained within the MTOC is another type of tubulin, γ-tubulin, which is distinct from the α and β subunits which compose the microtubules themselves. The γ-tubulin combines with several other associated proteins to form a circular structure known as the "γ-tubulin ring complex." This complex acts as a scaffold for α/β tubulin dimers to begin polymerization; it acts as a cap of the (−) end while microtubule growth continues away from the MTOC in the (+) direction.
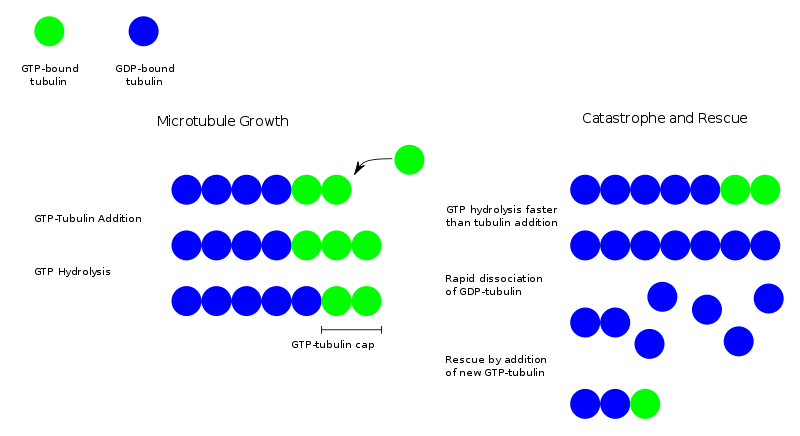
Dynamic instability
During polymerization, both the α- and β-subunits of the tubulin dimer are bound to a molecule of GTP. The GTP bound to α-tubulin is stable, but the GTP bound to β-tubulin may be hydrolized to GDP shortly after assembly. The kinetics of GDP-tubulin are different from those of GTP-tubulin; GDP-tubulin is prone to depolymerization. A GDP-bound tubulin subunit at the tip of a microtubule will fall off, though a GDP-bound tubulin in the middle of a microtubule cannot spontaneously pop out. Since tubulin adds onto the end of the microtubule only in the GTP-bound state, there is generally a cap of GTP-bound tubulin at the tip of the microtubule, protecting it from disassembly. When hydrolysis catches up to the tip of the microtubule, it begins a rapid depolymerization and shrinkage. This switch from growth to shrinking is called a catastrophe. GTP-bound tubulin can begin adding to the tip of the microtubule again, providing a new cap and protecting the microtubule from shrinking. This is referred to as rescue.
In vivo microtubule dynamics vary considerably. Assembly, disassembly and catastrophe rates depend on which microtubule-associated proteins (MAPs) are present.
Chemical effects on microtubule dynamics
Microtubule dynamics can also be altered by drugs. For example, the taxane drug class (e.g. paclitaxel or docetaxel), used in the treatment of cancer, blocks dynamic instability by stabilizing GDP-bound tubulin in the microtubule. Thus, even when hydrolysis of GTP reaches the tip of the microtubule, there is no depolymerization and the microtubule does not shrink back. Nocodazole and Colchicine have the opposite effect, blocking the polymerization of tubulin into microtubules.
Motor proteins
In addition to movement generated by the dynamic instability of the microtubule itself, the fibers are substrates along which motor proteins can move. The major microtubule motor proteins are kinesin, which generally moves towards the (+) end of the microtubule, and dynein, which generally moves towards the (−) end.
Microtubules and consciousness
Roger Penrose, a mathematician and Stuart Hameroff, an anesthesiologist developed a speculative theory linking microtubules to consciousness. Their theory, known as Orch-OR (orchestrated objective reduction), states that consciousness is a result of quantum computing in the brain, based on tubulin molecules in microtubules as qubits. There have been no empirical tests of the theory and it is regarded with skepticism in the scientific community.
Microtubules as drug target
http://www.scienceboard.net/community/perspectives.165.html
Additional images
-
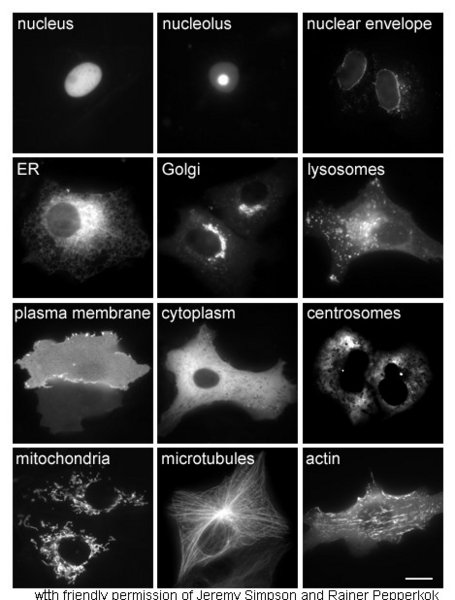
Proteins in different cellular compartments and structures tagged with green fluorescent protein.
ca:Microtúbul cs:Mikrotubulus da:Mikrotubuli de:Mikrotubulus it:Microtubulo lt:Mikrovamzdeliai mk:Микротубула nl:Microtubulus sk:Mikrotubuly sr:Микротубуле fi:Mikrotubulus